1. Introduction
Equine atypical myopathy (AM) is a seasonal and highly fatal [
1] intoxication caused by the ingestion and metabolism of two protoxins contained in some
Acer spp., including
Acer pseudoplatanus and
Acer negundo [
2,
3]: methylenecyclopropylalanine, also known as hypoglycin A (HGA), [
2] and methylenecyclopropylglycine (MCPrG) [
4].
Both HGA and MCPrG are non-proteinogenic amino acids [
5] that are not toxic
per se. Their metabolism into toxic metabolites requires the action of enzymes, mainly located in the mitochondria, involved in branched-chain amino acids (BCAAs) catabolic pathway [
6]. The first step is a transamination, catalysed by the branched-chain amino acid aminotransferase (BCAT) [
6,
7]. The second and irreversible step, catalysed by the branched-chain α-keto acid dehydrogenase complex (BCKDHc), is an oxidative decarboxylation with coenzyme A (CoA) added to oxidised products [
6,
8]. This results in the formation of methylenecyclopropylacetyl-CoA (MCPA-CoA) for HGA and methylenecyclopropylformyl-CoA (MCPF-CoA) for MCPrG [
6]. These toxic metabolites impair the
β-oxidation of fatty acids, leading to increased levels of acylcarnitine in tissues [
9], urine [
2,
10,
11] and blood [
5,
12,
13,
14,
15,
16]. The diagnosis of AM relies on a combination of clinical signs, acylcarnitine profiling, and the detection of toxic metabolites conjugated with carnitine or glycine, such as MCPA-carnitine [
3,
16,
17,
18,
19].
Both prokaryotic and eukaryotic cells can metabolise amino acids [
20]. Indeed, some bacteria have the above-mentioned enzymes: for example,
Lactococcus lactis and
Escherichia coli express BCAT [
21,
22], and
Bacillus subtilis,
Staphylococcus aureus and
Pseudomonas putida express BCKDHc [
23,
24,
25,
26,
27,
28,
29,
30,
31]. This raises the hypothesis that the intestinal microbiota may play a role in the metabolism of HGA and/or MCPrG, potentially affecting horses that have ingested these protoxins. Analysis of the faecal microbiota is of twofold interest in exploring this hypothesis: on top of being a non-invasive technique, the faecal microbiota is reflective of the colonic microbial population in equids [
32,
33,
34].
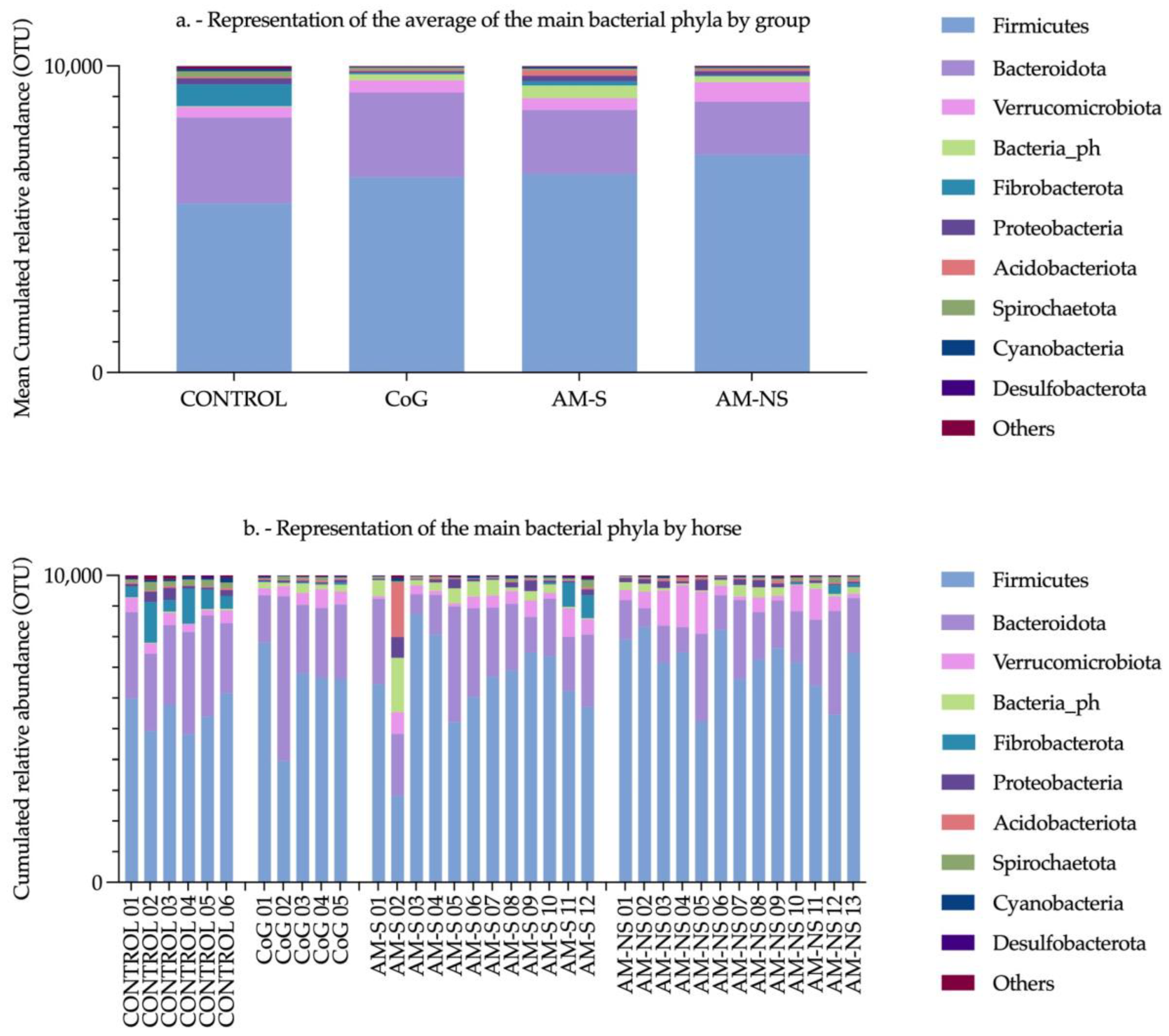
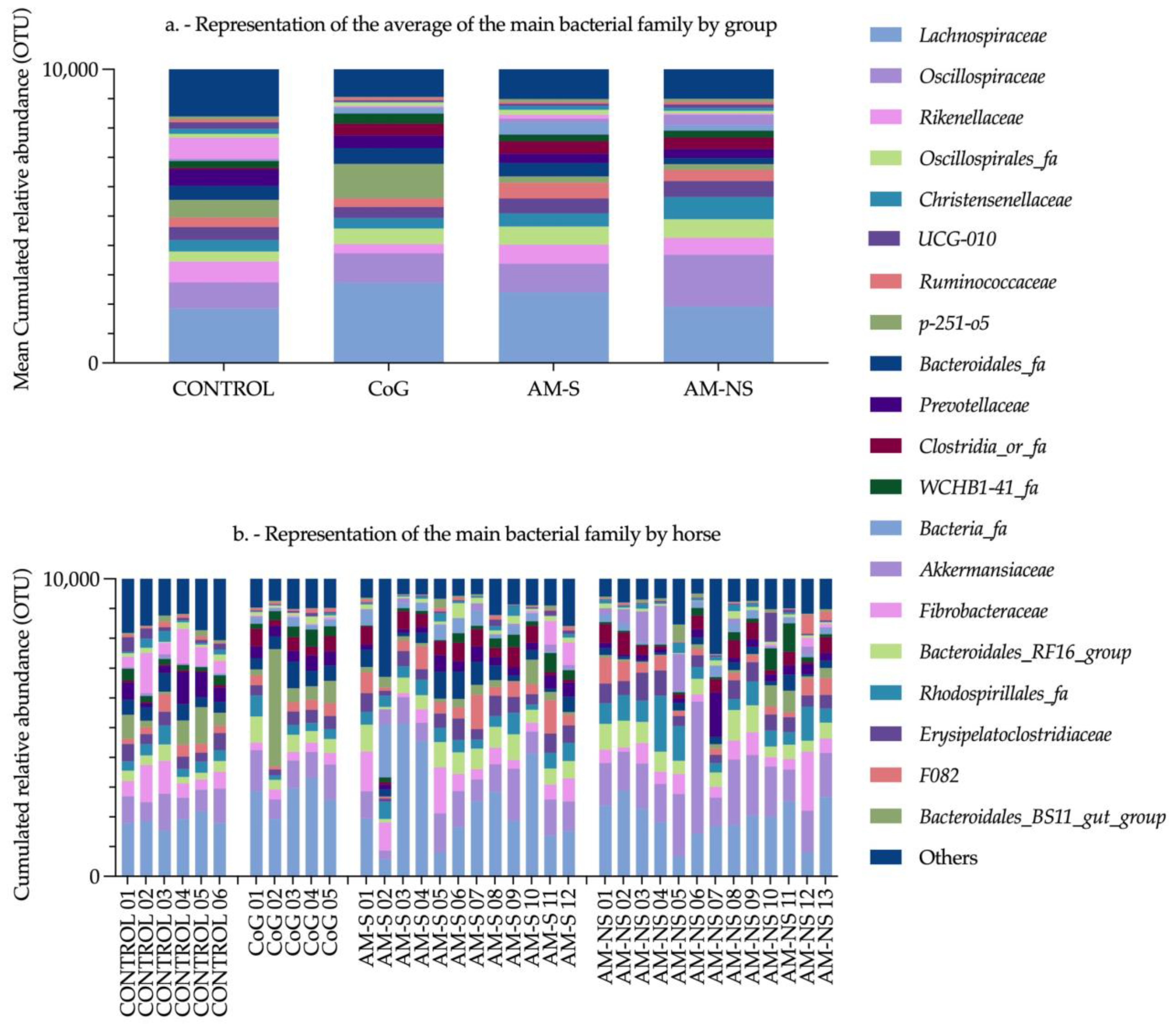
Wimmer-Scherr et al. (2021) conducted a study exploring the differences in faecal microbiota between horses with AM and a control group consisting of their cograzers (CoG) (i.e., horses grazing in the same pasture as a poisoned horse but having a normal physical examination). They described that (1) the relative abundance of families
