Safeguarding Bee Health: Insights from a Collaborative Monitoring and Prevention Project Against Pesticide Poisonings
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
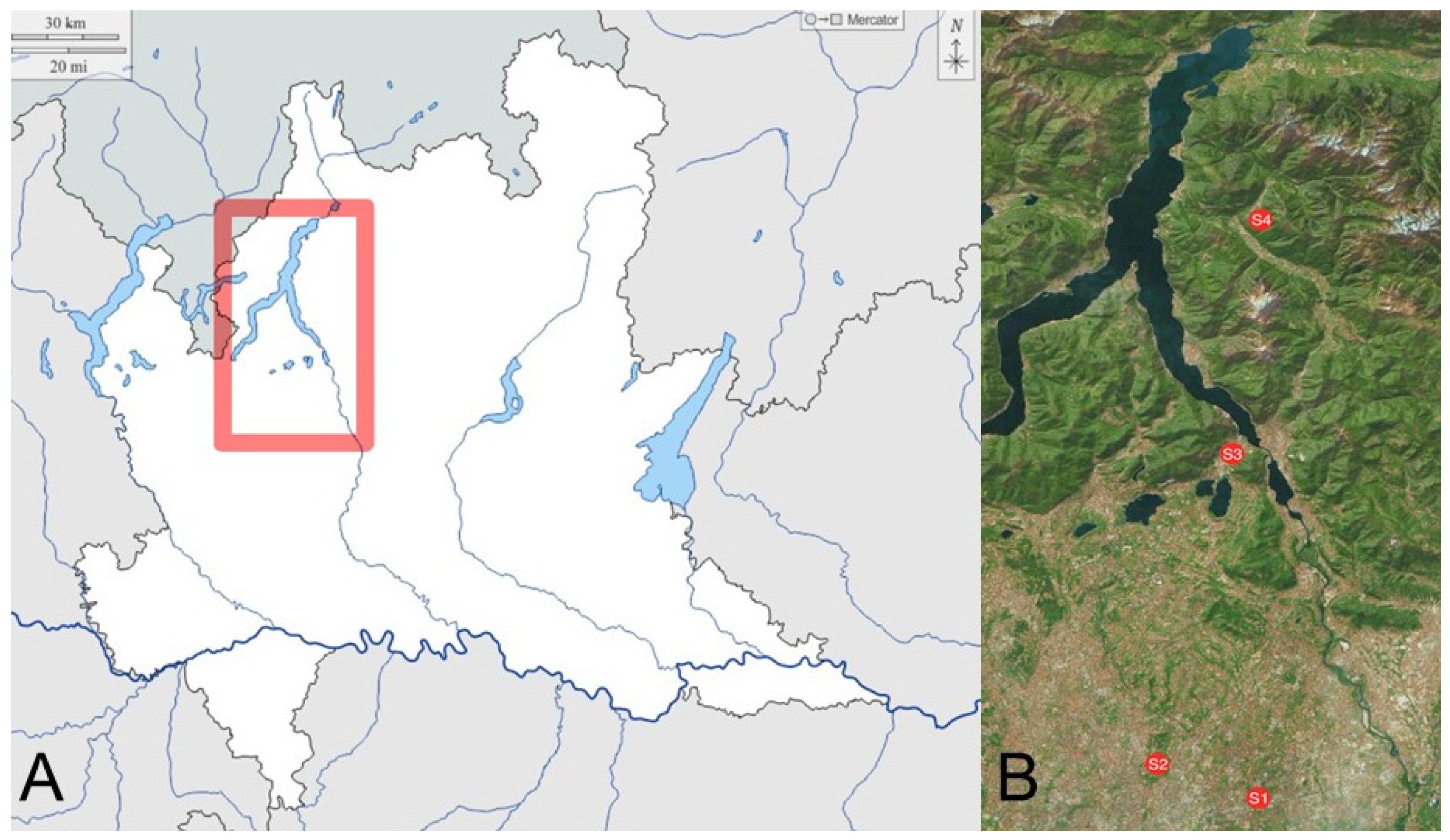
2.1. Sentinel Apiaries
2.2. Sampling Activities
2.3. Chemical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon-Delso, N.; San Martin, G.; Bruneau, E.; Minsart, L.A.; Mouret, C.; Hautier, L. Honeybee Colony Disorder in Crop Areas: The Role of Pesticides and Viruses. PLoS ONE 2014, 9, e103073. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Lin, Z.G.; Shen, S.Y.; Wang, K.; Ji, T. Biotic and abiotic stresses on honeybee health. Integr. Zool. 2023, 19, 442–457. [Google Scholar] [CrossRef]
- Sandrock, C.; Tanadini, M.; Tanadini, L.G.; Fauser-Misslin, A.; Potts, S.G.; Neumann, P. Impact of Chronic Neonicotinoid Exposure on Honeybee Colony Performance and Queen Supersedure. PLoS ONE 2014, 9, e103592. [Google Scholar] [CrossRef]
- Di Noi, A.; Casini, S.; Campani, T.; Cai, G.M.; Caliani, I. Review on Sublethal Effects of Environmental Contaminants in Honey Bees (Apis mellifera), Knowledge Gaps and Future Perspectives. Int. J. Environ. Res. Public Health 2021, 18, 1863. [Google Scholar] [CrossRef] [PubMed]
- Blettler, D.C.; Biurrun-Manresa, J.A.; Fagúndez, G.A. A review of the effects of agricultural intensification and the use of pesticides on honey bees and their products and possible palliatives. Span. J. Agric. Res. 2022, 20, e03R02. [Google Scholar] [CrossRef]
- Tosi, S.; Sfeir, C.; Carnesecchi, E.; vanEngelsdorp, D.; Chauzat, M.P. Lethal, sublethal, and combined effects of pesticides on bees: A meta-analysis and new risk assessment tools. Sci. Total Environ. 2022, 844, 156857. [Google Scholar] [CrossRef] [PubMed]
- Blacquière, T.; van der Steen, J.J.M. Three years of banning neonicotinoid insecticides based on sub-lethal effects: Can we expect to see effects on bees? Pest Manag. Sci. 2017, 73, 1299–1304. [Google Scholar] [CrossRef]
- D-maps.com Free Maps. Available online: https://d-maps.com/carte.php?num_car=8172&lang=en (accessed on 23 January 2025).
