The SCD5 Gene Modulates Adipogenic Differentiation via the WNT5B Signaling Pathway in Xinjiang Brown Cattle
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Sample Collection
2.2. Cell Isolation, Culture, and Differentiation
2.3. Oil Red O Staining
2.4. siRNA and Plasmid DNA Transfection
2.5. Cell Proliferation Test
2.6. RNA Extraction and RT-qPCR
2.7. Western Blot
2.8. Statistical Analysis
3. Results
3.1. Expression Profile of SCD5 During Adipogenic Differentiation
3.2. Overexpression and Knockdown of the SCD5
3.3. Overexpression of SCD5 Inhibits the Proliferation of Bovine Preadipocytes
3.4. SCD5 Knockdown Promotes Bovine Preadipocyte Proliferation
3.5. SCD5 Overexpression Suppresses Bovine Preadipocyte Differentiation
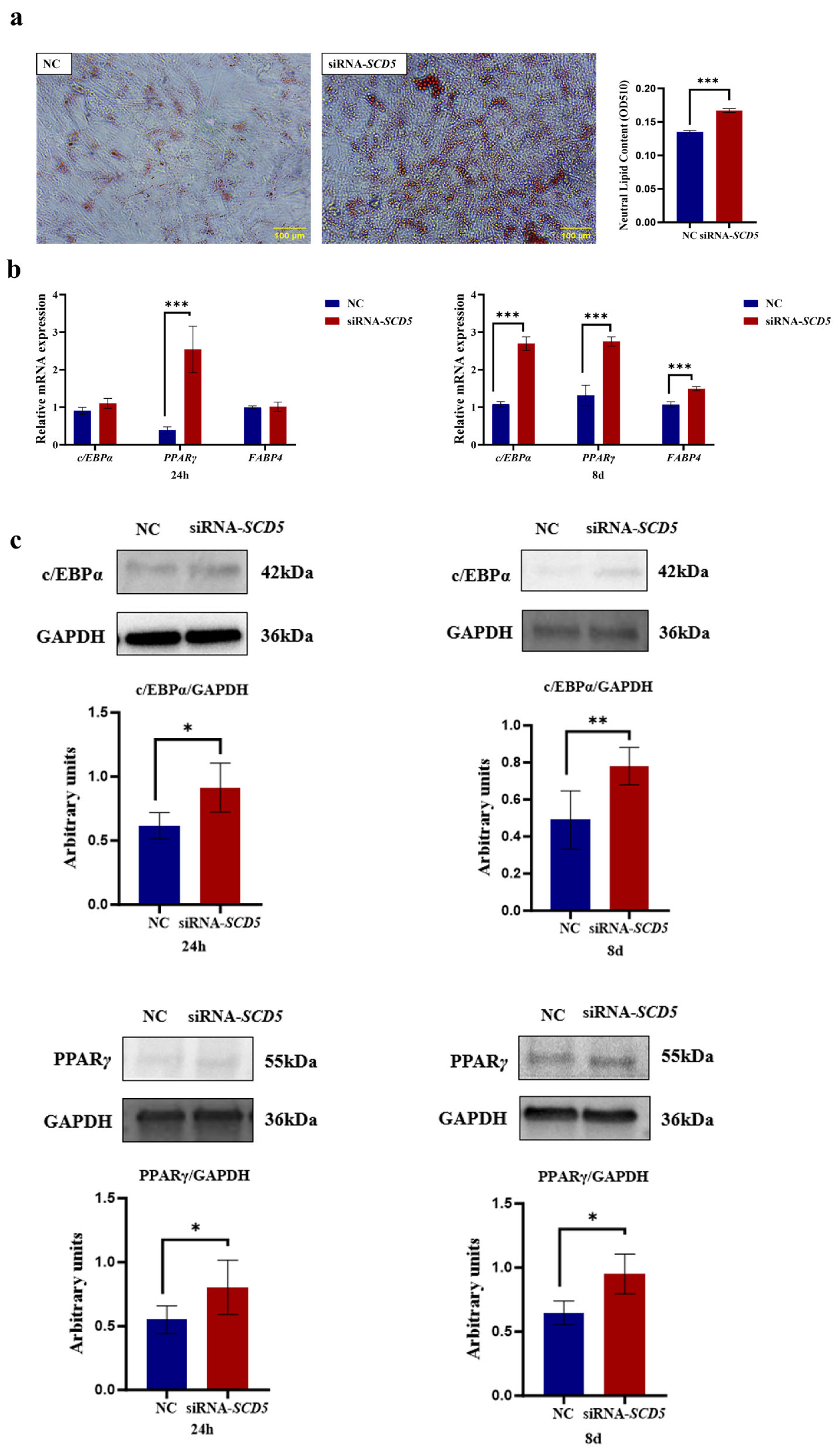
3.6. SCD5 Knockdown Promotes Bovine Preadipocyte Differentiation
3.7. Effect of the SCD5 on the WNT Signaling Pathway
3.8. SCD5 Regulates Adipogenesis Through the WNT5B Signaling Pathway
4. Discussion
4.1. Expression and Functional Study of the SCD5 in Adipogenic Differentiation
4.2. The SCD5 Suppresses the Proliferation of Bovine Preadipocytes
4.3. The SCD5 Inhibits the Differentiation of Bovine Preadipocytes
4.4. SCD5 Regulates Adipogenic Differentiation of Bovine Preadipocytes Through the WNT5B
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| β-catenin | Beta-Catenin |
| bGH | Bovine Growth Hormone |
| C16:0 | Palmitic Acid |
| C16:1n-7 | Palmitoleic Acid |
| C18:0 | Stearic Acid |
| C18:1n-9 | Oleic Acid |
| CCK-8 | Cell Counting Kit-8 |
| CDK1/2/6 | Cyclin-Dependent Kinase 1/2/6 |
| C/EBPα | CCAAT/Enhancer-Binding Protein Alpha |
| DMEM/F12 | Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 |
| EdU | 5-Ethynyl-2′-deoxyuridine |
| EGR2 | Early Growth Response Protein 2 |
| FA | Fatty Acid |
| FABP4 | Fatty Acid-Binding Protein 4 |
| FBS | Fetal Bovine Serum |
| FXR | Farnesoid X Receptor |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| IBMX | 3-Isobutyl-1-methylxanthine |
| IMF | Intramuscular Fat |
| PBS | Phosphate-Buffered Saline |
| PFA | Paraformaldehyde |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| RT-qPCR | Quantitative Reverse Transcription Polymerase Chain Reaction |
| SAT | Subcutaneous Adipose Tissue |
| SCD | Stearoyl-CoA Desaturase |
| SCD1 | Stearoyl-CoA Desaturase 1 |
| SCD5 | Stearoyl-CoA Desaturase 5 |
| SREBP1a | Sterol Regulatory Element-Binding Transcription Factor 1a |
| TG | Triglyceride |
| Wnt | Wingless-type MMTV integration site family |
| WNT3/3A/5A/5B/10B | Wnt Family Member 3/3A/5A/5B/10B |
References