Ruminococcaceae,
Christensenellaceae and
Akkermansiaceae was higher in horses suffering from AM compared to their CoG, and (2) the relative abundance of families
Lachnospiraceae,
Bacteroidales and
Clostridiales was lower in horses with AM compared to their CoG, especially in non-surviving AM animals [
35].
Clinically healthy CoG of HGA-poisoned horses are exposed to similar toxic pressure as horses affected by AM, yet they do not exhibit any clinical signs of poisoning [
36,
37,
38]. Due to their shared environment and, therefore, exposure to the same protoxins, their blood often contains detectable levels of HGA and, in some cases, MCPA-carnitine [
10,
17,
37,
39] at levels that sometimes overlap with those observed in clinically affected AM horses [
16,
38]. Despite the absence of overt clinical signs, acylcarnitines profiling—which is the diagnostic and prognostic gold standard of AM [
15,
16]—revealed that many CoG has increased levels of acylcarnitines compared to control horses (i.e., horses free of toxin) [
16]. This observation indicates the existence of subclinical cases among CoG, challenging their status as a healthy control group. Furthermore, another study on
Acer pseudoplatanus poisoning in herbivorous species highlighted the existence of subclinical poisoning in species other than equids and hypothesised a potential role of gut morphology and intestinal microbiota in the risk of HGA intoxication [
40]. Therefore, to study the potential role of microbiota in AM, it is necessary to include a control group composed of clinically healthy toxin-free horses and compare this group to both AM horses and their CoG.
As previously mentioned, some bacteria possess the enzymatic machinery necessary to metabolise HGA into MCPA. This raises the hypothesis that there could be a correlation between the host’s microbiota and blood parameters related to intoxication (such as HGA, MCPA-carnitine and the acylcarnitines profile). This hypothesis was not addressed in the study of Wimmer-Scherr et al. (2021), highlighting a second point for improvement in understanding the possible role of intestinal microbiota in AM [
35].
Therefore, the aims of this study were (1) to compare the faecal microbiota of AM horses (both survivors and non-survivors) and their CoG with that of control horses (i.e., grazing horses without protoxins or toxic metabolites in their blood) and (2) to analyse the correlation between faecal microbiota and blood parameters associated with AM intoxication in horses (i.e., HGA, MCPA-carnitine and the acylcarnitines profile).
