Evaluation of the Growth Performance and Meat Quality of Different F1 Crosses of Tengchong Snow and Xichou Black Bone Chicken Breeds
Simple Summary
 × TS
× TS  ), TX (TS
), TX (TS  × XBB
× XBB  ), and TT (TS
), and TT (TS  × TS
× TS  , control) were raised to 20 weeks of age. Results showed that the XT and TX groups had higher body weight, larger body size, and improved meat properties compared to the TT group. The XT group, in particular, exhibited better leg muscle color, unique muscle fibers, and lower abdominal fat, making it a promising line for future breeding. This study provides a technical reference for utilizing and protecting the Tengchong Snow germplasm.
, control) were raised to 20 weeks of age. Results showed that the XT and TX groups had higher body weight, larger body size, and improved meat properties compared to the TT group. The XT group, in particular, exhibited better leg muscle color, unique muscle fibers, and lower abdominal fat, making it a promising line for future breeding. This study provides a technical reference for utilizing and protecting the Tengchong Snow germplasm.Abstract
 × TS
× TS  ), (2) TX group (TS
), (2) TX group (TS  × XBB
× XBB  ), and (3) TT group (TS
), and (3) TT group (TS  × TS
× TS  ), with the TT group used as a control. A total of 725 healthy chicks (XT group: 247, TX group: 180, TT group: 298) were reared up to 20 weeks of age to estimate the growth performance and associated meat parameters. The results showed that the XT and TX groups had higher body weight and body size compared with the TT group (p < 0.05). Similarly, breast width, breast length, width of body, and carcass weights were also greater (p < 0.05) in the XT and TX groups compared with the TT group. Meat physical properties, including color, water-holding capacity, and tenderness, were improved (p < 0.05) for the XT and TX group compared to the TT group. The XT group had the better color of the leg muscles with the unique orientation of muscle fibers. Based on the results, the XT group is more in line with the future breeding direction as they have greater body weight, larger size, and lower abdominal fat. This study is a baseline technical reference for the protection, evaluation, and utilization of germplasm resources of Tengchong Snow chicken for screening the best matching lines and combinations with local chickens.
), with the TT group used as a control. A total of 725 healthy chicks (XT group: 247, TX group: 180, TT group: 298) were reared up to 20 weeks of age to estimate the growth performance and associated meat parameters. The results showed that the XT and TX groups had higher body weight and body size compared with the TT group (p < 0.05). Similarly, breast width, breast length, width of body, and carcass weights were also greater (p < 0.05) in the XT and TX groups compared with the TT group. Meat physical properties, including color, water-holding capacity, and tenderness, were improved (p < 0.05) for the XT and TX group compared to the TT group. The XT group had the better color of the leg muscles with the unique orientation of muscle fibers. Based on the results, the XT group is more in line with the future breeding direction as they have greater body weight, larger size, and lower abdominal fat. This study is a baseline technical reference for the protection, evaluation, and utilization of germplasm resources of Tengchong Snow chicken for screening the best matching lines and combinations with local chickens.1. Introduction
2. Materials and Methods
2.1. Experimental Animal and Feeding Management
 × TS
× TS ), TX (TS
), TX (TS × XBB
× XBB ), and TT (TS
), and TT (TS × TS
× TS , control). The TT group was used as a control in this study. The semen used for AI was fresh as well as undiluted for each cock and used at 2–day intervals. Egg collection was performed on the second day post-AI for 14 days and stored in the egg storeroom. The number of eggs set for incubation in a single hatch was 505, 536, and 400 for the XT, TX, and TT groups, respectively. A total of 725 healthy day-old chicks, 247, 180, and 298 for the XT, TX, and TT groups, respectively, were acquired, vaccinated, and transferred to the brooding pens. The rearing density was adjusted over time to 24–28 chicks/m2 by 4 weeks and 15–20 chicks/m2 from 4 to 7 weeks. The brooding conditions were standardized and identical across the groups. After 8 weeks, the chickens were transferred to triple cages until 20 weeks at 4–6 chicks/m2 density. The nutritional levels of the diets were maintained at the same level as the rearing and management conditions. The experimental chicken diets and chemical composition are shown in Table 1 and Table 2 respectively. The rearing conditions were homogenous across the groups and were in accordance with the established standards of the research poultry farm of the YAU. Briefly, the feed was offered twice (6:00 and 18:00 h) a day, 5% refusal was adjusted daily for ad libitum intake, fresh and clean water was ensured around the clock, and in-house air quality parameters (temperature fluctuated from 20.6 to 24.6 °C, whereas ammonia ranged from 9 to 24 ppm) were monitored regularly using the automatically installed system (Bestone Industrial Co., Ltd., Guangdong, China). The birds were immunized by following the established protocols of the YAU poultry research farm. The lighting program gradually reduced light exposure from 24 h at day-old to 10 h at 8 weeks old. The light was provided for 10 h every day for 12 weeks of growth. The lighting period was gradually raised by 1.5 h every week and achieved 16 h until 16 weeks of age. From 17 to 20 weeks, light was provided 17 h per day across the house.