- Document No. SANTE/11312/2021 v2-Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. Available online: https://food.ec.europa.eu/system/files/2023-11/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 23 January 2025).
- Albero, B.; Miguel, E.; García-Valcárcel, A.I. Acaricide residues in beeswax. Implications in honey, brood and honeybee. Environ. Monit. Assess. 2023, 195, 15. [Google Scholar] [CrossRef] [PubMed]
- Végh, R.; Csóka, M.; Mednyánszky, Z.; Sipos, L. Pesticide residues in bee bread, propolis, beeswax and royal jelly-A review of the literature and dietary risk assessment. Food Chem. Toxicol. 2023, 176, 19. [Google Scholar] [CrossRef] [PubMed]
- Bartling, M.-T.; Brandt, A.; Hollert, H.; Vilcinskas, A. Current Insights into Sublethal Effects of Pesticides on Insects. Int. J. Mol. Sci. 2024, 25, 6007. [Google Scholar] [CrossRef]
- van Engelsdorp, D.; Traynor, K.S.; Andree, M.; Lichtenberg, E.M.; Chen, Y.P.; Saegerman, C.; Cox-Foster, D.L. Colony Collapse Disorder (CCD) and bee age impact honey bee pathophysiology. PLoS ONE 2017, 12, e0179535. [Google Scholar] [CrossRef]
- Cullen, M.G.; Thompson, L.J.; Carolan, J.C.; Stout, J.C.; Stanley, D.A. Fungicides, herbicides and bees: A systematic review of existing research and methods. PLoS ONE 2019, 14, e0225743. [Google Scholar] [CrossRef] [PubMed]
- Bonmatin, J.M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef]
- Perugini, M.; Tulini, S.M.R.; Zezza, D.; Fenucci, S.; Conte, A.; Amorena, M. Occurrence of agrochemical residues in beeswax samples collected in Italy during 2013–2015. Sci. Total Environ. 2018, 625, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bayo, F.; Goka, K. Pesticide Residues and Bees—A Risk Assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Mosquito Control. Available online: https://www.epa.gov/mosquitocontrol (accessed on 13 January 2025).
- ATS Brianza. Guidelines for Mosquito Control. Available online: https://www.ats-brianza.it/images/pdf/veteriari/linee-guida-zanzare-con-allegati.pdf (accessed on 23 January 2025).