4. Discussion
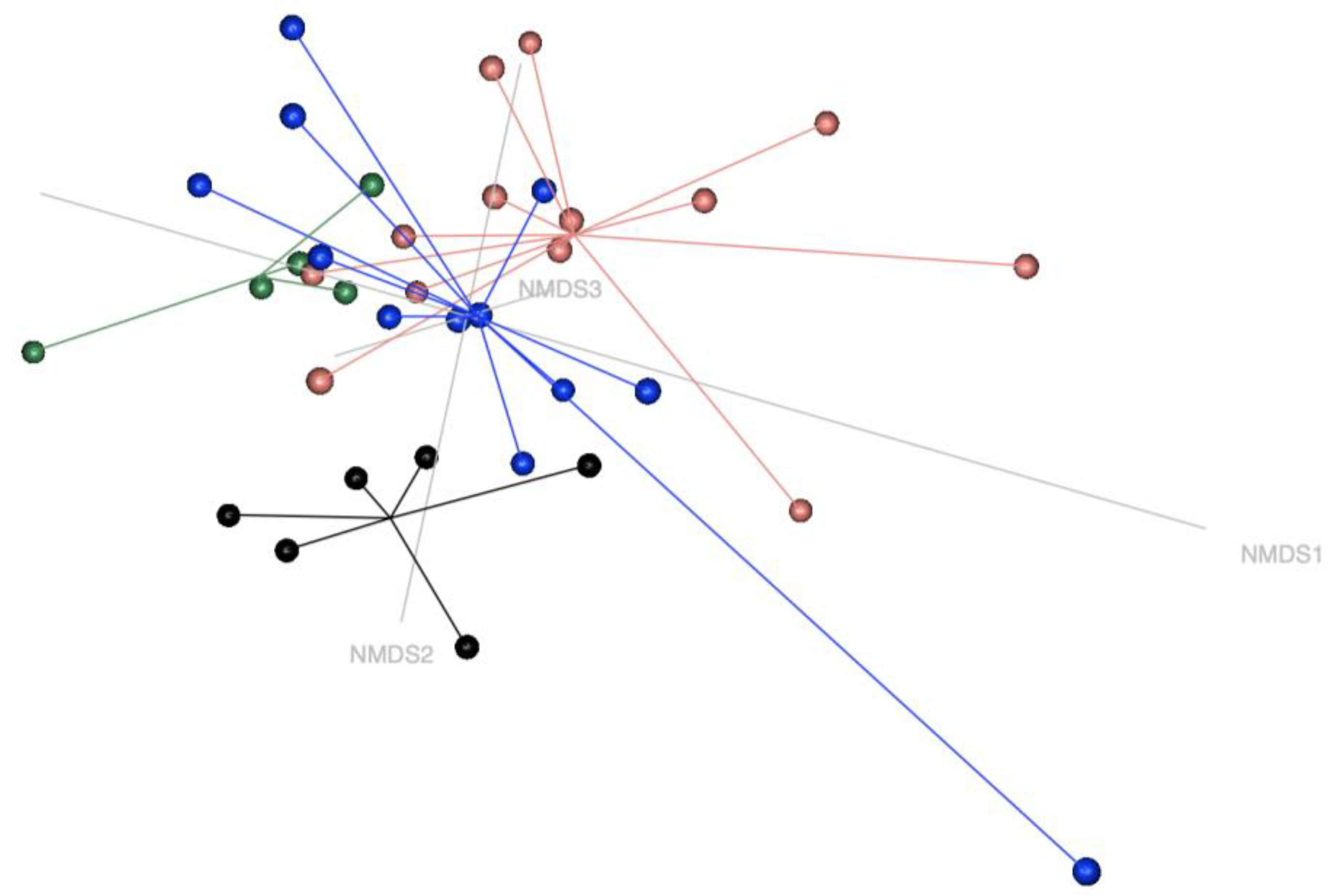
The present study reveals that (1) faecal microbiota differs between CONTROL horses (i.e., toxin-free horses) and horses suffering from both subclinical (CoG) and clinical (AM-S and AM-NS) Acer pseudoplatanus intoxication and (2) the blood concentrations of MCPA-carnitine and C14:1 (i.e., tetradecenoylcarnitine, a long-chain acylcarnitine) significantly correlate with the variation in faecal microbial composition observed between the different groups of horses.
Microbiota statistical analyses were performed at the genus level to detect differences at the highest possible taxonomic resolution. This approach allows for a more accurate identification of the microbial populations influencing the system under investigation using the most up-to-date bacterial taxonomy. Unfortunately, recent updates in bacterial taxonomy since the publication of Wimmer-Scherr et al. (2021) have complicated a direct comparison of the results between the two studies [
35]. Nevertheless, the findings of this new study will facilitate future comparisons with other research.
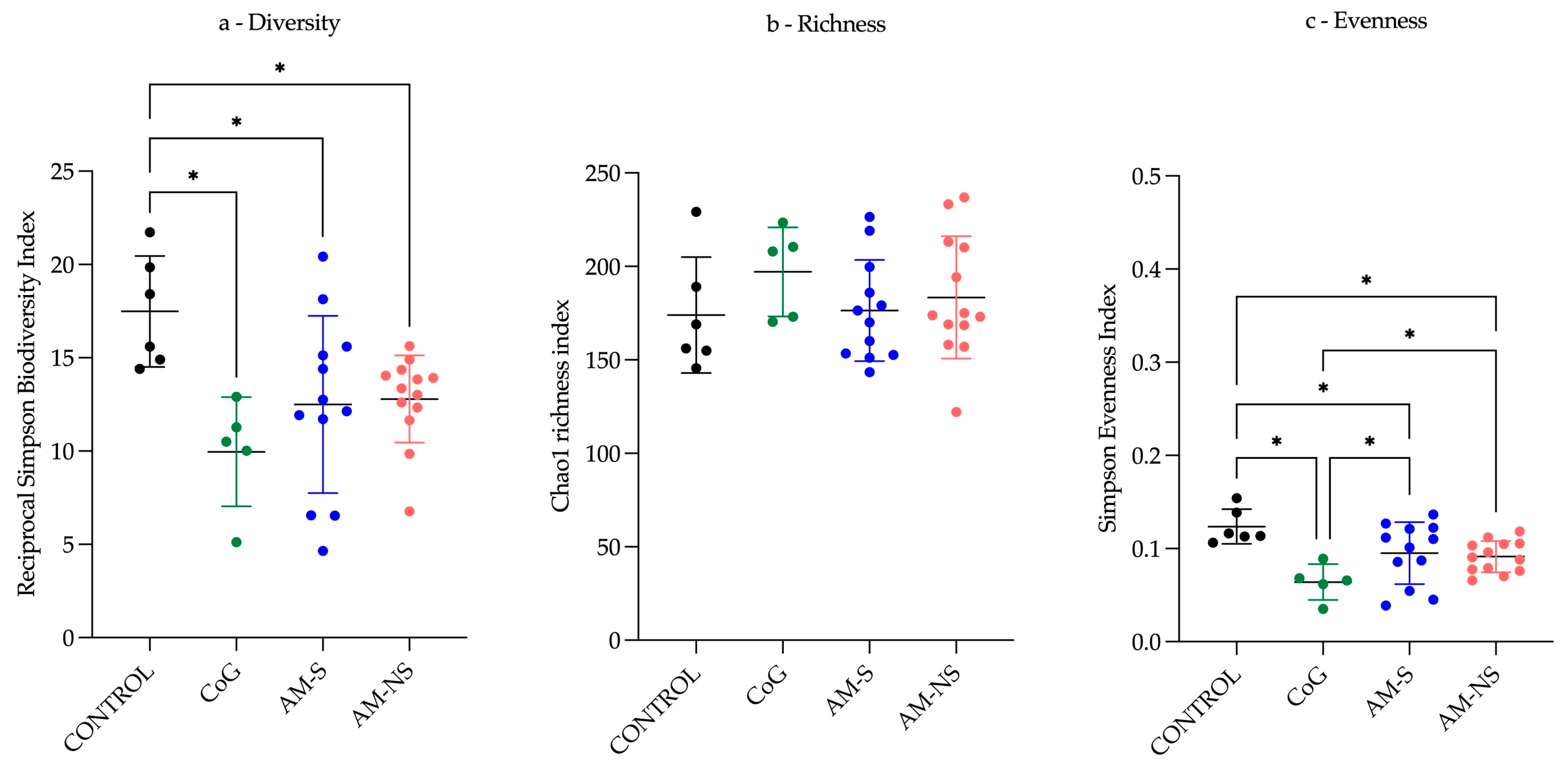
Regarding
α-diversity, the CONTROL group presents the highest
α-diversity and exhibits a more uniform distribution of populations compared to the three other groups, with an evenness index closer to one. The CoG group presents a lower
α-diversity and an evenness index close to zero, indicating the emergence of certain populations more abundant than others. “Dysbiosis” is defined as the loss of central mutualistic relationship among microbiota members, metabolic products, and the host immune system [
57]. Consequently, it is not possible to characterise the observed changes as dysbiosis only based on
α-diversity indicators.