, control). The TT group was used as a control in this study. The semen used for AI was fresh as well as undiluted for each cock and used at 2–day intervals. Egg collection was performed on the second day post-AI for 14 days and stored in the egg storeroom. The number of eggs set for incubation in a single hatch was 505, 536, and 400 for the XT, TX, and TT groups, respectively. A total of 725 healthy day-old chicks, 247, 180, and 298 for the XT, TX, and TT groups, respectively, were acquired, vaccinated, and transferred to the brooding pens. The rearing density was adjusted over time to 24–28 chicks/m2 by 4 weeks and 15–20 chicks/m2 from 4 to 7 weeks. The brooding conditions were standardized and identical across the groups. After 8 weeks, the chickens were transferred to triple cages until 20 weeks at 4–6 chicks/m2 density. The nutritional levels of the diets were maintained at the same level as the rearing and management conditions. The experimental chicken diets and chemical composition are shown in Table 1 and Table 2 respectively. The rearing conditions were homogenous across the groups and were in accordance with the established standards of the research poultry farm of the YAU. Briefly, the feed was offered twice (6:00 and 18:00 h) a day, 5% refusal was adjusted daily for ad libitum intake, fresh and clean water was ensured around the clock, and in-house air quality parameters (temperature fluctuated from 20.6 to 24.6 °C, whereas ammonia ranged from 9 to 24 ppm) were monitored regularly using the automatically installed system (Bestone Industrial Co., Ltd., Guangdong, China). The birds were immunized by following the established protocols of the YAU poultry research farm. The lighting program gradually reduced light exposure from 24 h at day-old to 10 h at 8 weeks old. The light was provided for 10 h every day for 12 weeks of growth. The lighting period was gradually raised by 1.5 h every week and achieved 16 h until 16 weeks of age. From 17 to 20 weeks, light was provided 17 h per day across the house.2.2. Body Weight Measurements
2.3. Slaughtering Performance Measurements
2.4. Meat Quality Physical Properties Analysis
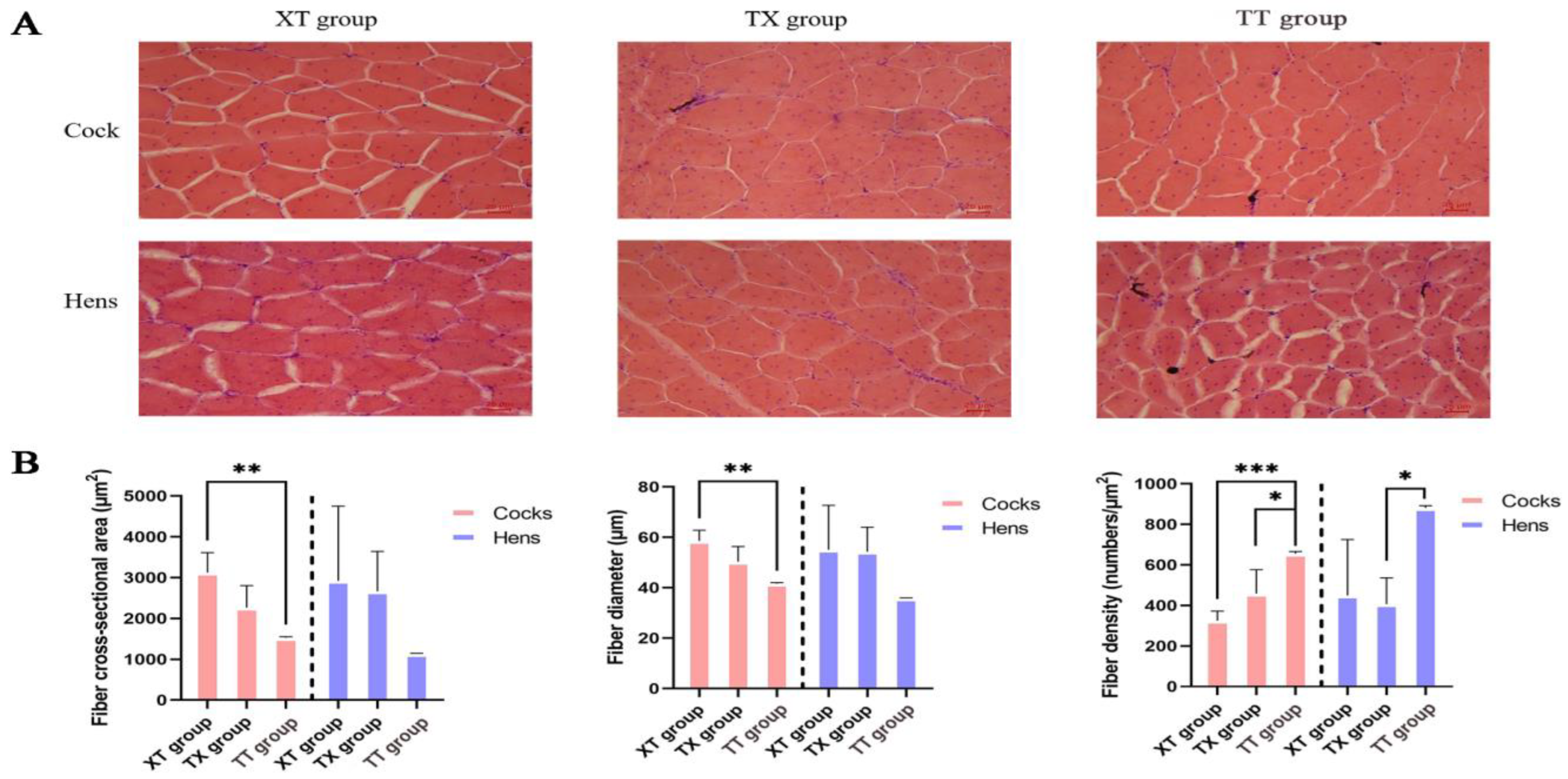
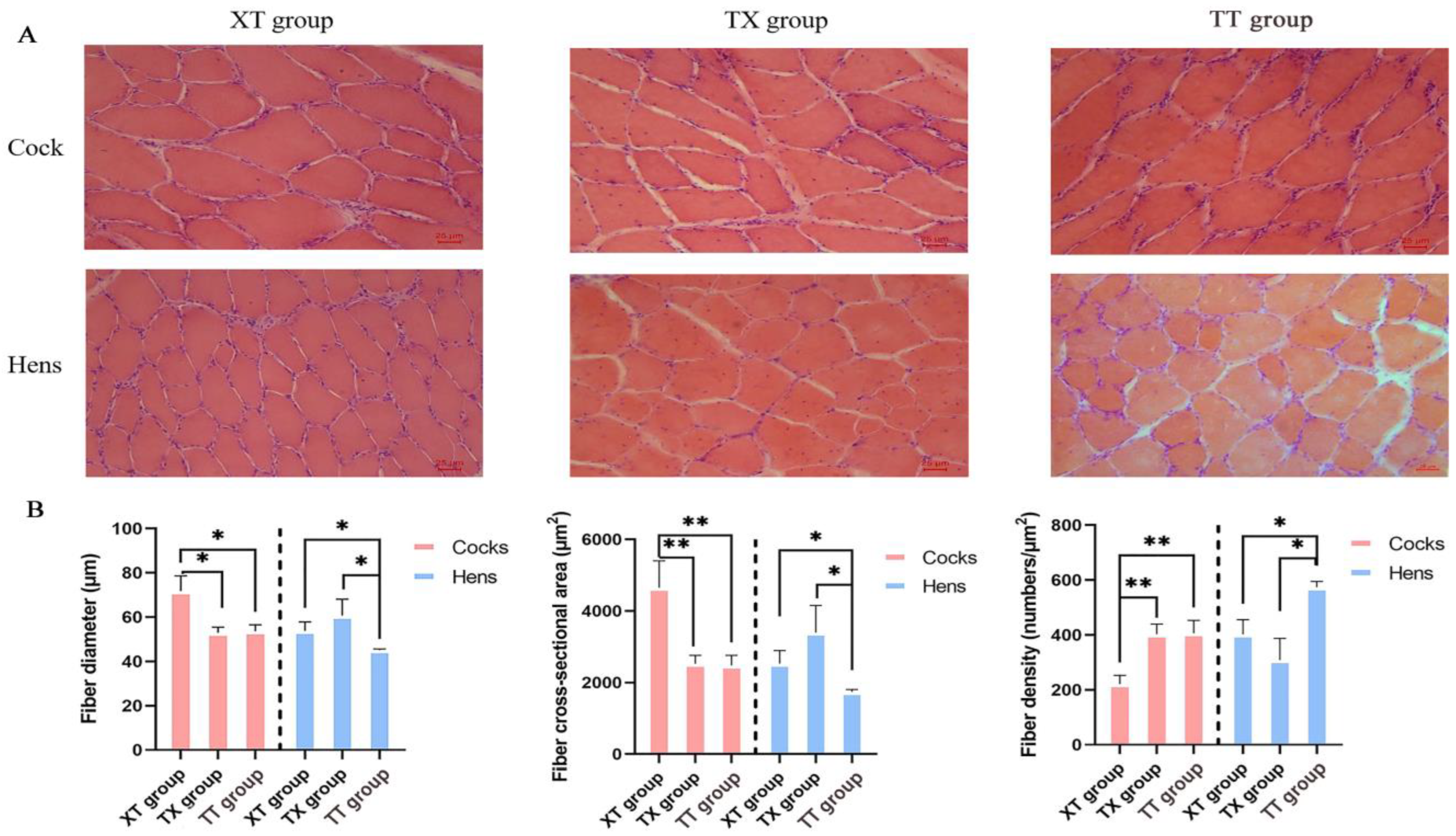
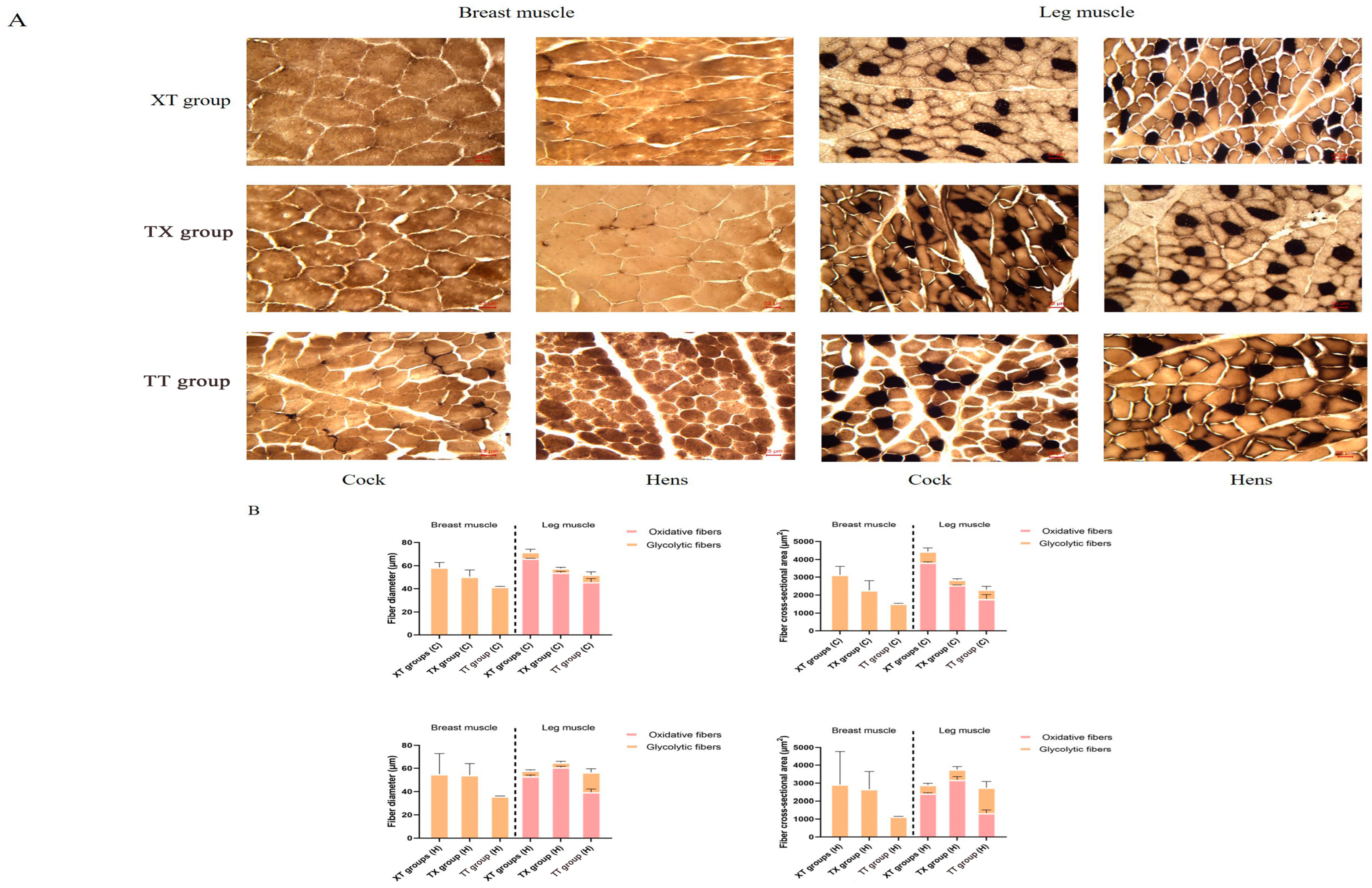
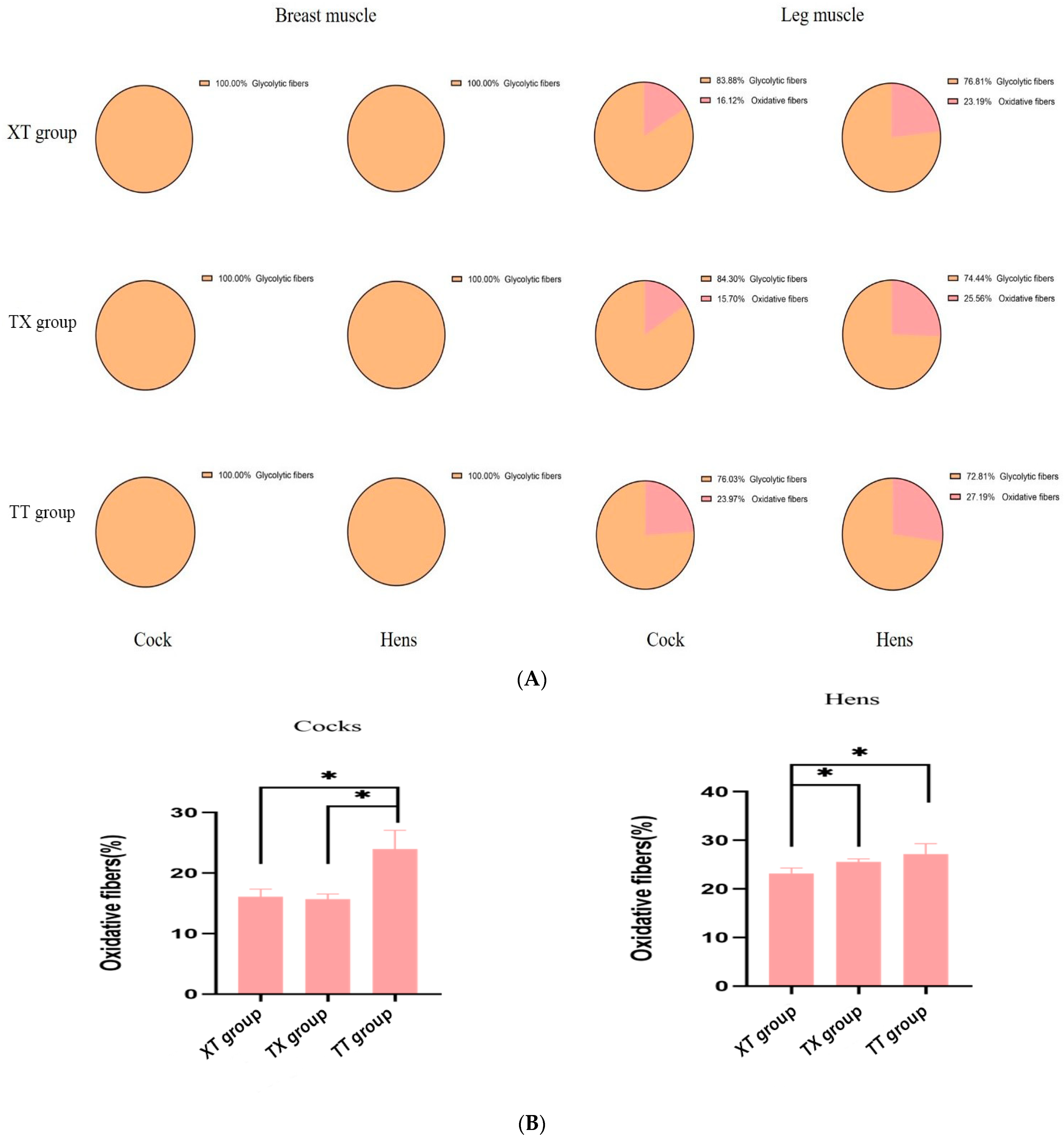
2.5. Histological Characterization of Muscle Fibers
2.6. Statistical Analysis
3. Results
3.1. Growth Performance and Carcass Attributes
3.2. Comparison of Muscle Fiber Properties with Physical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, M.; Lee, S.Y.; Park, J.Y.; Jung, S.; Jo, C.; Nam, K.-C. Comparison of functional compounds and micronutrients of chicken breast meat by breeds. Food Sci. Anim. Resour. 