Characterization of TCRβ and IGH Repertoires in the Spleen of Two Chicken Lines with Differential ALV-J Susceptibility Under Normal and Infection Conditions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal, Cell, and Virus
2.3. ALV-J Inoculation and Sample Collection
2.4. ALV-J Shedding, ALV-J Viremia, and ALV-J Antibody Were Detected by ELISA
2.5. RNA Isolation and Sequencing
2.6. Sequencing Data Analysis
2.7. Calculation of the Diversity Index and the Clonality Index
2.8. Statistical Analysis
3. Results
3.1. Detection of ALV-J Shedding, ALV-J Viremia, and ALV-J Antibody
3.2. Features of the Sequence Data
3.3. TCRβ Repertoire Diversity
3.4. TRB Clonal Homeostasis
3.5. Consistent Patterns in the CDR3 Length Distribution and V/J Gene Segment Usage Are Observed Between the E-Line and M-Line TCRβ Repertoires
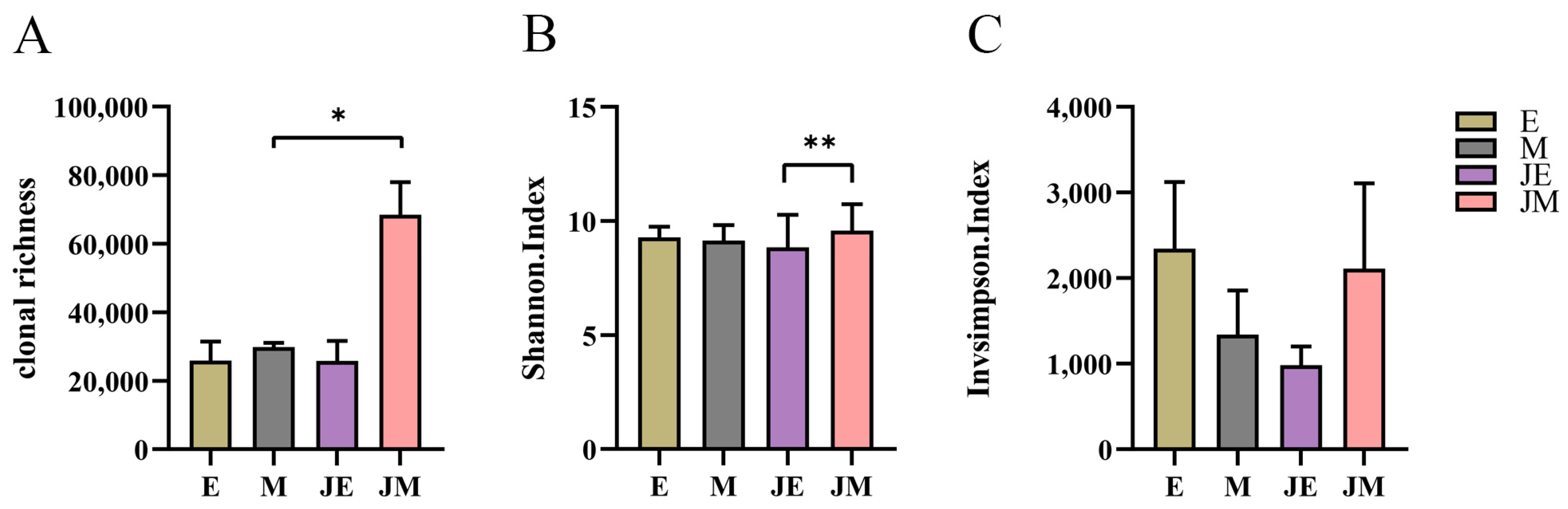
3.6. IGH Repertoire Diversity
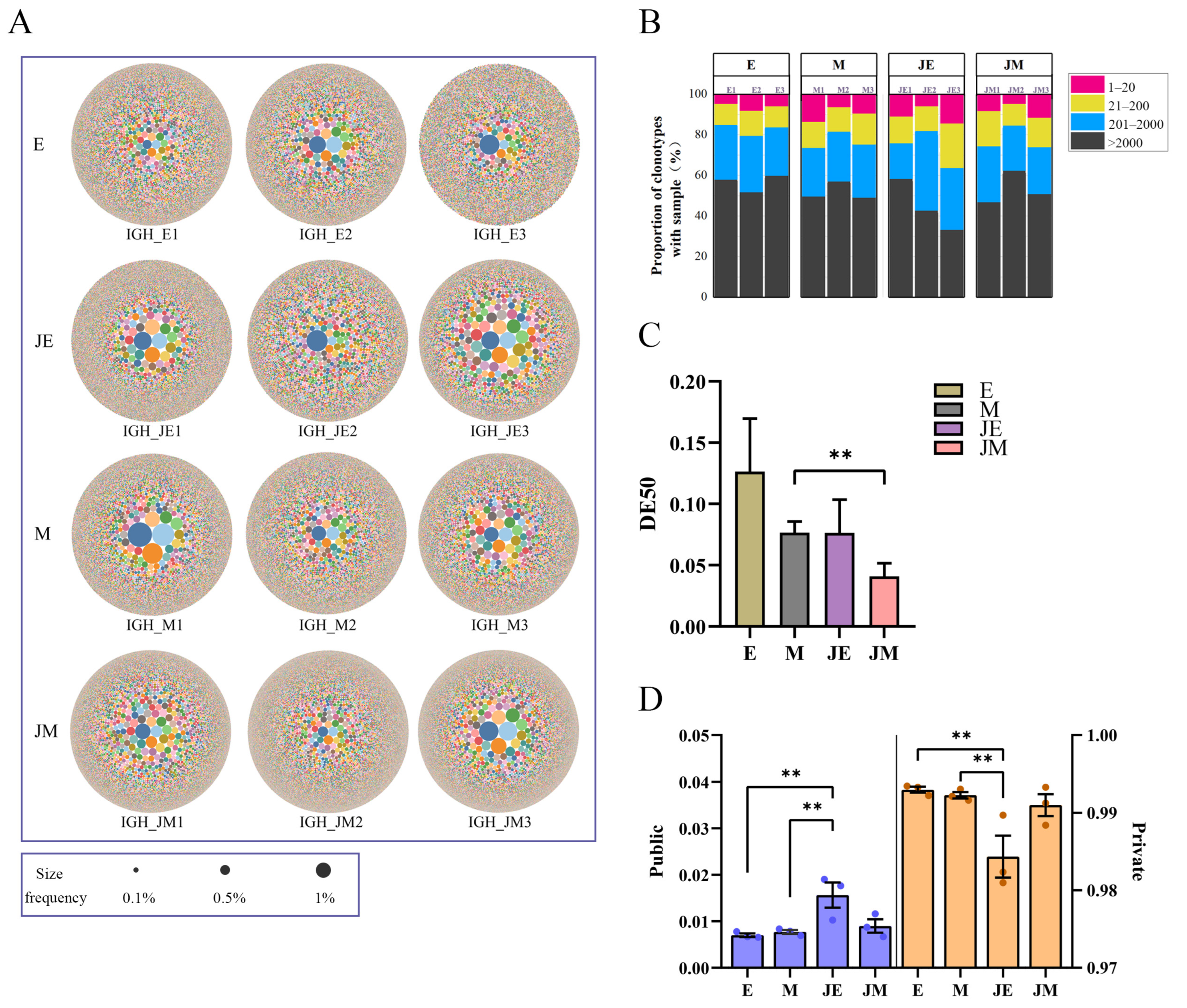
3.7. IGH Clonal Homeostasis
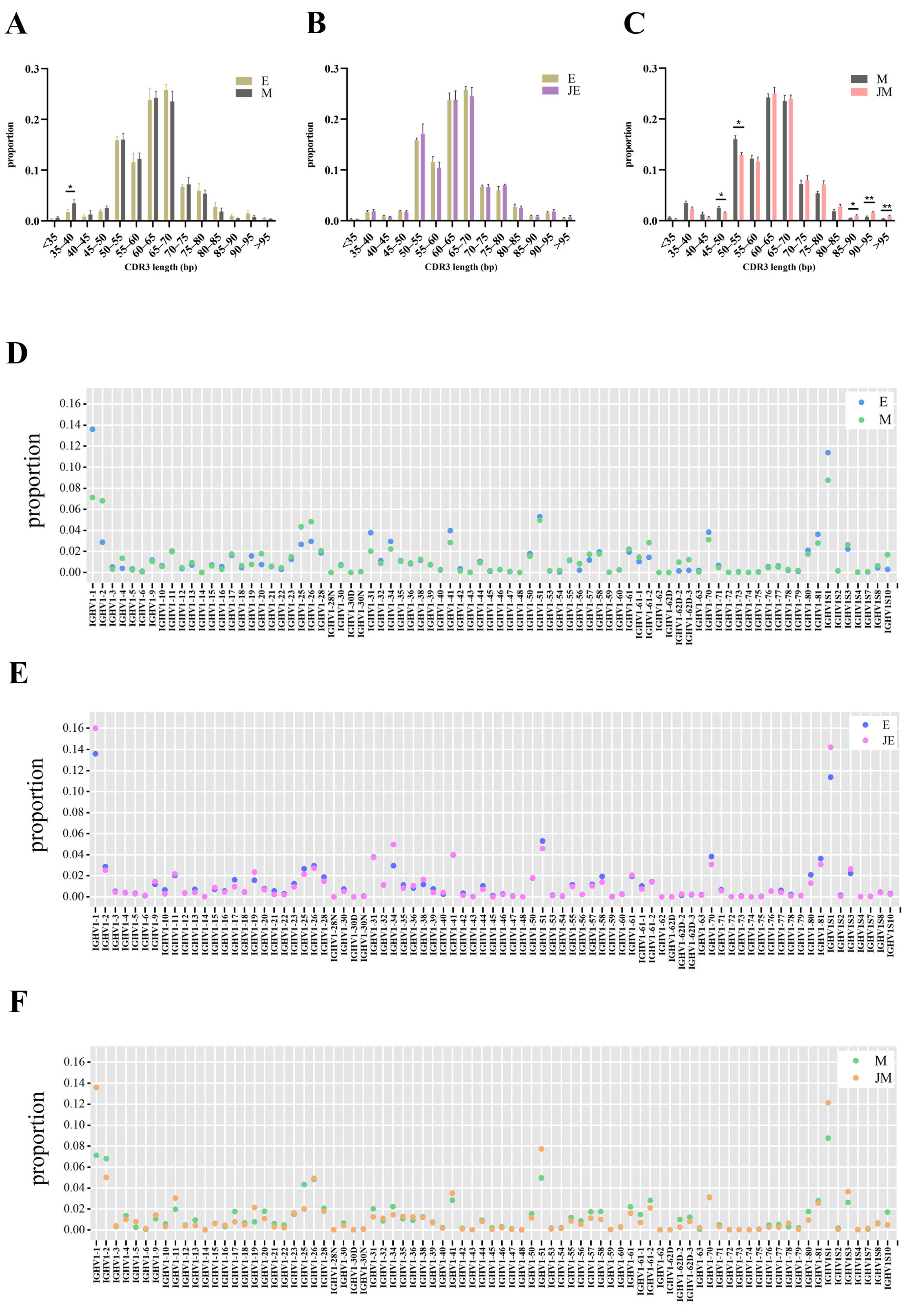
3.8. Similarity and Heterogeneity of the CDR3 Length Distribution and V/J Gene Segment Usage of the IGH Repertoire in Two Strains of Chickens
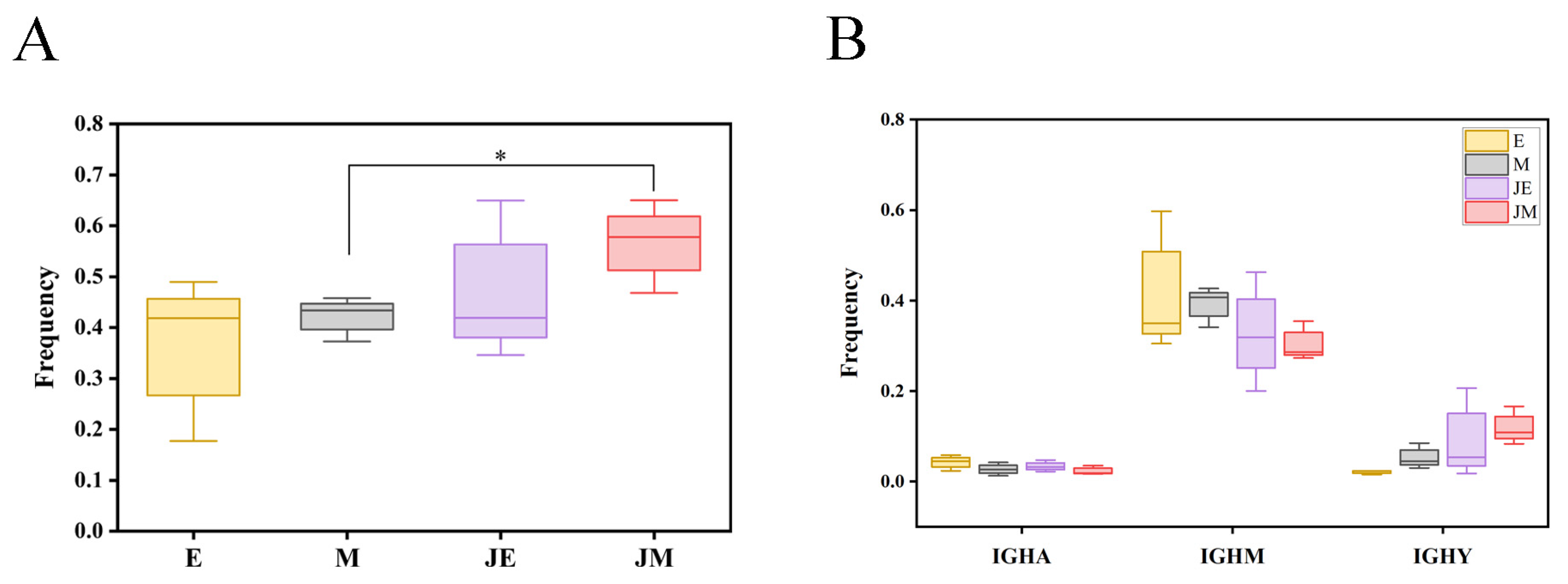
3.9. The Phenomena of Class-Switch Recombination (CSR) and Somatic Hypermutation (SHM) in the Immune System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, J.; Payne, L.N.; Skinner, M.A. HPRS-103 (exogenous avian leukosis virus, subgroup J) has an env gene related to those of endogenous elements EAV-0 and E51 and an E element found previously only in sarcoma viruses. J. Virol. 1995, 69, 779–784. [Google Scholar] [CrossRef]
- Payne, L.N.; Nair, V. The long view: 40 years of avian leukosis research. Avian Pathol. 2012, 41, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, M.; He, C.; Lu, Q.; Gao, Y.; Li, Q.; Shi, M.; Wang, P.; Wei, P. Genetic diversity of avian leukosis virus subgroup J (ALV-J): Toward a unified phylogenetic classification and nomenclature system. Virus Evol. 2021, 7, veab037. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, M.; Zhang, F.; Ji, H.; Zeng, Y.; Yang, Q.; Tan, J.; Huang, J.; Su, Q.; Huang, Y.; et al. Diversity of Avian leukosis virus subgroup J in local chickens, Jiangxi, China. Sci. Rep. 2021, 11, 4797. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhang, Y.; Zhang, L.; Wang, S.; Liu, Y.; Xu, Z.; Liu, P.; Chen, Y.; Guo, R.; Meng, L.; et al. N123I mutation in the ALV-J receptor-binding domain region enhances viral replication ability by increasing the binding affinity with chNHE1. PLoS Pathog. 2024, 20, e1011928. [Google Scholar] [CrossRef]
- Su, Q.; Li, Y.; Li, W.; Cui, S.; Tian, S.; Cui, Z.; Zhao, P.; Chang, S. Molecular characteristics of avian leukosis viruses isolated from indigenous chicken breeds in China. Poult. Sci. 2018, 97, 2917–2925. [Google Scholar] [CrossRef]
- Su, Q.; Cui, Z.; Zhang, Z.; Cui, Z.; Chang, S.; Zhao, P. Whole-genome analysis of an emerging recombinant avian leukosis virus in yellow chickens, south China. Transbound. Emerg. Dis. 2020, 67, 2254–2258. [Google Scholar] [CrossRef]
- Mo, G.; Hu, B.; Zhang, Q.; Ruan, Z.; Li, W.; Liang, J.; Shen, Y.; Mo, Z.; Zhang, Z.; Wu, Z.; et al. dPRLR causes differences in immune responses between early and late feathering chickens after ALV-J infection. Vet. Res. 2022, 53, 1. [Google Scholar] [CrossRef]
- Chuwatthanakhajorn, S.; Chang, C.-S.; Ganapathy, K.; Tang, P.-C.; Chen, C.-F. Comparison of Immune-Related Gene Expression in Two Chicken Breeds Following Infectious Bronchitis Virus Vaccination. Animals 2023, 13, 1642. [Google Scholar] [CrossRef]
- Stepanek, O.; Draber, P.; Drobek, A.; Horejsi, V.; Brdicka, T. Nonredundant Roles of Src-Family Kinases and Syk in the Initiation of B-Cell Antigen Receptor Signaling. J. Immunol. 2013, 190, 1807–1818. [Google Scholar] [CrossRef]