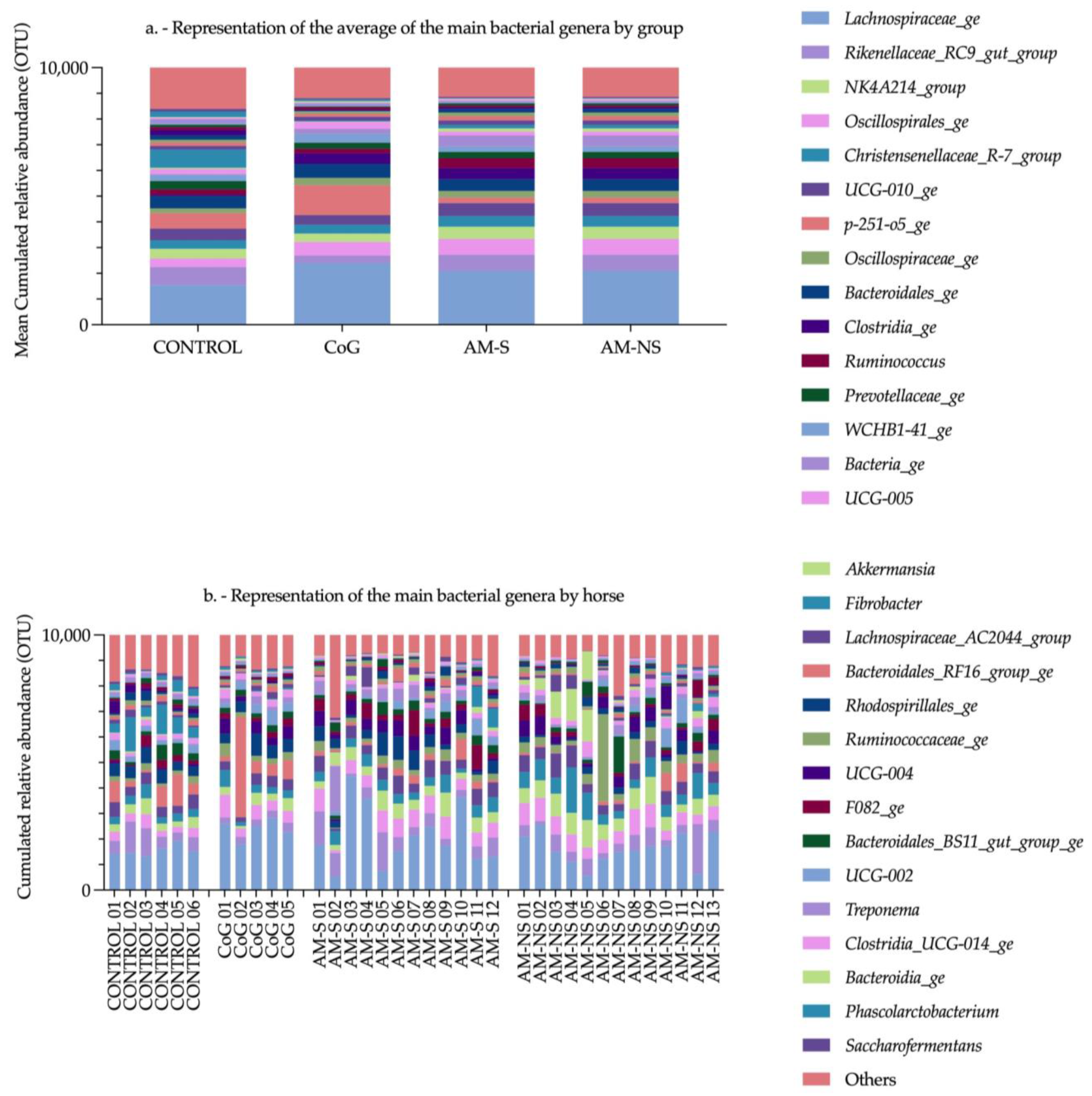
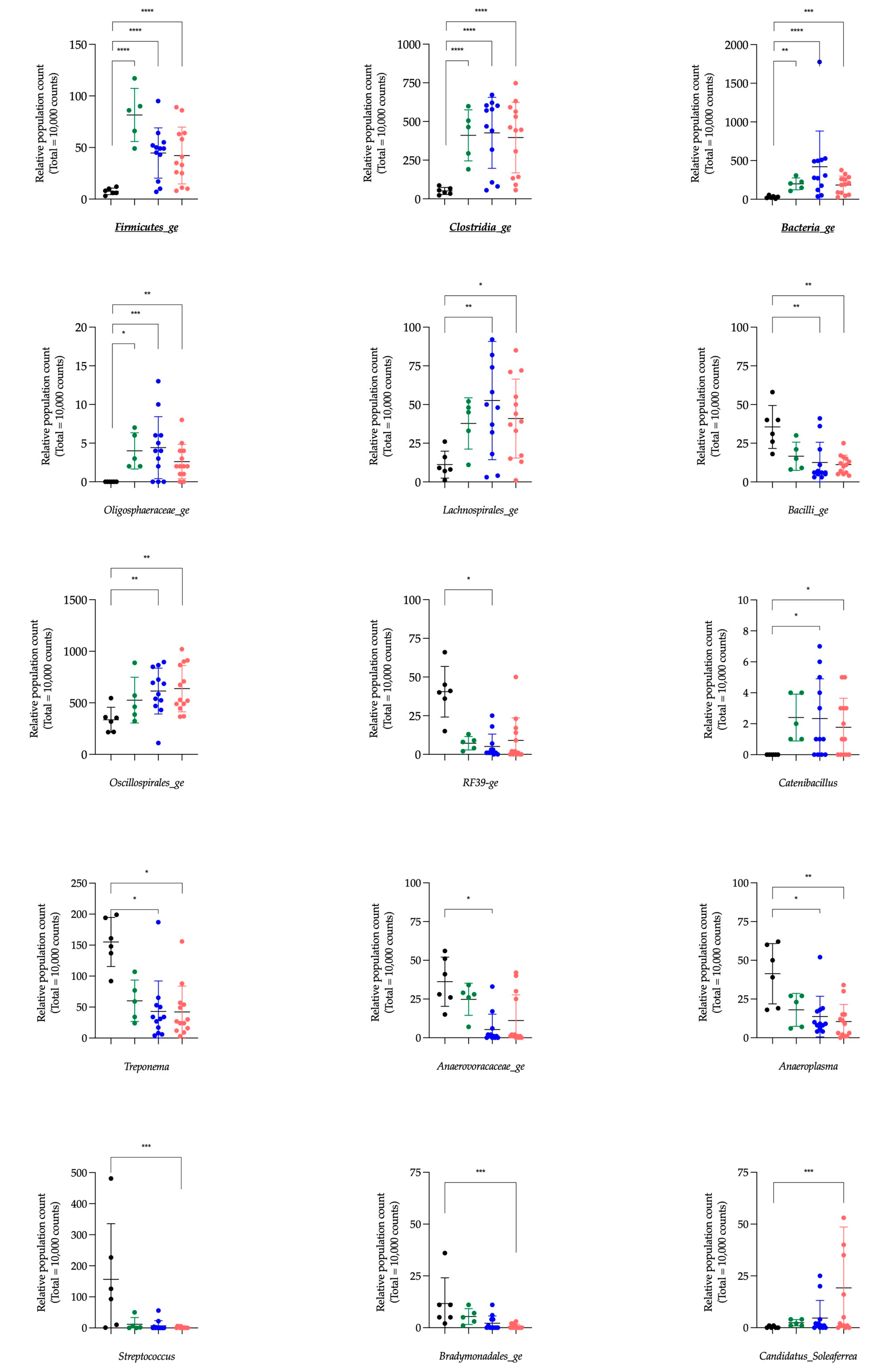
The Aldex function identified six genera having a significant impact in the global system (i.e., Clostridia_ge, Bacteria_ge, Firmicutes_ge, Phascolarctobacterium, Fibrobacter and NK4A214_group) compared to the null statistical distribution empirically determined by this function. The paired tests (Deseq2) compared bacterial genera between the CONTROL group and the three other groups and revealed 34 significantly different genera. Among these bacterial genera, five were also highlighted by the Aldex function. Indeed, Clostridia_ge, Bacteria_ge, and Firmicutes_ge presented a significantly lower relative abundance in the CONTROL group compared to CoG, AM-S and AM-NS groups, and NK4A214_group and Fibrobacter in the CONTROL group exhibit a significantly lower and higher relative abundance, respectively, compared to AM-NS horses.
The genera
Clostridia_ge,
Bacteria_ge, and
Firmicutes_ge are composed of bacteria with features belonging to the
Clostridia class, Bacteria kingdom or Firmicutes phyla, respectively, without the possibility of further characterisation. The genus
Fibrobacter is a major and highly specialised cellulolytic bacterial genus and is detected in the intestinal tract of several herbivorous animals, including horses [
58,
59]. Cellulose is not digested and not absorbed in the mammal’s gut, so cellulolytic bacteria play a vital role by providing energy to the host via the metabolism of cellulose into short-chain fatty acids (SCFAs). The SCFAs have beneficial effects on colonocytes, intestinal membrane integrity and local intestinal immunity [
60,
61]. The toxic metabolites of protoxins (i.e., MCPA-CoA and MCPF-CoA) inhibit the
β-oxidation of fatty acids with subsequent decreased mitochondrial respiration and uncoupled phosphorylation [
5,
12,
13]. Therefore, lipids can no longer be used as an energy substrate, but the glycolytic pathway is preserved [
14,
62,
63]. Consequently, it is generally recommended to give a complete mix of grains or glucose in another form to horses affected by AM [
64]. Although the appetite of diseased horses is preserved, the clinical picture (weakness, recumbency, depression, stiffness, etc. [
1,
36,
65]) can make an inadequate ingestion of food. It is hypothesised that the inhibition of
β-oxidation by toxic metabolites alters the overall energy metabolism of the host and the intestinal environment, potentially leading to conditions that are less favourable for the growth and activity of cellulolytic bacteria, thus explaining the observed decrease in the relative abundance of this genus from CONTROL horses to intoxicated horses (from CoG to AM-NS).
The genus
NK4A214_group is associated with the degradation of structural carbohydrates, such as cellulose and hemicellulose, other less complex polysaccharide substrates and the production of butyrate (i.e., SCFAs) [