2019, 39, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Chumngoen, W.; Tan, F.J. Relationships between descriptive sensory attributes and physicochemical analysis of broiler and Taiwan native chicken breast meat. Asian-Australas. Anim. Sci. 2015, 28, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Dou, T.; Zhao, S.; Rong, H.; Gu, D.; Li, Q.; Huang, Y.; Xu, Z.; Chu, X.; Tao, L.; Liu, L.; et al. Biological mechanisms discriminating growth rate and adult body weight phenotypes in two Chinese indigenous chicken breeds. BMC Genomics 2017, 18, 469. [Google Scholar] [CrossRef]
- Guo, X.; Fang, Q.; Ma, C.; Zhou, B.; Wan, Y.; Jiang, R. Whole-genome resequencing of Xishuangbanna fighting chicken to identify signatures of selection. Genet. Sel. Evol. 2016, 48, 62. [Google Scholar] [CrossRef]
- Huang, X.; Weng, Z.; He, Y.; Miao, Y.; Luo, W.; Zhang, X.; Zhong, F.; Du, B. Mitochondrial DNA diversity and demographic history of Black-boned chickens in China. Mitochondrial DNA B Resour. 2021, 6, 1462–1467. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, M.; Wang, W.; Wu, H.; Fu, Z.; Lin, L. Determination of carnosine in Black-Bone Silky Fowl (Gallus gallus domesticus Brisson) and common chicken by HPLC. Eur. Food Res. Technol. 2007, 226, 311–314. [Google Scholar] [CrossRef]