- Nutt, S.L.; Hodgkin, P.D.; Tarlinton, D.M.; Corcoran, L.M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 2015, 15, 160–171. [Google Scholar] [CrossRef]
- Allman, D.; Wilmore, J.R.; Gaudette, B.T. The continuing story of T-cell independent antibodies. Immunol. Rev. 2019, 288, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Vos, Q.; Lees, A.; Wu, Z.Q.; Snapper, C.M.; Mond, J.J. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol. Rev. 2000, 176, 154–170. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Xu, Y.; Zhou, D.; Liu, K.; Geng, N.; Lu, J.; Liu, Y.; Liu, J. Novel carbon quantum dots can serve as an excellent adjuvant for the gp85 protein vaccine against avian leukosis virus subgroup J in chickens. Poult. Sci. 2019, 98, 5315–5320. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Kelsoe, G.; Rajewsky, K.; Weiss, U. Intraclonal generation of antibody mutants in germinal centres. Nature 1991, 354, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Victora, G.D.; Nussenzweig, M.C. Germinal Centers. Annu. Rev. Immunol. 2012, 30, 429–457. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, R.; Sims, G.P.; Fairhurst, A.-M.; Robbins, R.; da Silva, Y.S.; Spolski, R.; Leonard, W.J.; Lipsky, P.E. IL-21 Induces Differentiation of Human Naive and Memory B Cells into Antibody-Secreting Plasma Cells. J. Immunol. 2005, 175, 7867–7879. [Google Scholar] [CrossRef]
- Shulman, Z.; Gitlin, A.D.; Weinstein, J.S.; Lainez, B.; Esplugues, E.; Flavell, R.A.; Craft, J.E.; Nussenzweig, M.C. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science 2014, 345, 1058–1062. [Google Scholar] [CrossRef]
- Rajewsky, K. Clonal selection and learning in the antibody system. Nature 1996, 381, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Zemlin, M.; Schelonka, R.L.; Bauer, K.; Schroeder, H.W., Jr. Regulation and chance in the ontogeny of B and T cell antigen receptor repertoires. Immunol. Res. 2002, 26, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Schatz, D.G.; Swanson, P.C. V(D)J Recombination: Mechanisms of Initiation. Annu. Rev. Genet. 