- Igal, R.A.; Sinner, D.I. Stearoyl-CoA desaturase 5 (SCD5), a Δ-9 fatty acyl desaturase in search of a function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158840. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, L.; Yao, K.; Wang, Y.; Shao, W.; Yang, M.; Zhang, X.; Wei, Y.; Ren, W. Exploration of Genes Related to Intramuscular Fat Deposition in Xinjiang Brown Cattle. Genes 2024, 15, 1121. [Google Scholar] [CrossRef]
- Crespo-Piazuelo, D.; Criado-Mesas, L.; Revilla, M.; Castelló, A.; Noguera, J.L.; Fernández, A.I.; Ballester, M.; Folch, J.M. Identification of strong candidate genes for backfat and intra-muscular fatty acid composition in three crosses based on the Iberian pig. Sci. Rep. 2020, 10, 13962. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Michal, J.J.; Tobey, D.J.; Daniels, T.F.; Rule, D.C.; Macneil, M.D. Significant associations of stearoyl-CoA desaturase (SCD1) gene with fat deposition and composition in skeletal muscle. Int. J. Biol. Sci. 2008, 4, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Rezamand, P.; Watts, J.S.; Yavah, K.M.; Mosley, E.E.; Ma, L.; Corl, B.A.; McGuire, M.A. Relationship between stearoyl-CoA desaturase 1 gene expression, relative protein abundance, and its fatty acid products in bovine tissues. J. Dairy Res. 2014, 81, 333–339. [Google Scholar] [CrossRef]
- Liu, R.; Fang, X.; Lu, X.; Liu, Y.; Li, Y.; Bai, X.; Ding, X.; Yang, R. Polymorphisms of the SCD1 Gene and Its Association Analysis with Carcass, Meat Quality, Adipogenic Traits, Fatty Acid Composition, and Milk Production Traits in Cattle. Animals 2024, 14, 1759. [Google Scholar] [CrossRef]
- Miyazaki, M.; Jacobson, M.J.; Man, W.C.; Cohen, P.; Asilmaz, E.; Friedman, J.M.; Ntambi, J.M. Identification and characterization of murine SCD4, a novel heart-specific stearoyl-CoA desaturase isoform regulated by leptin and dietary factors. J. Biol. Chem. 2003, 278, 33904–33911. [Google Scholar] [CrossRef]
- Gamarra, D.; Aldai, N.; Arakawa, A.; Barron, L.J.R.; López-Oceja, A.; de Pancorbo, M.M.; Taniguchi, M. Distinct correlations between lipogenic gene expression and fatty acid composition of subcutaneous fat among cattle breeds. BMC Vet. Res. 2018, 14, 167. [Google Scholar] [CrossRef]
- Rios-Esteves, J.; Resh, M.D. Stearoyl CoA desaturase is required to produce active, lipid-modified WNT proteins. Cell Rep. 2013, 4, 1072–1081. [Google Scholar] [CrossRef]
- Bagchi, D.P.; Li, Z.; Corsa, C.A.; Hardij, J.; Mori, H.; Learman, B.S.; Lewis, K.T.; Schill, R.L.; Romanelli, S.M.; MacDougald, O.A. WNTless regulates lipogenic gene expression in adipocytes and protects against diet-induced metabolic dysfunction. Mol. Metab. 2020, 39, 100992. [Google Scholar] [CrossRef]
- Christodoulides, C.; Lagathu, C.; Sethi, J.K.; Vidal-Puig, A. Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 2009, 20, 16–24. [Google Scholar] [CrossRef]