- Lengkidworraphiphat, P.; Wongpoomchai, R.; Bunmee, T.; Chariyakornkul, A.; Chaiwang, N.; Jaturasitha, S. Taste-active and nutritional components of Thai native chicken meat: A perspective of consumer satisfaction. Food Sci. Anim. Resour. 2021, 41, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Tunim, S.; Phasuk, Y.; Aggrey, S.E.; Duangjinda, M. Increasing fat deposition via upregulates the transcription of peroxisome proliferator-activated receptor gamma in native crossbred chickens. Animals 2021, 11, 90. [Google Scholar] [CrossRef]
- El-Tahawy, W.S.; Habashy, W.S. Genetic effects on growth and egg production traits in two-way crosses of Egyptian and commercial layer chickens. S. Afr. J. Anim. Sci. 2021, 51, 349–354. [Google Scholar] [CrossRef]
- Laenoi, W.; Buranawit, K. Productive performance of purebred Thai native black-bone chickens (Chee Fah and Fah Luang) and their crossbreds. Indian J. Anim. Res. 2022, 56, 637–641. [Google Scholar] [CrossRef]
- Isa, A.M.; Sun, Y.; Shi, L.; Jiang, L.; Li, Y.; Fan, J.; Wang, P.; Ni, A.; Huang, Z.; Ma, H.; et al. Hybrids generated by crossing elite laying chickens exhibited heterosis for clutch and egg quality traits. Poult. Sci. 2020, 99, 6332–6340. [Google Scholar] [CrossRef] [PubMed]
- NY/T 823-2020; People’s Republic of China Agricultural Industry Standard: Terminology and Statistical Methods for Poultry Production Performance. China Poultry Industry Guide. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2006; Volume 15, pp. 45–46.