2011, 45, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Bassing, C.H.; Swat, W.; Alt, F.W. The mechanism and regulation of chromosomal V(D)J recombination. Cell 2002, 109, S45–S55. [Google Scholar] [CrossRef]
- Brecht, R.M.; Liu, C.C.; Beilinson, H.A.; Khitun, A.; Slavoff, S.A.; Schatz, D.G. Nucleolar localization of RAG1 modulates V(D)J recombination activity. Proc. Natl. Acad. Sci. 2020, 117, 4300–4309. [Google Scholar] [CrossRef] [PubMed]
- Schatz, D.G.; Ji, Y. Recombination centres and the orchestration of V(D)J recombination. Nat. Rev. Immunol. 2011, 11, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Davison, F. Chapter 1—The Importance of the Avian Immune System and its Unique Features. In Avian Immunology, 2nd ed.; Schat, K.A., Kaspers, B., Kaiser, P., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 1–9. [Google Scholar]
- Chen, L.; Fakiola, M.; Staines, K.; Butter, C.; Kaufman, J. Functional Alleles of Chicken BG Genes, Members of the Butyrophilin Gene Family, in Peripheral T Cells. Front. Immunol. 2018, 9, 930. [Google Scholar] [CrossRef]
- Ratcliffe, M.J.; Jacobsen, K.A. Rearrangement of immunoglobulin genes in chicken B cell development. Semin. Immunol. 1994, 6, 175–184. [Google Scholar] [CrossRef]
- Koslová, A.; Trefil, P.; Mucksová, J.; Reinišová, M.; Plachý, J.; Kalina, J.; Kučerová, D.; Geryk, J.; Krchlíková, V.; Lejčková, B.; et al. Precise CRISPR/Cas9 editing of the NHE1 gene renders chickens resistant to the J subgroup of avian leukosis virus. Proc. Natl. Acad. Sci. 2020, 117, 2108–2112. [Google Scholar] [CrossRef] [PubMed]