66,
67]. The relative increase in this genus from CONTROL horses to intoxicated horses, as previously reported [
35], could be linked to the preservation of the glycolytic pathway and its ability to deal with less complex polysaccharides, as mentioned above. This preservation may provide a more favourable environment for the
NK4A214_group by maintaining substrate availability for carbohydrate fermentation despite the metabolic disruptions caused by toxic exposure.
Other clearly identified genera, such as
Akkermansia,
Bacteroides,
Prevotella, and
Treponema, might be considered interesting in the context of AM. The genus
Akkermansia attracts the attention of scientists for its anti-inflammatory and anti-obesity effects in mice and men by reducing insulin resistance, glucose intolerance, and gut permeability. One of the distinguishing features of
Akkermansia is its ability to (1) promote the renewal and thickening of the mucin layer, which reduces intestinal permeability, and (2) degrade intestinal mucin glycoproteins and to use them as a source of carbon and nitrogen when dietary fibres are defective; this process leads to an increase of the intestinal permeability and to the production of SCFAs [
68,
69,
70,
71,
72]. This ability to use mucin as a source of carbon and nitrogen may give
Akkermansia an advantage when facing disruptions in host energy metabolism in the context of AM, despite the potential repercussions on intestinal microbiota. On the other hand, the resulting increase in intestinal permeability represents a disadvantage, as this could increase the absorption of HGA and other toxins present in the digestive tract and into the bloodstream. Lastly, the resulting production of SCFAs—which account for 65% of the horse’s energy production [
73,
74]—cannot be used as an energy source by the horse. Indeed, in the case of AM, the muscle cannot use them effectively as energy substrates following the inhibition of acyl-CoA dehydrogenases [
5,
12,
75,
76,
77].
The genus
Bacteroides, which increases in AM-NS compared to CONTROL horses, is known for its ability to metabolise polysaccharides and oligosaccharides such as starch [
78,
79], which could make sense in the framework of the modification of the metabolic pathways available in horses affected by AM. This genus has interesting features to thrive in the gut as (1) complex systems to sense and adapt to available nutrients, (2) systems to get rid of toxic substances, and (3) the ability to control the environment by interacting with the host’s immune system, which is an opportunity to control of other pathogens considered as competitors by
Bacteroides [
78]. Moreover, the relative abundance of
Prevotella is inversely correlated with that of the
Bacteroides in human gut microbiota [
80], and this is what is observed in the faecal microbiota analysis between CONTROL and AM-NS horses.