- Takada, I.; Kouzmenko, A.P.; Kato, S. WNT and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat. Rev. Rheumatol. 2009, 5, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.N.; Ross, S.E.; Longo, K.A.; Bajnok, L.; Hemati, N.; Johnson, K.W.; Harrison, S.D.; MacDougald, O.A. Regulation of WNT signaling during adipogenesis. J. Biol. Chem. 2002, 277, 30998–31004. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, S.; Fujimori, K. Promotion of lipogenesis by PPARγ-activated FXR expression in adipocytes. Biochem. Biophys. Res. Commun. 2020, 527, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lengi, A.J.; Corl, B.A. Regulation of the bovine SCD5 promoter by EGR2 and SREBP1. Biochem. Biophys. Res. Commun. 2012, 421, 375–379. [Google Scholar] [CrossRef]
- Beswick, N.S.; Kennelly, J.J. Influence of bovine growth hormone and growth hormone-releasing factor on messenger RNA abundance of lipoprotein lipase and stearoyl-CoA desaturase in the bovine mammary gland and adipose tissue. J. Anim. Sci. 2000, 78, 412–419. [Google Scholar] [CrossRef]
- Liang, H.; Xu, L.; Zhao, X.; Pan, K.; Yi, Z.; Bai, J.; Qi, X.; Xin, J.; Li, M.; Ouyang, K.; et al. RNA-Seq analysis reveals the potential molecular mechanisms of daidzein on adipogenesis in subcutaneous adipose tissue of finishing Xianan beef cattle. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1–11. [Google Scholar] [CrossRef]
- Keating, A.F.; Kennelly, J.J.; Zhao, F.Q. Characterization and regulation of the bovine stearoyl-CoA desaturase gene promoter. Biochem. Biophys. Res. Commun. 2006, 344, 233–240. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Liang, X.; Wu, X.; Liu, J.; Yang, S.; Tao, C.; Zhang, J.; Tian, J.; Zhao, J.; et al. Stearoyl-CoA Desaturase is Essential for Porcine Adipocyte Differentiation. Int. J. Mol. Sci. 2020, 21, 2446. [Google Scholar] [CrossRef]
- Seale, P.; Conroe, H.M.; Estall, J.; Kajimura, S.; Frontini, A.; Ishibashi, J.; Cohen, P.; Cinti, S.; Spiegelman, B.M. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Investig. 2011, 121, 96–105. [Google Scholar] [CrossRef]
- Matson, J.P.; Cook, J.G. Cell cycle proliferation decisions: The impact of single cell analyses. FEBS J. 2017, 284, 362–375. [Google Scholar] [CrossRef]
- Gao, S.W.; Liu, F. Novel insights into cell cycle regulation of cell fate determination. J. Zhejiang Univ. Sci. B 2019, 20, 467–475. [Google Scholar] [CrossRef]
- An, E.J.; Kim, Y.; Lee, S.H.; Ko, H.M.; Chung, W.S.; Jang, H.J. Anti-Cancer Potential of Oxalis obtriangulata in Pancreatic Cancer Cell through Regulation of the ERK/Src/STAT3-Mediated Pathway. Molecules 2020, 25, 2301. [Google Scholar] [CrossRef]