- Kheimar, A.; Klinger, R.; Bertzbach, L.D.; Sid, H.; Yu, Y.; Conradie, A.M.; Schade, B.; Böhm, B.; Preisinger, R.; Nair, V.; et al. A Genetically Engineered Commercial Chicken Line Is Resistant to Highly Pathogenic Avian Leukosis Virus Subgroup J. Microorganisms 2021, 9, 1066. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, L.; Dou, J.; Li, L.; Lu, Q.; Jin, X.; Shao, H.; Cheng, Z.; Zhang, T.; Luo, Q.; et al. Identification of a New B-Cell Epitope on the Capsid Protein of Avian Leukosis Virus and Its Application. Curr. Issues Mol. Biol. 2024, 46, 5866–5880. [Google Scholar] [CrossRef] [PubMed]
- Matoušková, M.; Plachý, J.; Kučerová, D.; Pecnová, Ľ.; Reinišová, M.; Geryk, J.; Karafiát, V.; Hron, T.; Hejnar, J. Rapid adaptive evolution of avian leukosis virus subgroup J in response to biotechnologically induced host resistance. PLoS Pathog. 2024, 20, e1012468. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, D.N.; Gambón-Cerdá, S.; Gambón-Deza, F. Evolution of V genes from the TRV loci of mammals. Immunogenetics 2015, 67, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Göbel, T.W. Chapter 6—Avian T cells: Antigen Recognition and Lineages. In Avian Immunology, 3rd ed.; Kaspers, B., Schat, K.A., Göbel, T.W., Vervelde, L., Eds.; Academic Press: Boston, MA, USA, 2022; pp. 121–134. [Google Scholar]
- Zhuo, Y.; Yang, X.; Shuai, P.; Yang, L.; Wen, X.; Zhong, X.; Yang, S.; Xu, S.; Liu, Y.; Zhang, Z. Evaluation and comparison of adaptive immunity through analyzing the diversities and clonalities of T-cell receptor repertoires in the peripheral blood. Front. Immunol. 2022, 13, 916430. [Google Scholar] [CrossRef]
- Liang, C.; Sun, L.; Zhu, Y.; Wu, J.; Zhao, A.; Huang, T.; Yan, F.; He, K. Local chicken breeds exhibit abundant TCR-V segments but similar repertoire diversity. Dev. Comp. Immunol. 2024, 157, 105196. [Google Scholar] [CrossRef]
- Chen, E.C.; Gilchuk, P.; Zost, S.J.; Suryadevara, N.; Winkler, E.S.; Cabel, C.R.; Binshtein, E.; Chen, R.E.; Sutton, R.E.; Rodriguez, J.; et al. Convergent antibody responses to the SARS-CoV-2 spike protein in convalescent and vaccinated individuals. Cell Rep. 2021, 36, 109604. [Google Scholar] [CrossRef]
