Germline-Restricted Chromosome (GRC) in Diploid and Polyploid Spermatocytes of the Eurasian Bullfinch, Pyrrhula pyrrhula (Fringillidae, Passeriformes, Aves)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Synaptonemal Complex (SC) Spreading and Staining
2.3. Generation of DNA Probe for the Bullfinch GRC
2.4. FISH with GRC DNA Probe
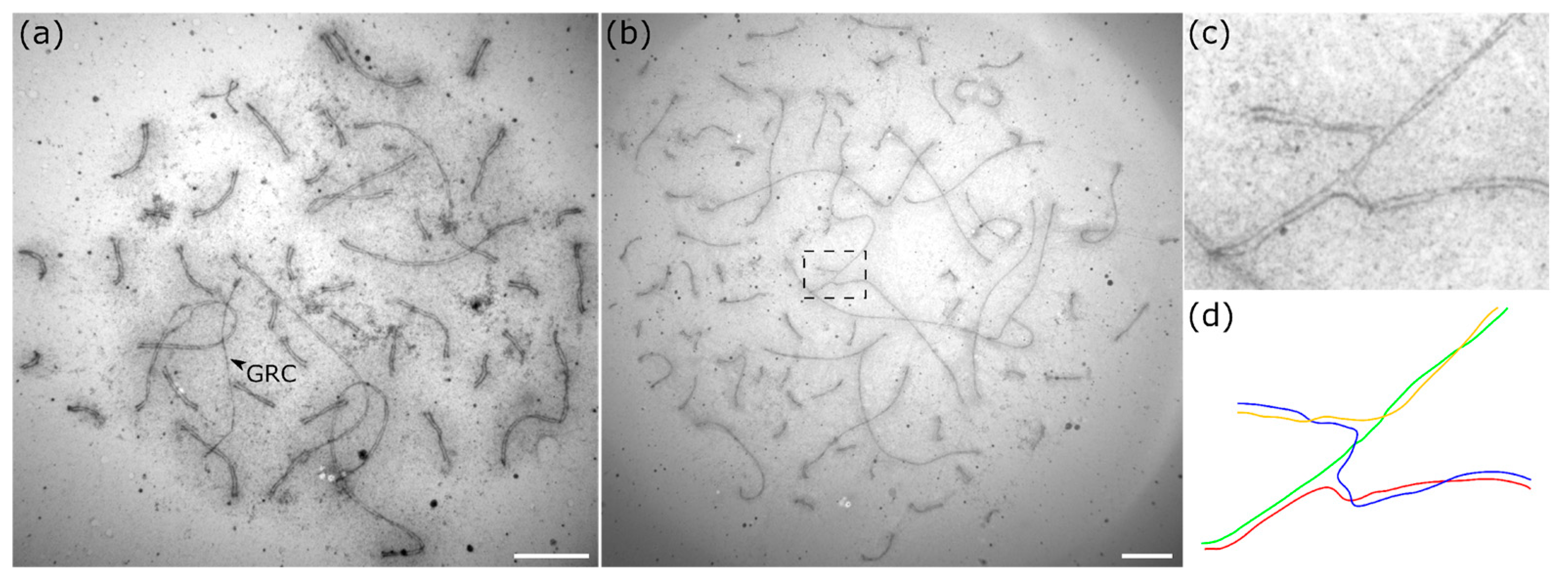
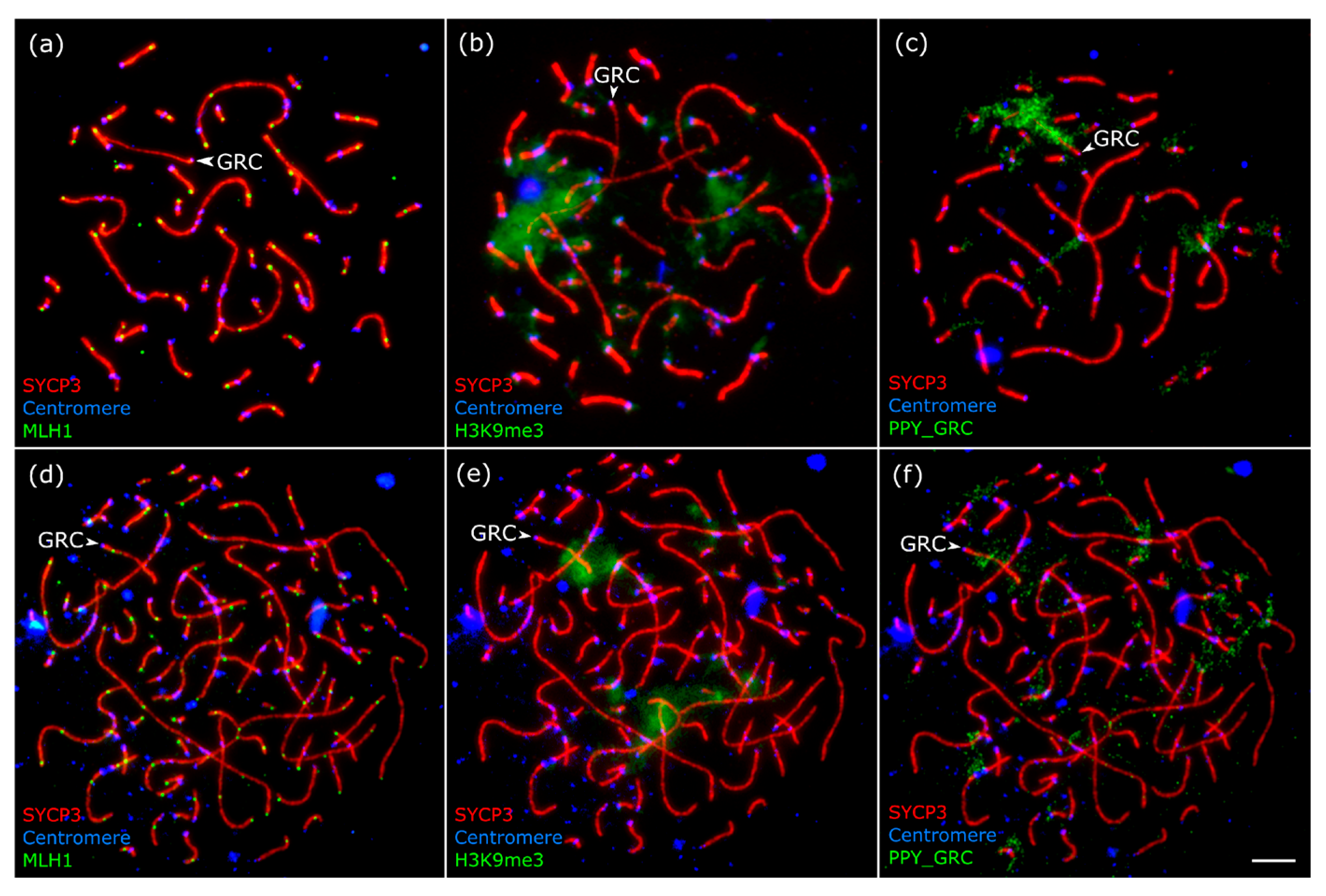
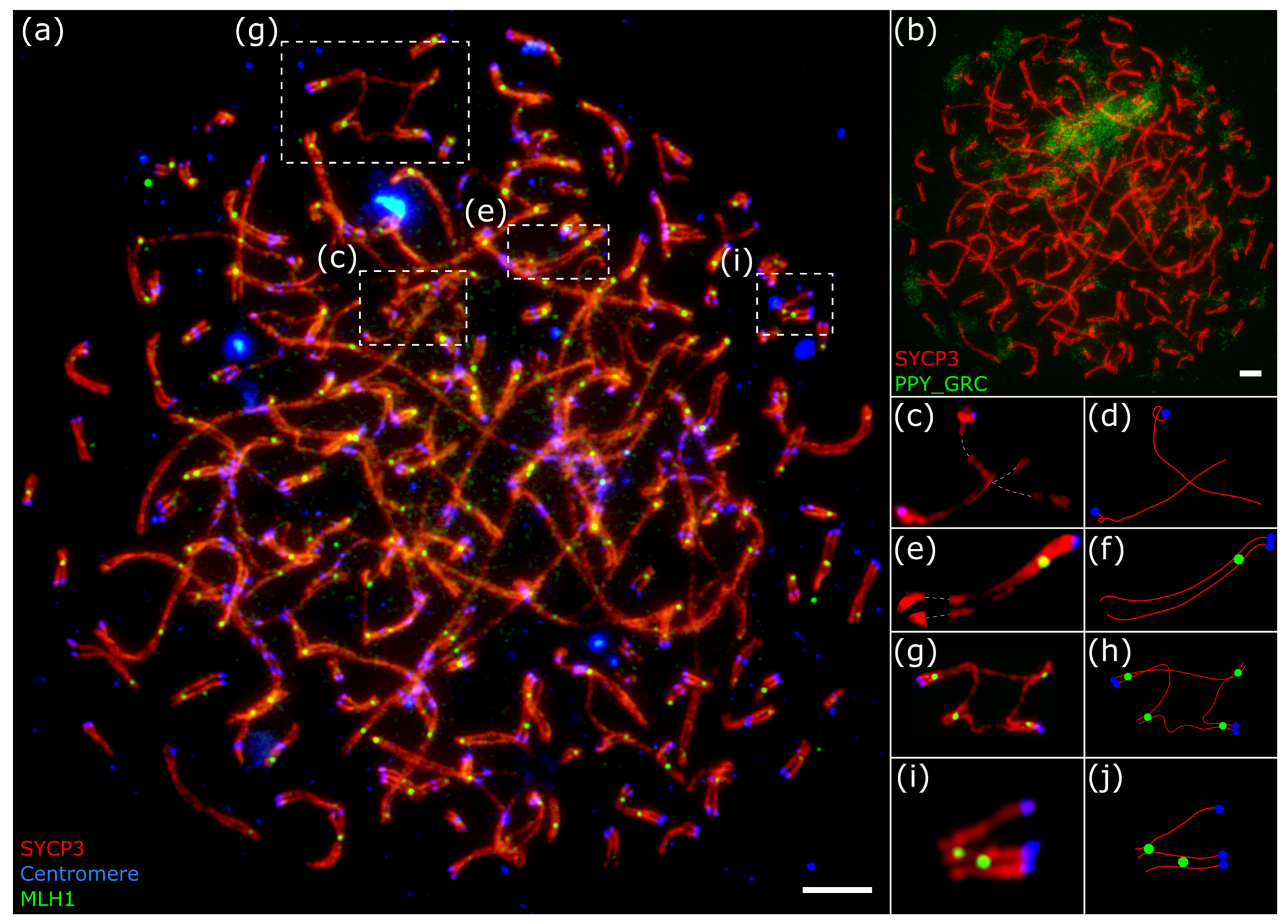