- Onwuchekwa-Henry, C.B.; Coe, R.; Van Ogtrop, F.; Roche, R.; Tan, D.K.Y. Efficacy of Pendimethalin Rates on Barnyard Grass (Echinochloa crus-galli) (L.) Beauv) and Their Effect on Photosynthetic Performance in Rice. Agronomy 2023, 13, 582. [Google Scholar] [CrossRef]
- Morales, M.M.; Ramos, M.J.G.; Vázquez, P.P.; Galiano, F.J.D.; Valverde, M.G.; López, V.G.; Flores, J.M.; Fernández-Alba, A.R. Distribution of chemical residues in the beehive compartments and their transfer to the honeybee brood. Sci. Total Environ. 2020, 710, 136288. [Google Scholar] [CrossRef]
- Mitton, G.A.; Arcerito, F.M.; Cooley, H.; de Landa, G.F.; Eguaras, M.J.; Ruffinengo, S.R.; Maggi, M.D. More than sixty years living with Varroa destructor: A review of acaricide resistance. Int. J. Pest Manag. 2022, 1–18. [Google Scholar] [CrossRef]
- Premrov Bajuk, B.; Babnik, K.; Snoj, T.; Milčinski, L.; Pislak Ocepek, M.; Škof, M.; Jenčič, V.; Filazi, A.; Štajnbaher, D.; Kobal, S. Coumaphos residues in honey, bee brood, and beeswax after Varroa treatment. Apidologie 2017, 48, 588–598. [Google Scholar] [CrossRef]
- Bischoff, K.; Moiseff, J. The role of the veterinary diagnostic toxicologist in apiary health. J. Vet. Diagn. Investig. 2023, 35, 597–616. [Google Scholar] [CrossRef] [PubMed]
| Year | Sentinel Apiary | April | May/1 | May/2 | June/1 | June/2 | July | August/1 | August/2 | September/1 | September/2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2021 | S1 | 0 | 0.016 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S3 | 0 | 0.015 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S4 | 0 | 0.012 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2022 | S1 | 0.015 | 0.014 | 0 | 0 | 0 | 0 | 0.012 | 0 | 0 | 0 |
| S2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S3 | 0 | 0.012 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Year | Target | April | May/1 | May/2 | June/1 | June/2 | July | August/1 | August/2 | September/1 | September/2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2021 | Cypermethrin | 0 | 0 | 0 | 0.110 | 0.021 | 0.031 | 0 | 0 | 0 | 0 |
| Permethrin | 0 | 0 | 0 | 0 | 0.017 | 0 | 0 | 0 | 0 | 0 | |
| 2022 | Cypermethrin | 0.013 | 0 | 0.014 | 0.012 | 0.015 | 0.160 | 0.110 | 0.050 | 0.140 | 0.032 |
| Permethrin | 0.011 | 0 | 0.017 | 0.018 | 0.017 | 0 | 0 | 0 | 0 | 0 |
| Year | Sentinel Apiary | April/1 | April/2 | May/1 | May/2 | June/1 | June/2 | July | August | September/1 | September/2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2021 | S1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.021 | 0.018 |
| S2 | 0.029 | 0.022 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S3 | 0.029 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.032 | 0 | |
| 2022 | S1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.022 |
| S2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.024 | |
| S3 | 0 | 0 | 0 | 0 | 0 | 0 | 0.046 | 0.088 | 0.180 | 0.130 | |
| S4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.020 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasparini, M.; Prestini, G.; Rainini, F.; Cancemi, G.; De Palo, S.; Colombari, L.; Mortarino, M. Safeguarding Bee Health: Insights from a Collaborative Monitoring and Prevention Project Against Pesticide Poisonings. Animals 2025, 15, 449. https://doi.org/10.3390/ani15030449
Gasparini M, Prestini G, Rainini F, Cancemi G, De Palo S, Colombari L, Mortarino M. Safeguarding Bee Health: Insights from a Collaborative Monitoring and Prevention Project Against Pesticide Poisonings. Animals. 2025; 15(3):449. https://doi.org/10.3390/ani15030449
Chicago/Turabian StyleGasparini, Mara, Giovanni Prestini, Franco Rainini, Gabriella Cancemi, Silvia De Palo, Livio Colombari, and Michele Mortarino. 2025. "Safeguarding Bee Health: Insights from a Collaborative Monitoring and Prevention Project Against Pesticide Poisonings" Animals 15, no. 3: 449. https://doi.org/10.3390/ani15030449
APA StyleGasparini, M., Prestini, G., Rainini, F., Cancemi, G., De Palo, S., Colombari, L., & Mortarino, M. (2025). Safeguarding Bee Health: Insights from a Collaborative Monitoring and Prevention Project Against Pesticide Poisonings. Animals, 15(3), 449. https://doi.org/10.3390/ani15030449