- Huo, W.; Weng, K.; Gu, T.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Effect of muscle fiber characteristics on meat quality in fast- and slow-growing ducks. Poult. Sci. 2021, 100, 101264. [Google Scholar] [CrossRef]
- Xue, J.; Fang, C.; Mu, R.; Zhuo, R.; Xiao, Y.; Qing, Y.; Tang, J.; Fang, R. Potential mechanism and effects of different selenium sources and different effective microorganism supplementation levels on growth performance, meat quality, and muscle fiber characteristics of Three-Yellow chickens. Front. Nutr. 2022, 9, 869540. [Google Scholar] [CrossRef] [PubMed]
- Weng, K.; Huo, W.; Li, Y.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Fiber characteristics and meat quality of different muscular tissues from slow- and fast-growing broilers. Poult. Sci. 2022, 101, 101537. [Google Scholar] [CrossRef] [PubMed]
- Picard, B.; Gagaoua, M. Muscle fiber properties in cattle and their relationships with meat qualities: An overview. J. Agric. Food Chem. 2020, 68, 6021–6039. [Google Scholar] [CrossRef]
- Hagan, B.A.; Asumah, C.; Yeboah, E.D.; Lamptey, V.K. Modeling the growth of four commercial broiler genotypes reared in the tropics. Trop. Anim. Health Prod. 2022, 54, 75. [Google Scholar] [CrossRef]
- Deng, S.; Xing, T.; Li, C.; Xu, X.; Zhou, G. The effect of breed and age on the growth performance, carcass traits and metabolic profile in breast muscle of Chinese indigenous chickens. Foods 2022, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Nutautaité, M.; Alijošius, S.; Bliznikas, S.; Šašytė, V.; Vilienė, V.; Pockevičius, A.; Racevičiūtė-Stupelienė, A. Effect of betaine, a methyl group donor, on broiler chicken growth performance, breast muscle quality characteristics, oxidative status and amino acid content. Ital. J. Anim. Sci. 2020, 19, 621–629. [Google Scholar] [CrossRef]
- Sungkhapreecha, P.; Chankitisakul, V.; Duangjinda, M.; Boonkum, W. Combining abilities, heterosis, growth performance, and carcass characteristics in a diallel cross from Black-Bone chickens and Thai native chickens. Animals 2022, 12, 1602. [Google Scholar] [CrossRef]
- Chao, X.; Guo, L.; Wang, Q.; Huang, W.; Liu, M.; Luan, K.; Jiang, J.; Lin, S.; Nie, Q.; Luo, W.; et al. miR-429-3p/LPIN1 axis promotes chicken abdominal fat deposition via PPARγ pathway. Front. Cell Dev. Biol. 2020, 8, 595637. [Google Scholar] [CrossRef]
- Yin, H.D.; Gilbert, E.R.; Chen, S.Y.; Wang, Y.; Zhang, Z.C.; Zhao, X.L.; Zhang, Y.; Zhu, Q. Effect of hybridization on carcass traits and meat quality of Erlang Mountainous chickens. Asian-Australas. J. Anim. Sci. 2013, 26, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Koomkrong, N.; Theerawatanasirikul, S.; Boonkaewwan, C.; Jaturasitha, S.; Kayan, A. Breed-related number and size of muscle fibres and their response to carcass quality in chickens. Ital. J. Anim. Sci. 2015, 14, 165–168. [Google Scholar] [CrossRef]
- Lukasiewicz, M.; Niemiec, J.; Wnuk, A.; Mroczek-Sosnowska, N. Meat quality and the histological structure of breast and leg muscles in Ayam Cemani chickens, Ayam Cemani × Sussex hybrids and slow-growing Hubbard JA 957 chickens. J. Sci. Food Agric. 2015, 95, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.R.; Weng, K.Q.; Li, Y.; Zhang, Y.; Zhang, Y.; Xu, Q.; Chen, G. Comparison of muscle fiber characteristics and glycolytic potential between slow- and fast-growing broilers. Poult. Sci. 2022, 101, 101649. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.M.; He, Y.; Li, X.Z.; Du, Y.; Zhao, J.; Ge, C. Regulation of non-coding RNA in the growth and development of skeletal muscle in domestic chickens. Genes 2022, 13, 1033. [Google Scholar] [CrossRef]
- Quinting, T.; Heymann, A.K.; Bicker, A.; Nauth, T.; Bernardini, A.; Hankeln, T.; Fandrey, J.; Schreiber, T. Myoglobin protects breast cancer cells due to its ROS and NO scavenging properties. Front. Endocrinol. 2021, 12, 732190. [Google Scholar] [CrossRef]