- Böttcher, L.; Wald, S.; Chou, T. Mathematical Characterization of Private and Public Immune Receptor Sequences. Bull. Math. Biol. 2023, 85, 102. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Liu, S.; Meng, J.; Huang, J.; Huang, J.; Tang, D.; Dai, Y. Profiling the TRB and IGH repertoire of patients with H5N6 Avian Influenza Virus Infection by high-throughput sequencing. Sci. Rep. 2019, 9, 7492. [Google Scholar] [CrossRef] [PubMed]
- Dascalu, S.; Preston, S.G.; Dixon, R.J.; Flammer, P.G.; Fiddaman, S.; Boyd, A.; Sealy, J.E.; Sadeyen, J.-R.; Kaspers, B.; Velge, P.; et al. The influences of microbial colonisation and germ-free status on the chicken TCRβ repertoire. Front. Immunol. 2023, 13, 1052297. [Google Scholar] [CrossRef] [PubMed]
- den Hartog, G.; Crooijmans, R.P.M.A.; Parmentier, H.K.; Savelkoul, H.F.J.; Bos, N.A.; Lammers, A. Ontogeny of the avian intestinal immunoglobulin repertoire: Modification in CDR3 length and conserved VH-pseudogene usage. Mol. Immunol. 2013, 56, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Chothia, C.; Lesk, A.M. Canonical structures for the hypervariable regions of immunoglobulins. J. Mol. Biol. 1987, 196, 901–917. [Google Scholar] [CrossRef] [PubMed]
- Ademokun, A.; Wu, Y.C.; Martin, V.; Mitra, R.; Sack, U.; Baxendale, H.; Kipling, D.; Dunn-Walters, D.K. Vaccination-induced changes in human B-cell repertoire and pneumococcal IgM and IgA antibody at different ages. Aging Cell 2011, 10, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Tjoelker, L.W.; Carlson, L.M.; Lee, K.; Lahti, J.; McCormack, W.T.; Leiden, J.M.; Chen, C.L.; Cooper, M.D.; Thompson, C.B. Evolutionary conservation of antigen recognition: The chicken T-cell receptor beta chain. Proc. Natl. Acad. Sci. USA 1990, 87, 7856–7860. [Google Scholar] [CrossRef] [PubMed]