The genus
Treponema is associated with the degradation of structural carbohydrates and correlates positively with different dietary fibres in pigs and horses [
66,
81]. In this study,
Treponema was present in faecal microbiota in CONTROL horses and significantly decreased in AM-NS, which could also be explained by the modification of energetic metabolism in AM horses.
The explanations provided remain hypothetical. At this stage, it is not possible to draw definitive conclusions about the role of these bacterial populations in the context of AM based solely on relative abundance. By definition, the analysis of relative abundance reveals populations that are increasing and others that are decreasing relative to each other. This phenomenon is illustrated by the inverse correlation observed between
Prevotella and
Bacteroides in humans [
80]. This illustration of the ecological niche can also explain variations in populations of genera like
Campylobacter,
Endomicrobium, and
Streptococcus. It is important to remain cautious when interpreting increases or decreases in bacterial populations expressed in relative abundance values: only an absolute quantification of these populations would make it possible to truly quantify the increases and decreases (for example, via RT-PCR). Furthermore, the bacteria present in the gut microbiota are also influenced by the host’s metabolism. As a result, observations made in vivo, at the level of the faeces, may not necessarily reflect changes in the microbiota itself following exposure to the protoxins studied. Finally, it is possible that bacteria identified as significantly different between groups are involved in host-related metabolic functions rather than in the direct metabolism of HGA. The discovery of the subclinical character of metabolic processes in horses that have ingested toxins involved in AM [
16] suggests that the gut microbiota also has time to modify before the appearance of the clinical phase, which remains acute.
The enzymes responsible for HGA metabolism (i.e., BCAT and BCKDHc) are involved in the catabolism of BCAAs in bacteria [
82], leading to the hypothesis that bacteria can transform HGA into toxic metabolic compounds. In mammals, the BCAT exists in two isoenzymes: a mitochondrial (BCATm) ubiquitously present (mainly in skeletal muscle, brain, kidney, and intestine in humans), and a cytosolic (BCATc) isoenzyme, mainly presents in the brain in humans and rats, and in ovary and placenta in rats [
83,
84,
85,
86,
87,
88,
89,
90]. In contrast to the mitochondrial and cytosolic isoenzymes found in higher eukaryotes, a single form of BCAT is ubiquitously expressed in bacteria. Moreover, this BCAT is involved in the final step of anabolism and in the first step of catabolism of BCAAs, which explains that many bacterial species possess this BCAT. Finally, bacterial BCAT is also distinguished from eukaryotes by broad substrate specificity [
91,
92,
93]. The mammals BCKDHc is a multienzyme complex of three separate subunits located on the inner surface of the inner mitochondrial membrane [
89,
94]. The bacterial BCKDHc seems to mainly consist of three subunits, as described for mammalian species [
82,
95] and is also found in several bacterial species [
25,
27,
28,
31,
96,
97]. Among the bacterial genera highlighted by Aldex function and/or Deseq2 analysis, some of them have in their known genome content one or more copies of the orthologous sequences encoding for the BCAT and/or subunits of BCKDHc (i.e., enzymes involved in the metabolisation of HGA). However, no bacterial genus appears to possess all the expected enzymes, and consequently, the question of the ability of these genera to completely transform HGA in MCPA-linked to carnitine or glycine arises. Interestingly, (1) a pattern of distribution can be observed with the same ortholog missing in groups of genera owning 5/6, 4/6 or 3/6 orthologous sequences and (2) if we compare the pattern of distribution of genera identified in the scientific literature as having the BCKDHc (i.e.,
Bacillus,
Pseudomonas,
Staphylococcus [
24,
25,
26,
28,
29,
30,
31,
96], this pattern is exactly the same as the pattern encountered for the genera having 5/6 orthologous sequences with only the K11381 missing (i.e., 2-oxoisovalerate dehydrogenase E1 component). Unfortunately, these OTUs presented this interesting pattern belonging to the non-clearly identified genera
Bacilli_ge and
Bacteria_ge. Indeed, the identification of these OTUs is stopped at the
Bacilli class and Bacteria kingdom, respectively, preventing further possible characterisation and explanations. Nevertheless,
Bacteria_ge was also highlighted by Aldex function (i.e., having a significant impact on the global system) and Deseq2 function (i.e., being significantly higher in CoG, AM-S and AM-NS horses vs. CONTROL horses). It will be valuable to investigate these specific OTUs from
Bacteria_ge and compare them across groups to determine whether these specific OTUs are significantly different. The
Bacilli_ge was highlighted by the Deseq2 function and was significantly different between CONTROL and AM-S and AM-NS but in a decreasing way from CONTROL to AM-NS: the opposite of the
Bacteria_ge. This last fact seems to be contradictory (i.e., having the machinery to metabolise HGA but presenting an opposite evolution between the groups). However, some bacteria might possess analogous (non-orthologous) enzymes capable of performing similar functions.
The “Age” parameter did not seem significant in the horse population of the present study, contrary to recent findings in the scientific literature, which reported that diseased horses were either younger than 2 years or older than 10 years, while 90% of CoG were between 2 and 10 years of age [
16]. In our study, there was an age overlap between groups. Regarding the “Sex” parameter, Renaud and collaborators found that, among horses exposed to the protoxins, geldings were less susceptible to developing AM compared to intact males and females [
16]. However, in our study, neither “Sex” nor the parameters “Health Status” or “Final Outcome” were significantly associated. This may be explained by the lower number of individuals in our study as compared to the above-mentioned one.