- Suski, J.M.; Ratnayeke, N.; Braun, M.; Zhang, T.; Strmiska, V.; Michowski, W.; Can, G.; Simoneau, A.; Snioch, K.; Cup, M.; et al. CDC7-independent G1/S transition revealed by targeted protein degradation. Nature 2022, 605, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Ganner, A.; Philipp, A.; Lagies, S.; Wingendorf, L.; Wang, L.; Pilz, F.; Welte, T.; Grand, K.; Lienkamp, S.S.; Klein, M.; et al. SCD5 Regulation by VHL Affects Cell Proliferation and Lipid Homeostasis in ccRCC. Cells 2023, 12, 835. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, L.; Li, X.; Wang, J.; Zhu, Y.; Jia, Y.; Tong, Z. SCD5 expression correlates with prognosis and response to neoadjuvant chemotherapy in breast cancer. Sci. Rep. 2021, 11, 8976. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Vellon, L.; Colomer, R.; Lupu, R. Oleic acid, the main monounsaturated fatty acid of olive oil, suppresses Her-2/neu (erbB-2) expression and synergistically enhances the growth inhibitory effects of trastuzumab (Herceptin) in breast cancer cells with Her-2/neu oncogene amplification. Ann. Oncol. 2005, 16, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xi, Q.Y.; Wei, S.; Wu, D.; Ye, R.S.; Chen, T.; Qi, Q.E.; Jiang, Q.Y.; Wang, S.B.; Wang, L.N.; et al. Critical role of miR-125b in lipogenesis by targeting stearoyl-CoA desaturase-1 (SCD-1). J. Anim. Sci. 2016, 94, 65–76. [Google Scholar] [CrossRef]
- Shao, X.; Wang, M.; Wei, X.; Deng, S.; Fu, N.; Peng, Q.; Jiang, Y.; Ye, L.; Xie, J.; Lin, Y. Peroxisome Proliferator-Activated Receptor-γ: Master Regulator of Adipogenesis and Obesity. Curr. Stem Cell Res. Ther. 2016, 11, 282–289. [Google Scholar] [CrossRef]
- Xiang, X.; Han, S.; Xu, D.; Chen, Q.; Ji, R.; Zhao, Z.; Du, J.; Mai, K.; Ai, Q. Oleic and palmitic acids induce hepatic angiopoietin-like 4 expression predominantly via PPAR-γ in Larimichthys crocea. Br. J. Nutr. 2023, 129, 1657–1666. [Google Scholar] [CrossRef]
- Cheng, H.S.; Tan, W.R.; Low, Z.S.; Marvalim, C.; Tan, N.S. Exploration and development of PPAR modulators in health and disease: An update of clinical evidence. Int. J. Mol. Sci. 2019, 20, 5055. [Google Scholar] [CrossRef]
- Jing, E.; Gesta, S.; Kahn, C.R. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab. 2007, 6, 105–114. [Google Scholar] [CrossRef]
- Wang, F.; Tong, Q. SIRT2 suppresses adipocyte differentiation by deacetylating FOXO1 and enhancing FOXO1’s repressive interaction with PPARγ. Mol. Biol. Cell 2009, 20, 801–808. [Google Scholar] [CrossRef]