- Ramanathan, R.; Suman, S.P.; Faustman, C. Biomolecular interactions governing fresh meat color in post-mortem skeletal muscle: A review. J. Agric. Food Chem. 2020, 68, 12779–12787. [Google Scholar] [CrossRef]
- Chriki, S.; Gardner, G.E.; Jurie, C.; Picard, B.; Micol, D.; Brun, J.-P.; Journaux, L.; Hocquette, J.-F. Cluster analysis application identifies muscle characteristics of importance for beef tenderness. BMC Biochem. 2012, 13, 29. [Google Scholar] [CrossRef]
- Li, C.; Tian, D.; Tang, B.; Liu, X.; Teng, X.; Zhao, W.; Zhang, Z.; Song, S. Genome Variation Map: A worldwide collection of genome variations across multiple species. Nucleic Acids Res. 2021, 49, 1186–1191. [Google Scholar] [CrossRef]

| Components | 1–7 Weeks (%) | 8–20 Weeks (%) |
|---|---|---|
| Corn | 63.26 | 67.19 |
| Soybean meal | 30.2 | 18.88 |
| Wheat bran | 0.00 | 10.00 |
| Fishmeal | 2.50 | 0.00 |
| Coarse stone powder | 0.40 | 0.46 |
| Fine stone powder | 0.71 | 0.60 |
| Dicalcium phosphate | 1.50 | 1.50 |
| Methionine | 0.08 | 0.07 |
| Salt | 0.35 | 0.30 |
| 1 Commercial premix | 1.00 | 1.00 |
| Nutrient Contents | 1–7 Weeks (%) | 8–20 Weeks (%) |
|---|---|---|
| Dry matter (%) | 89.90 | 90.10 |
| Crude protein (%) | 22.15 | 17.56 |
| Metabolizable energy (Kcal/kg DM) | 3112.89 | 3057.23 |
| Crude fiber (%) | 3.55 | 4.00 |
| Ether extract (%) | 2.17 | 2.01 |
| Ash (%) | 3.46 | 3.01 |
| Calcium (%) | 0.76 | 0.98 |
| Phosphorous (%) | 0.36 | 0.43 |
| Indices | XT Group | TX Group | TT Group | |||
|---|---|---|---|---|---|---|
| Cocks | Hens | Cocks | Hens | Cocks | Hens | |
| Body slope length (mm) | 197.38 ± 14.21 A | 182.38 ± 10.20 a | 192.15 ± 10.27 B | 177.23 ± 9.58 b | 194.64 ± 10.97 | 164.60 ± 10.30 c |
| Breast width (mm) | 82.52 ± 5.27 A | 71.36 ± 4.59 a | 76.81 ± 3.65 B | 72.51 ± 3.92 a | 71.87 ± 9.35 C | 66.50 ± 4.7 b |
| Breast depth (mm) | 90.13 ± 6.14 A | 80.41 ± 7.49 a | 87.62 ± 4.31 B | 79.20 ± 7.39 a | 82.15 ± 7.37 C | 72.23 ± 5.32 b |
| Pelvis width (mm) | 87.07 ± 4.73 A | 92.60 ± 5.74 a | 82.57 ± 3.71 B | 84.65 ± 4.90 b | 76.33 ± 9.51 C | 75.30 ± 5.90 c |
| Keel bone length (mm) | 118.28 ± 9.57 A | 102.86 ± 4.01 | 116.23 ± 7.82 A | 102.80 ± 2.78 | 123.05 ± 14.34 B | 102.10 ± 5.9 |
| Shank length (mm) | 100.52 ± 11.76 | 80.67 ± 5.01 | 102.28 ± 5.37 A | 81.91 ± 6.20 a | 98.49 ± 8.78 B | 79.10 ± 3.5 b |
| Shank girth (mm) | 4.43 ± 0.25 A | 3.79 ± 0.17 a | 4.70 ± 0.21 B | 3.91 ± 0.12 b | 4.41 ± 0.60 A | 3.64 ± 0.40 c |
| Body weight (g) | 2326.90 ± 148.66 A | 1888.8 ± 265.99 a | 2311.40 ± 250.17 A | 1907.8 ± 143.52 a | 2078.2 ± 263.40 B | 1660.8 ± 339.5 b |
| Carcass weight (g) | 2037.20 ± 187.43 A | 1614.40 ± 234.94 a | 2032.95 ± 220.23 A | 1687.40 ± 117.68 a | 1834.15 ± 237.56 B | 1419.95 ± 336.7 b |
| Half eviscerated weight (g) | 1900.20 ± 153.76 A | 1502.75 ± 267.88 a | 1911.15 ± 230.38 A | 1514.33 ± 117.48 a | 1761.46 ± 245.67 B | 1329.70 ± 302.83 b |
| Eviscerated weight (g) | 1639.10 ± 147.42 A | 1206.25 ± 204.80 | 1582.45 ± 210.06 | 1240.10 ± 109.52 | 1518.85 ± 197.12 B | 1211.81 ± 238.18 |
| Breast weight (g) | 228.25 ± 24.15 | 215.69 ± 91.40 a | 223.65 ± 33.83 | 201.85 ± 30.26 | 224.15 ± 38.38 | 181.89 ± 49.55 b |
| Thighs weight (g) | 425.18 ± 22.85 A | 224.05 ± 40.12 a | 398.94 ± 22.25 B | 268.00 ± 52.76 b | 422.35 ± 78.81 A | 235.65 ± 35.34 a |
| Abdominal fat weight (g) | 44.95 ± 32.68 A | 119.95 ± 74.35 a | 51.40 ± 25.63 A | 88.10 ± 29.82 b | 66.31 ± 15.74 B | 106.25 ± 52.11 |
| Wings weight (g) | 197.10 ± 21.92 | 138.80 ± 23.14 | 194.10 ± 32.96 | 135.35 ± 50.38 | 193.15 ± 35.67 | 138.9 ± 21.19 |
| Head weight (g) | 99.00 ± 12.99 A | 47.40 ± 5.50 a | 95.70 ± 21.72 A | 47.65 ± 4.80 a | 129.03 ± 32.97 B | 51.65 ± 8.16 b |
| Claws weight (g) | 84.80 ± 10.17 A | 51.45 ± 9.33 a | 101.85 ± 37.08 B | 55.75 ± 8.01 b | 111.3 ± 31.41 B | 58.75 ± 8.86 b |