As found previously [
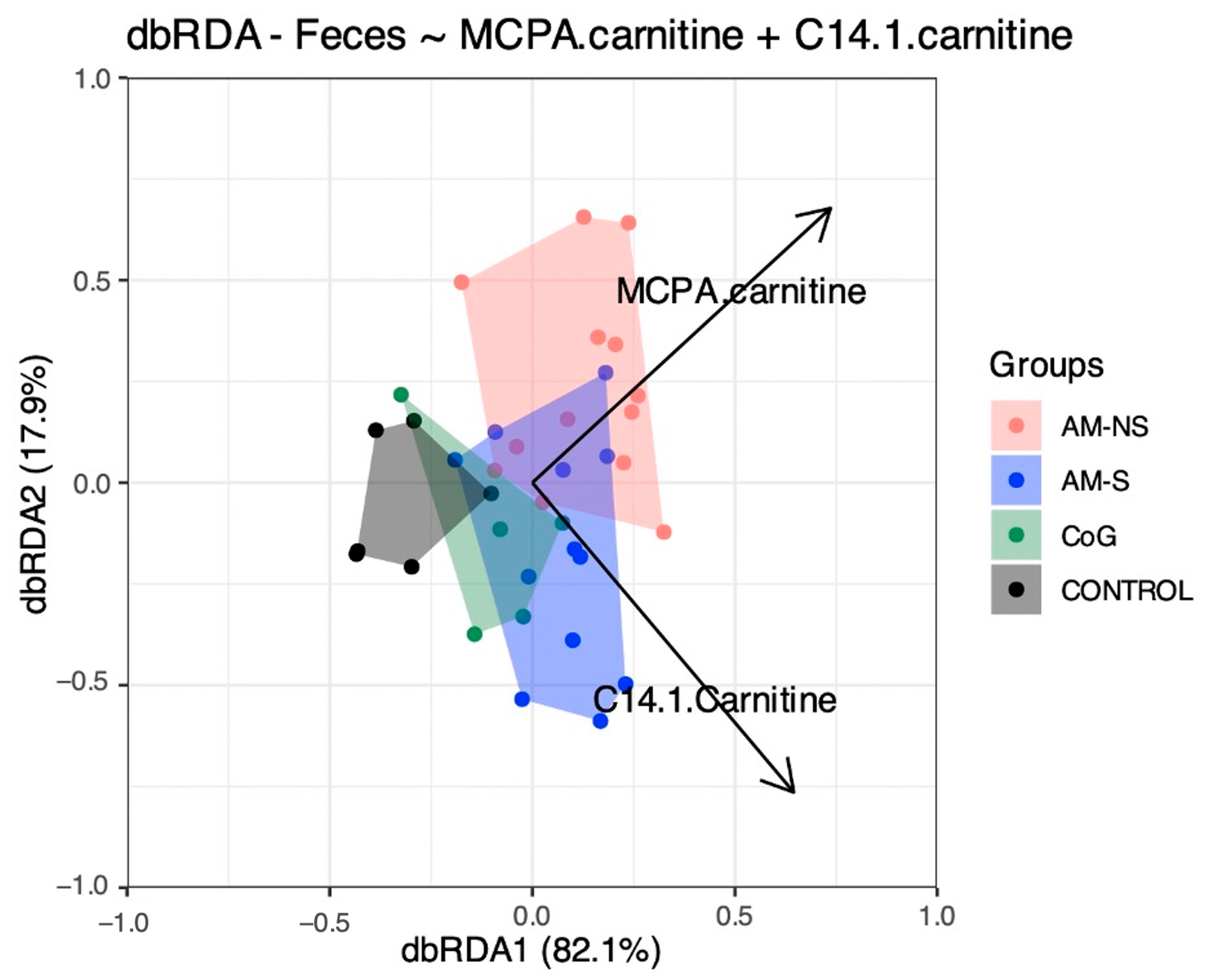
16], the mean serum concentrations of HGA and MCPA-carnitine were significantly different when comparing CoG and diseased horses. Among the latter, only MCPA-carnitine could differentiate AM-S from AM-NS. Interestingly, MCPA-carnitine was identified as one of the most important blood variables to explain the variation in microbial composition between groups and seemed to correlate better with AM-NS vs. AM-S.
Acylcarnitines are esters formed by the conjugation of acyl groups (notably fatty acids) with carnitine. They are typically divided into four groups: short-chain (C2–C5), medium-chain (C6–C12), long-chain (C13–C20) and very-long-chain (>C21) acylcarnitines. The primary biological function of acylcarnitines is to facilitate the transport of acyl groups from the cytosol into the mitochondrial matrix for
β-oxidation, which in turn contributes to cellular energy production. Atypical myopathy is characterised by a severe alteration of the serum acylcarnitines profile with an increase in nearly all acylcarnitines, regardless of their chain length [
4,
11,
13,
14,
15,
16,
63,
98,
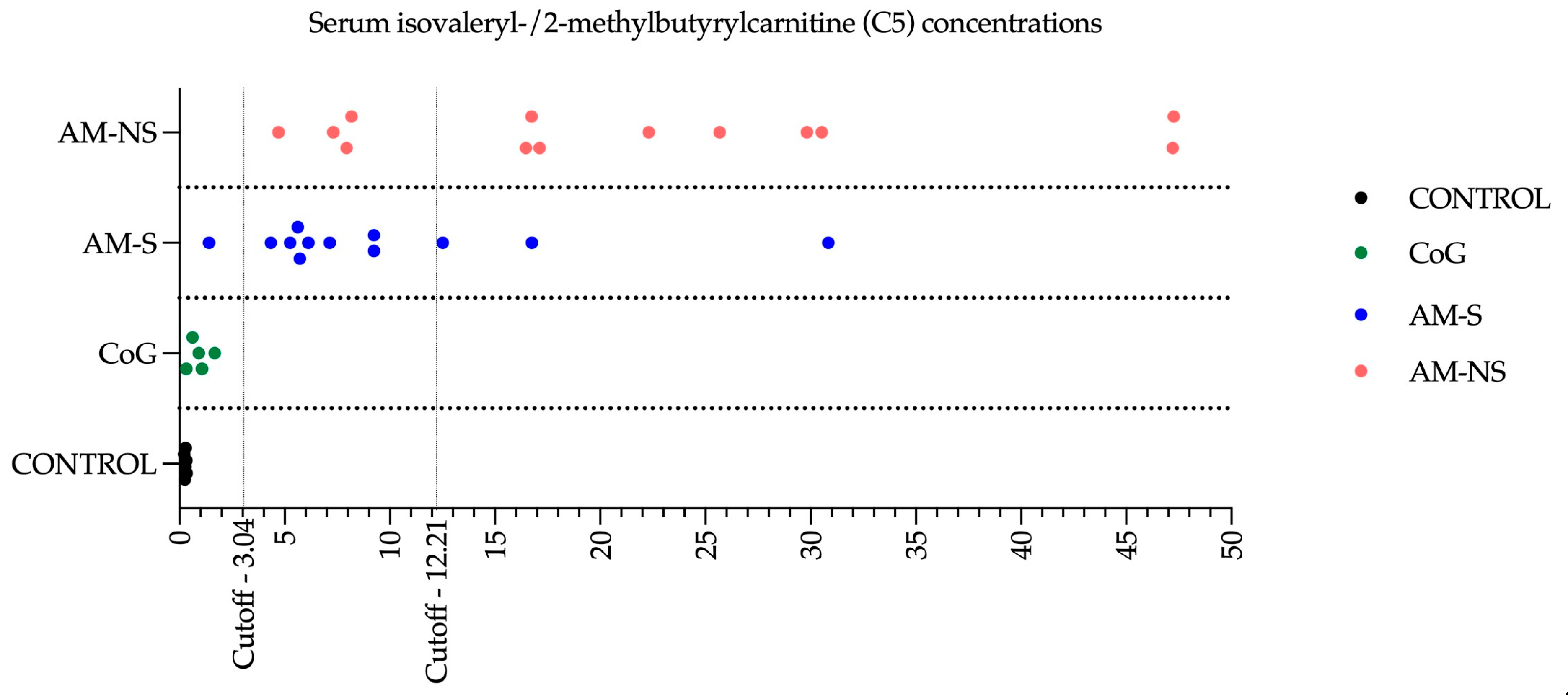
99]. In the present study, acylcarnitines profiling revealed significant changes comparable to those previously described in the literature. Moreover, C5-carnitine has been highlighted as the best candidate for helping in both the diagnosis and prognosis of the disease. According to the model described by Renaud et al. (2024), a serum concentration of C5-carnitine lower than 3.04 μmol/L would make it possible to identify a diseased horse vs. a cograzer in more than 90% of cases. A second threshold at 12.21 µmol/L of C5-carnitine would identify an animal likely to die (i.e., negative predictive value) in 76% of cases and a surviving animal (i.e., positive predictive value) in 81% of cases [
16]. In this study, the analysis of the C5 concentration identified one horse in the AM-S group (i.e., AM-S 04) with a profile similar to that of a CoG, as well as four other cases with prognostic survival estimates that differed from the real outcome: nevertheless, these results are consistent with the percentages announced in the literature. Of the four diseased horses that ultimately died despite having C5 levels indicative of a positive outcome, it should be noted that 3 of them were euthanised following the worsening of clinical signs despite the intensive treatment put in place, and the last was also euthanised, but the reason (i.e., medical or financial constraints) was not clearly identified. Moreover, to the authors’ knowledge, none of the CoG developed symptoms of AM during the sampling season, supporting the results obtained from the analysis of these horses’ blood concentration of C5 carnitine. Rapid access to C5 assay results remains a significant challenge, preventing clinicians from promptly updating prognoses in cases characterised by severe clinical signs.