- Puglisi, R.; Bellenghi, M.; Pontecorvi, G.; Gulino, A.; Petrini, M.; Felicetti, F.; Bottero, L.; Mattia, G.; Carè, A. SCD5 restored expression favors differentiation and epithelial-mesenchymal reversion in advanced melanoma. Oncotarget 2018, 9, 7567–7581. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.K.Y.; Kweon, S.M.; Chi, F.; Hwang, E.; Kabe, Y.; Higashiyama, R.; Qin, L.; Yan, R.; Wu, R.P.; Lai, K.; et al. Stearoyl-CoA Desaturase Promotes Liver Fibrosis and Tumor Development in Mice via a WNT Positive-Signaling Loop by Stabilization of Low-Density Lipoprotein-Receptor-Related Proteins 5 and 6. Gastroenterology 2017, 152, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Q.; Li, Z.; Yang, Q.; Liu, Y.; Du, Z.; Zhang, G.; Song, Y. Circular RNA CDR1as promotes adipogenic and suppresses osteogenic differentiation of BMSCs in steroid-induced osteonecrosis of the femoral head. Bone 2020, 133, 115258. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, T.C.; Macdougald, O.A. WNT/β-catenin signaling in adipogenesis and metabolism. Curr. Opin. Cell Biol. 2007, 19, 612–617. [Google Scholar] [CrossRef]
- Rawson, P.; Stockum, C.; Peng, L.; Manivannan, B.; Lehnert, K.; Ward, H.E.; Berry, S.D.; Davis, S.R.; Snell, R.G.; McLauchlan, D.; et al. Metabolic proteomics of the liver and mammary gland during lactation. J. Proteom. 2012, 75, 4429–4435. [Google Scholar] [CrossRef]
- Silva-García, O.; Valdez-Alarcón, J.J.; Baizabal-Aguirre, V.M. WNT/β-Catenin Signaling as a Molecular Target by Pathogenic Bacteria. Front. Immunol. 2019, 10, 2135. [Google Scholar] [CrossRef]
- van Tienen, F.H.; Laeremans, H.; van der Kallen, C.J.; Smeets, H.J. WNT5b stimulates adipogenesis by activating PPARγ, and inhibiting the β-catenin dependent WNT signaling pathway together with WNT5a. Biochem. Biophys. Res. Commun. 2009, 387, 207–211. [Google Scholar] [CrossRef]
- Bagchi, D.P.; MacDougald, O.A. WNT Signaling: From Mesenchymal Cell Fate to Lipogenesis and Other Mature Adipocyte Functions. Diabetes 2021, 70, 1419–1430. [Google Scholar] [CrossRef]
- Kamizaki, K.; Katsukawa, M.; Yamamoto, A.; Fukada, S.I.; Uezumi, A.; Endo, M.; Minami, Y. Ror2 signaling regulated by differential WNT proteins determines pathological fate of muscle mesenchymal progenitors. Cell Death Dis. 2024, 15, 784. [Google Scholar] [CrossRef]
- Liang, Z.; Li, S.; Wang, Z.; Zhou, J.; Huang, Z.; Li, J.; Bao, H.; Yam, J.W.P.; Xu, Y. Unraveling the Role of the Wnt Pathway in Hepatocellular Carcinoma: From Molecular Mechanisms to Therapeutic Implications. J. Clin. Transl. Hepatol. 2025, 13, 315–326. [Google Scholar] [CrossRef]








| Item | Sense (5′ to 3′) | Antisense (5′ to 3′) | |
|---|---|---|---|
| SCD5 | siRNA1 | ACUCCAUGGCUUUCCAGAATT | UUCUGGAAAGCCAUGGAGUTT |
| siRNA2 | GGUCCGGUUCCAGAGAAAGTT | CUUUCUCUGGAACCGGACCTT | |
| siRNA3 | GUCGCUCACAUGUACGGAATT | UUCCGUACAUGUGAGCGACTT | |
| WNT5B | siRNA1 | CUCGCCUUGCUGUUCGCCUTT | AGGCGAACAGCAAGGCGAGTT |
| siRNA2 | UCCGUCUUUGGGAGAGUCCTT | GGACUCUCCCAAAGACGGATT | |
| siRNA3 | GGGCUGUGUACAAGACGGCTT | GCCGUCUUGUACACAGCCCTT | |
| NC | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