| Dressing (%) | 91.08 ± 2.72 A | 90.72 ± 2.03 a | 89.80 ± 2.63 B | 89.63 ± 3.25 a | 88.26 ± 2.56 C | 85.44 ± 3.32 b |
| Half-eviscerated yield (%) | 85.42 ± 1.67 A | 79.87 ± 3.26 | 84.31 ± 1.47 B | 80.44 ± 4.19 | 81.70 ± 2.13 C | 79.83 ± 1.34 |
| Eviscerated yield (%) | 72.46 ± 3.02 A | 63.72 ± 3.62 a | 73.14 ± 3.78 A | 69.17 ± 3.29 b | 66.67 ± 3.16 B | 67.70 ± 2.14 c |
| Breast muscle (%) | 14.14 ± 1.54 A | 15.80 ± 1.77 a | 11.73 ± 1.35 B | 13.31 ± 1.52 b | 14.76 ± 1.27 C | 15.00 ± 2.11 c |
| Leg muscle (%) | 25.94 ± 4.51 | 18.03 ± 1.42 a | 25.21 ± 3.54 | 21.48 ± 2.72 b | 24.80 ± 4.32 | 23.34 ± 4.21 c |
| Item | pH | Meat Color | Pressing Loss (%) | Cooking Loss (%) | Shear Force (N) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 45 min | 24 h | L* | a* | b* | ||||||
| XT Group | Cocks | Breast | 6.13 ± 0.09 | 6.05 ± 0.21 | 41.01 ± 3.13 A | 7.00 ± 1.89 A | 8.73 ± 1.44 A | 21.07 ± 3.29 A | 14.46 ± 2.79 B | 3.65 ± 0.89 A |
| Leg | 6.32 ± 0.11 A | 6.27 ± 0.14 A | 39.35 ± 2.32 A | 9.06 ± 1.02 A | 7.31 ± 1.45 A | 20.41 ± 4.19 A | 14.22 ± 1.09 A | 3.19 ± 0.66 A | ||
| Hens | Breast | 6.12 ± 0.13 a | 6.19 ± 0.21 | 42.91 ± 2.79 a | 5.58 ± 1.67 a | 8.72 ± 2.19 a | 19.91 ± 3.24 a | 13.04 ± 2.11 a | 3.82 ± 1.1 a | |
| Leg | 6.34 ± 0.24 a | 6.35 ± 0.18 a | 41.95 ± 3.27 a | 3.28 ± 0.89 a | 7.59 ± 2.19 a | 21.56 ± 1.22 a | 14.85 ± 2.26 a | 3.12 ± 1.43 a | ||
| TX Group | Cocks | Breast | 6.10 ± 0.12 | 6.04 ± 0.17 | 40.39 ± 2.89 A | 6.88 ± 1.19 A | 7.77 ± 0.79 B | 22.08 ± 2.88 A | 15.54 ± 2.62 A | 4.37 ± 1.02 B |
| Leg | 6.20 ± 0.19 B | 6.25 ± 0.09 A | 39.20 ± 2.01 A | 9.39 ± 1.14 A | 6.20 ± 1.18 B | 21.25 ± 3.21 A | 14.93 ± 0.89 A | 2.32 ± 0.73 B | ||
| Hens | Breast | 6.20 ± 0.07 b | 6.21 ± 0.15 | 40.59 ± 3.01 b | 5.32 ± 1.89 a | 7.19 ± 1.85 b | 23.09 ± 4.32 b | 16.16 ± 3.69 b | 4.42 ± 1.23 b | |
| Leg | 6.16 ± 0.16 b | 6.12 ± 0.21 b | 37.52 ± 4.19 b | 4.27 ± 1.06 b | 7.51 ± 2.22 a | 21.98 ± 1.39 a | 14.27 ± 2.13 a | 3.41 ± 1.26 a | ||
| TT Group | Cocks | Breast | 6.17 ± 0.29 | 6.04 ± 0.30 | 38.33 ± 4.41 B | 3.08 ± 1.34 B | 5.70 ± 1.48 C | 24.89 ± 4.45 B | 13.71 ± 2.29 B | 3.02 ± 1.01 C |
| Leg | 6.24 ± 0.21 B | 6.17 ± 0.22 B | 33.94 ± 4.32 B | 14.92 ± 2.38 B | 4.73 ± 2.12 C | 26.42 ± 3.89 B | 18.98 ± 3.65 B | 3.00 ± 1.21 A | ||
| Hens | Breast | 6.20 ± 0.29 b | 6.17 ± 0.27 | 39.96 ± 3.38 b | 4.01 ± 1.68 b | 5.91 ± 2.99 c | 31.97 ± 3.67 c | 18.61 ± 2.16 c | 1.84 ± 0.57 c | |
| Leg | 6.30 ± 0.18 a | 6.16 ± 0.19 b | 37.38 ± 7.43 b | 4.69 ± 0.89 c | 3.60 ± 1.08 b | 31.99 ± 5.90 b | 21.63 ± 3.15 b | 1.79 ± 0.96 b | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Mushtaq, M.; Khan, M.; Fu, J.; Rahman, A.; Long, Y.; Liu, Y.; Zi, X.; Sun, D.; Ge, C.; et al. Evaluation of the Growth Performance and Meat Quality of Different F1 Crosses of Tengchong Snow and Xichou Black Bone Chicken Breeds. Animals 2024, 14, 3099. https://doi.org/10.3390/ani14213099
Li Z, Mushtaq M, Khan M, Fu J, Rahman A, Long Y, Liu Y, Zi X, Sun D, Ge C, et al. Evaluation of the Growth Performance and Meat Quality of Different F1 Crosses of Tengchong Snow and Xichou Black Bone Chicken Breeds. Animals. 2024; 14(21):3099. https://doi.org/10.3390/ani14213099
Chicago/Turabian StyleLi, Zijian, Maida Mushtaq, Muhammad Khan, Jing Fu, Abdur Rahman, Yingxiang Long, Yong Liu, Xiannian Zi, Dawei Sun, Changrong Ge, and et al. 2024. "Evaluation of the Growth Performance and Meat Quality of Different F1 Crosses of Tengchong Snow and Xichou Black Bone Chicken Breeds" Animals 14, no. 21: 3099. https://doi.org/10.3390/ani14213099
APA StyleLi, Z., Mushtaq, M., Khan, M., Fu, J., Rahman, A., Long, Y., Liu, Y., Zi, X., Sun, D., Ge, C., & Wang, K. (2024). Evaluation of the Growth Performance and Meat Quality of Different F1 Crosses of Tengchong Snow and Xichou Black Bone Chicken Breeds. Animals, 14(21), 3099. https://doi.org/10.3390/ani14213099