Lastly, the C14:1 was identified as one important blood variable to explain the variation in faecal microbiota between groups. The elevated C14:1 level in neonates may indicate a very-long-chain acyl-coenzyme A dehydrogenase deficiency known under the following abbreviation VLCADD, an autosomal recessive disease [
100]. In horses, AM is recognised as an acquired multiple acyl-CoA dehydrogenase deficiency [
14,
63,
98]. The long-chain carnitine C14:1 concentration was above the reference range in AM horses [
3,
13], though serum concentrations showed no significant difference between surviving and deceased horses [
15]. In a recent study involving a larger group of AM-affected horses, C14:1 was also identified as one of the variables with the most significant impact on distinguishing groups (comparable to those used to compare faecal microbiota in this paper), further corroborating the results presented here. However, it did not prove to be a reliable diagnostic or prognostic indicator [
16]. In humans, tetradecenoylcarnitine (i.e., C14:1), as other long-chain acylcarnitines, plays a role in insulin resistance and in the development of cardiovascular diseases [
101]. Most horses affected by AM show elevated plasma concentrations of cardiac troponin I, a specific biomarker of myocardial injury [
36,
102], and some horses exhibit specific alterations in electrocardiogram (ECG) recordings and cardiac ultrasound examination similar to those observed in mice and humans with VLCADD [
103]. Its role in muscle insulin resistance [
104] might contribute to explaining the hyperglycaemia observed in AM horses.
In equids, individual variations in faecal microbiota are influenced by factors such as nutrition, management practices, seasonal variation, medications, animal-related factors, pathological conditions, and stress-related factors [
105]. To reduce these individual variations, horses included in the present study were all pasturing for a minimum of 6 h per day during a high-risk season for AM (i.e., autumn and spring), including the CONTROL horses that were sampled in November 2020, as 94% of “autumnal” cases occurred between October and December [
106]. Seasonal variation and associated weather conditions are known to influence gut microbiota composition in horses [
107,
108,
109]. This seasonal effect could be attributed to changes in the composition of environmental bacteria (e.g., soil and grass/haylage microbiota) or in the nutrient composition of pasture which are in turn influenced by climatic conditions [
107,
109,
110]. In this study, AM-S and AM-NS horses were sampled in autumn and spring, while CoG and CONTROL horses were sampled in autumn. However, seasonal variations between winter and summer are reported to have only a minor impact on faecal microbiota, accounting for 2.8% of the variation, according to Theelen et al. (2021) [
108]. Similarly, the variations between autumn and spring in the present study could also be considered minor. It is important to note that the year of sampling differed between groups (2016, 2017, and 2018 for clinical cases of AM, 2018 for selected CoG, and 2020 for CONTROL horses), which may introduce potential biases. To minimise the impact of this temporal variation, samples were collected under a strict protocol, stored immediately in a conservative medium, and frozen at −20 °C until analysis [
42,
111,
112]. Moreover, analyses were conducted on a regular basis over the years to ensure that samples did not remain frozen for extended periods. Finally, sampling was also standardised using a protocol described by Stewart et al. (2018) [
42], further minimising potential sampling bias.
There are some limitations in this study. Some of them are already described by Wimmer-Scherr et al. (2021): the potential bias introduced by euthanasia for ethical reasons based on the severity of the clinical signs in the AM-NS group as well as the possible medications received by some horses prior to referral to the clinic [
35]. The method used for microbiota assessment also presents inherent limitations, potentially favouring or underestimating certain bacterial taxa due to the lack of absolute quantification and selection bias during the process (e.g., DNA extraction, primer selection, PCR amplification, and bioinformatics parameters) [
113].
When a modification of the intestinal microbiota is associated with a pathology, the question arises whether this modification reflects the state of health of the host or influences the host’s health. Recently, Renaud et al. (2022) suggested that protoxins may be transformed by rumen microbiota, particularly in species with a long retention time, which would protect these species from developing AM-clinical signs. Indeed, the hypothesis proposed is that having a proximal fermentation compartment located before the absorption site of amino acids might be protective while having a distal fermentation compartment located after the absorption site of protoxins—as is the case in horses—might make the species more sensitive to poisoning [
40]. This hypothesis can be partly explained by the fact that (1) certain bacteria can metabolise peptides and amino acids, and the protoxins are non-proteinogenic amino acids [
114,
115], and (2) some bacteria possess the enzymes involved in the metabolism of BCAAs [
6,
114], HGA, and MCPrG [
6].
The identification of bacteria within the equine microbiota that can metabolise HGA and MCPrG into their corresponding toxic metabolites or potentially degrade these protoxins could be valuable in understanding species sensitivity. Moreover, this could contribute to a broader comprehension of intoxication and potentially aid in the discovery of molecules and/or bacteria capable of preventing this intoxication. To study the intestinal microbiota in this context, the use of alternative models, such as in vitro dynamic (i.e., SHIME
® or static fermentation models (i.e., batch), makes sense [
116,
117,
118]. In addition to aligning with the three R’s—replacement, reduction, and refinement—this type of model would eliminate the direct influence of host metabolism on the microbiota by using faeces of healthy donors (i.e., CONTROL horses) and by adding the studied challenge (for example, the addition of HGA). As such, the changes observed in the digestive microbiota would be directly linked to the challenge applied to the system: the addition of protoxins in the AM model. The identification of bacteria that could play a role in HGA and/or MCPrG poisoning would then be easier. Another benefit of this kind of in vitro dynamic or static fermentation model is that it can offer opportunities for studying treatments by also adding targeted molecules. Finally, another possibility would be to use metagenomic shotgun analysis. This analysis makes it possible to describe the taxonomic composition of a community of organisms and its diversity, as well as its genes and, therefore, its functional capacities.