| Gene | mRNA RefSeq ID | Primer Sequence (5′ to 3′) | Product Size |
|---|---|---|---|
| SCD5 | NM_001076945.1 | F: TGGGTGCCATTGGTGAAGGT | 124 bp |
| R: CCCAGCCAACACATGAAGTC | |||
| PPARγ | NM_181024.2 | F: TGGAGACCGCCCAGGTTTGC | 111 bp |
| R: AGCTGGGAGGACTCGGGGTG | |||
| C/EBPα | NM_176784.2 | F: TGGGCAAGAGCCGGGACAAG | 166 bp |
| R: ACCAGGGAGCTCTCGGGCAG | |||
| FABP4 | NM_174314.2 | F: TCCTTCAAATTGGGCCAGGAA | 218 bp |
| R: CCCTTGGCTTATGCTCTCTCA | |||
| CDK1 | NM_174016.2 | F: GTGGAAACCAGGAAGCTTAGC | 201 bp |
| R: TGCTCTTGACACAACACAGGGA | |||
| CDK2 | NM_001014934.1 | F: GGCATTCCTCTTCCGCTCAT | 142 bp |
| R: CTGCTAGCTTGATGGACCCA | |||
| CDK6 | NM_001192301.2 | F: GGAGTGCCCACTGAAACCAT | 131 bp |
| R: ATTTGTCCACTGCTGGTCACC | |||
| CyclinB | NM_001045872.1 | F: ACACCTACACCAAGTTTCAAATCA | 182 bp |
| R: ATCGTAGTCCAGCATAGTTAGTTCC | |||
| CyclinD | NM_001046273.2 | F: ATGAAGGAGACCATCCCCCT | 123 bp |
| R: CGCCAGGTTCCACTTGAGTT | |||
| CyclinE | NM_001192776.1 | F: CCTCCAAAGTTGCACCAGTT | 195 bp |
| R: AGGATACTGAGGCAGGAGCA | |||
| WNT10B | XM_010805029.4 | F: GTCTCCTGTTCCTGGCGTTGTG | 103 bp |
| R: CACACGGTGTTGGCGGTCAG | |||
| WNT3 | XM_005220917.5 | F: CTGGGAACGGGTGAAGTGTGTG | 120 bp |
| R: GCGTCTGGCAAGAGTCCTGATTC | |||
| WNT3A | XM_024995392.2 | F: AGTTCGGCGGGATGGTGTCTC | 123 bp |
| R: GGTGCATGTGACTGGCGATGG | |||
| WNT5A | XM_005222857.5 | F: TCGGATCGCTAGGTCACACTCTC | 110 bp |
| R: CATTCGCTGGGTCGGACACTTG | |||
| WNT5B | XM_059886227.1 | F: AGCCAGTAGCCACTCAAGACACC | 144 bp |
| R: AGCAGGAGACAGGACCACAGATG | |||
| GAPDH | NM_001034034.2 | F: TGCCCGTTCGACAGATAGCC | 148 bp |
| R: GCGACGATGTCCACTTTGCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ren, W.; Shao, W.; Zhou, Y.; Liu, Y.; Cao, J.; Wang, F.; Bi, J.; Yang, L. The SCD5 Gene Modulates Adipogenic Differentiation via the WNT5B Signaling Pathway in Xinjiang Brown Cattle. Animals 2025, 15, 3547. https://doi.org/10.3390/ani15243547
Wang Y, Ren W, Shao W, Zhou Y, Liu Y, Cao J, Wang F, Bi J, Yang L. The SCD5 Gene Modulates Adipogenic Differentiation via the WNT5B Signaling Pathway in Xinjiang Brown Cattle. Animals. 2025; 15(24):3547. https://doi.org/10.3390/ani15243547
Chicago/Turabian StyleWang, Yiran, Wanping Ren, Wei Shao, Yuxin Zhou, Yili Liu, Junwei Cao, Fengju Wang, Jingdong Bi, and Liang Yang. 2025. "The SCD5 Gene Modulates Adipogenic Differentiation via the WNT5B Signaling Pathway in Xinjiang Brown Cattle" Animals 15, no. 24: 3547. https://doi.org/10.3390/ani15243547
APA StyleWang, Y., Ren, W., Shao, W., Zhou, Y., Liu, Y., Cao, J., Wang, F., Bi, J., & Yang, L. (2025). The SCD5 Gene Modulates Adipogenic Differentiation via the WNT5B Signaling Pathway in Xinjiang Brown Cattle. Animals, 15(24), 3547. https://doi.org/10.3390/ani15243547