- Tweedie, S.; Braschi, B.; Gray, K.; Jones, T.E.M.; Seal, R.L.; Yates, B.; Bruford, E.A. Genenames.org: The HGNC and VGNC resources in 2021. Nucleic Acids Res. 2021, 49, D939–D946. [Google Scholar] [CrossRef]
- Schina, A.; Sztupinszki, Z.; Svane, I.M.; Szallasi, Z.; Jönsson, G.; Donia, M. Intratumoral T-cell and B-cell receptor architecture associates with distinct immune tumor microenvironment features and clinical outcomes of anti-PD-1/L1 immunotherapy. J. Immunother. Cancer 2023, 11, e006941. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Jing, C.; Ye, A.Y.; Kratochvil, S.; Cottrell, C.A.; Koo, J.-H.; Williams, A.C.; Francisco, L.V.; Batra, H.; Lamperti, E.; et al. Humanized V(D)J-rearranging and TdT-expressing mouse vaccine models with physiological HIV-1 broadly neutralizing antibody precursors. Proc. Natl. Acad. Sci. 2022, 120, e2217883120. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Li, X.; Liang, Y.; Jiang, Y.; Zhang, Y.; Xu, P.; Deng, L.; Wang, Z.; Sun, T.; et al. T-cell receptor sequencing reveals hepatocellular carcinoma immune characteristics according to Barcelona Clinic liver cancer stages within liver tissue and peripheral blood. Cancer Sci. 2023, 115, 94–108. [Google Scholar] [CrossRef]
- Mathew, N.R.; Jayanthan, J.K.; Smirnov, I.V.; Robinson, J.L.; Axelsson, H.; Nakka, S.S.; Emmanouilidi, A.; Czarnewski, P.; Yewdell, W.T.; Schön, K.; et al. Single-cell BCR and transcriptome analysis after influenza infection reveals spatiotemporal dynamics of antigen-specific B cells. Cell Rep. 2022, 41, 111764. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yadavalli, A.D.; Matos-Rodrigues, G.; Xu, D.; Pintado-Urbanc, A.P.; Simon, M.D.; Wu, W.; Nussenzweig, A.; Schatz, D.G. Transcription elongation factor ELOF1 is required for efficient somatic hypermutation and class switch recombination. bioRxiv 2024. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, B.-R.; Wang, Y.; Curman, P.; Beilinson, H.A.; Brecht, R.M.; Liu, C.C.; Farrell, R.J.; de Juan-Sanz, J.; Charbonnier, L.-M.; et al. Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) activity is required for V(D)J recombination. J. Exp. Med. 2021, 218, e20201708. [Google Scholar] [CrossRef] [PubMed]