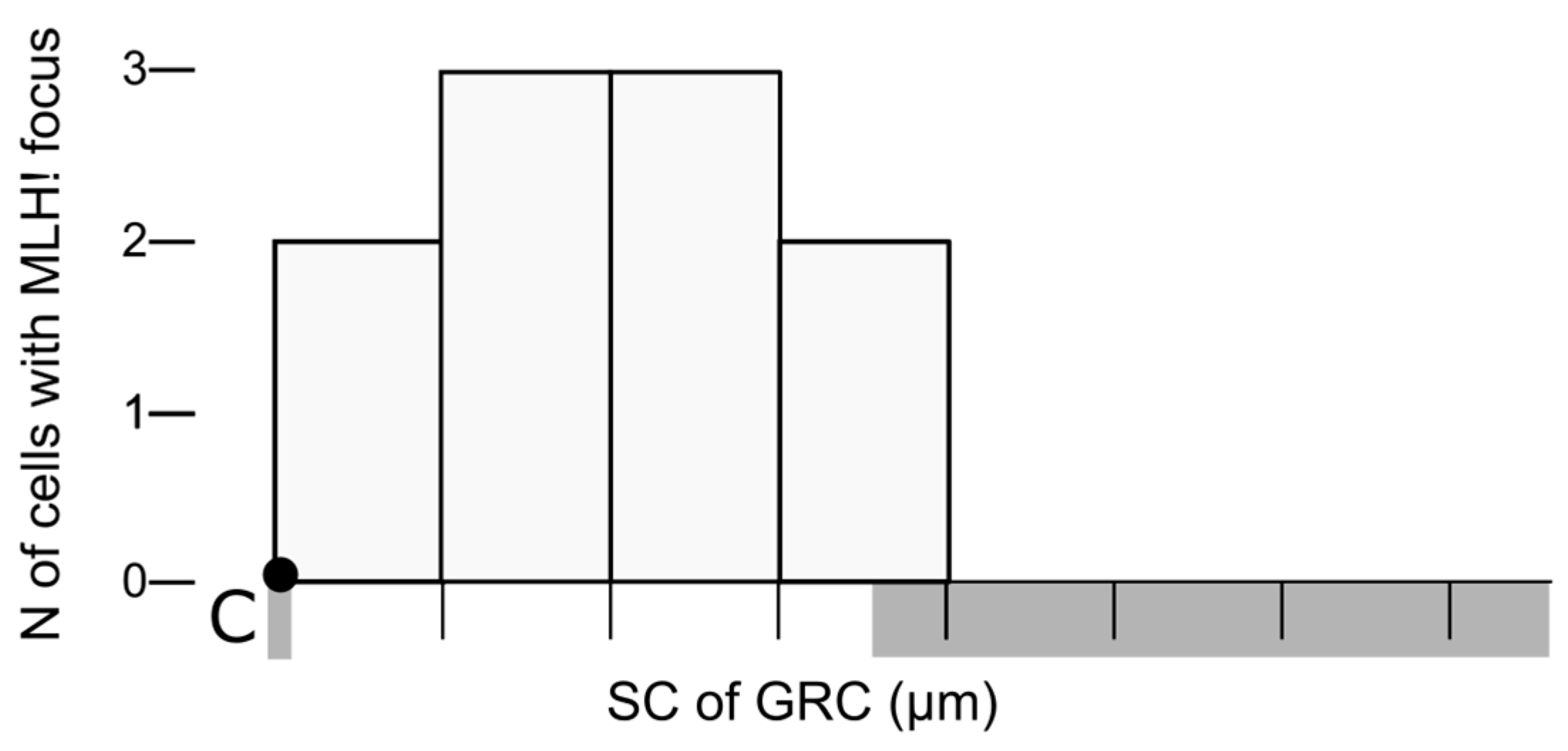
2.5. Image Analysis
- (i)
- The nucleus is well-isolated from other nuclei, exhibits a continuous regularly shaped outline and all SCs within the nucleus are at the same stage of meiotic prophase.
- (ii)
- At the early and mid-late pachytene stage, the nucleus contains more than 70 SCs (approximately double the diploid chromosome number). At the zygotene stage, the nucleus contains two synapsed or closely located GRCs.