- Koning, M.T.; Vletter, E.M.; Rademaker, R.; Vergroesen, R.D.; Trollmann, I.J.M.; Parren, P.; van Bergen, C.A.M.; Scherer, H.U.; Kiełbasa, S.M.; Toes, R.E.M.; et al. Templated insertions at VD and DJ junctions create unique B-cell receptors in the healthy B-cell repertoire. Eur. J. Immunol. 2020, 50, 2099–2101. [Google Scholar] [CrossRef]
- Thai, T.H.; Kearney, J.F. Isoforms of terminal deoxynucleotidyltransferase: Developmental aspects and function. Adv. Immunol. 2005, 86, 113–136. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, Q.; Ju, R.; Xia, J.; Xu, C.; Chen, W.; Zhang, X. Characterization of TCRβ and IGH Repertoires in the Spleen of Two Chicken Lines with Differential ALV-J Susceptibility Under Normal and Infection Conditions. Animals 2025, 15, 334. https://doi.org/10.3390/ani15030334
Wang M, Zhang Q, Ju R, Xia J, Xu C, Chen W, Zhang X. Characterization of TCRβ and IGH Repertoires in the Spleen of Two Chicken Lines with Differential ALV-J Susceptibility Under Normal and Infection Conditions. Animals. 2025; 15(3):334. https://doi.org/10.3390/ani15030334
Chicago/Turabian StyleWang, Meihuizi, Qihong Zhang, Rongyang Ju, Junliang Xia, Chengxun Xu, Weiding Chen, and Xiquan Zhang. 2025. "Characterization of TCRβ and IGH Repertoires in the Spleen of Two Chicken Lines with Differential ALV-J Susceptibility Under Normal and Infection Conditions" Animals 15, no. 3: 334. https://doi.org/10.3390/ani15030334
APA StyleWang, M., Zhang, Q., Ju, R., Xia, J., Xu, C., Chen, W., & Zhang, X. (2025). Characterization of TCRβ and IGH Repertoires in the Spleen of Two Chicken Lines with Differential ALV-J Susceptibility Under Normal and Infection Conditions. Animals, 15(3), 334. https://doi.org/10.3390/ani15030334