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. A Curious Normality of Chromosome Pairing and Recombination in the Polyploid Bullfinch Spermatocytes
4.2. GRC Behavior in Polyploid Bullfinch Spermatocytes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pigozzi, M.I.; Solari, A.J. Germ cell restriction and regular transmission of an accessory chromosome that mimics a sex body in the zebra finch, Taeniopygia guttata. Chromosome Res. 1998, 6, 105–113. [Google Scholar] [CrossRef]
- Torgasheva, A.A.; Malinovskaya, L.P.; Zadesenets, K.S.; Karamysheva, T.V.; Kizilova, E.A.; Akberdina, E.A.; Pristyazhnyuk, I.E.; Shnaider, E.P.; Volodkina, V.A.; Saifitdinova, A.F.; et al. Germline-restricted chromosome (GRC) is widespread among songbirds. Proc. Natl. Acad. Sci. USA 2019, 116, 11845–11850. [Google Scholar] [CrossRef]
- Biederman, M.K.; Nelson, M.M.; Asalone, K.C.; Pedersen, A.L.; Saldanha, C.J.; Bracht, J.R. Discovery of the first germline-restricted gene by subtractive transcriptomic analysis in the zebra finch, Taeniopygia guttata. Curr. Biol. 2018, 28, 1620–1627. [Google Scholar] [CrossRef]
- Kinsella, C.M.; Ruiz-Ruano, F.J.; Dion-Côté, A.-M.; Charles, A.J.; Gossmann, T.I.; Cabrero, J.; Kappei, D.; Hemmings, N.; Simons, M.J.P.; Camacho, J.P.M.; et al. Programmed DNA elimination of germline development genes in songbirds. Nat. Commun. 2019, 10, 5468. [Google Scholar] [CrossRef]
- Mueller, J.C.; Schlebusch, S.A.; Pei, Y.; Poignet, M.; Vontzou, N.; Ruiz-Ruano, F.J.; Albrecht, T.; Reifová, R.; Forstmeier, W.; Suh, A.; et al. Micro germline-restricted chromosome in blue tits: Evidence for Meiotic Functions. Mol. Biol. Evol. 2023, 40, msad096. [Google Scholar] [CrossRef]
- Ruiz-Ruano, F.J.; Schlebusch, S.A.; Vontzou, N.; Moreno, H.; Biegler, M.T.; Kutschera, V.E.; Ekman, D.; Borges, I.; Pei, Y.; Rossini, R.; et al. Programmed DNA elimination drives rapid genomic innovation in two thirds of all bird species. bioRxiv 2025. [Google Scholar] [CrossRef]
- Fang, B.; Edwards, S.V. Avian germline-restricted chromosomes are reservoirs for active long-terminal-repeat retroviruses. bioRxiv 2025. [Google Scholar] [CrossRef]
- Borodin, P.; Chen, A.; Forstmeier, W.; Fouché, S.; Malinovskaya, L.; Pei, Y.; Reifová, R.; Ruiz-Ruano, F.J.; Schlebusch, S.A.; Sotelo-Muñoz, M.; et al. Mendelian Nightmares: The Germline-Restricted Chromosome of Songbirds. Chromosome Res. 2022, 30, 255–272. [Google Scholar] [CrossRef]
- del Priore, L.; Pigozzi, M.I. Histone modifications related to chromosome silencing and elimination during male meiosis in Bengalese finch. Chromosoma 2014, 123, 293–302. [Google Scholar] [CrossRef]
- Malinovskaya, L.P.; Zadesenets, K.S.; Karamysheva, T.V.; Akberdina, E.A.; Kizilova, E.A.; Romanenko, M.V.; Shnaider, E.P.; Scherbakova, M.M.; Korobitsyn, I.G.; Rubtsov, N.B.; et al. Germline-restricted chromosome (GRC) in the sand martin and the pale martin (Hirundinidae, Aves): Synapsis, recombination and copy number variation. Sci. Rep. 2020, 10, 1058. [Google Scholar] [CrossRef]
- Pei, Y.; Forstmeier, W.; Ruiz-Ruano, F.J.; Mueller, J.C.; Cabrero, J.; Camacho, J.P.M.; Alché, J.D.; Franke, A.; Hoeppner, M.; Börno, S.; et al. Occasional paternal inheritance of the germline-restricted chromosome in songbirds. Proc. Natl. Acad. Sci. USA 2022, 119, e2103960119. [Google Scholar] [CrossRef]
- Grishko, E.; Malinovskaya, L.; Slobodchikova, A.; Kotelnikov, A.; Torgasheva, A.; Borodin, P. Cytological analysis of crossover frequency and distribution in male meiosis of Cardueline finches (Fringillidae, Aves). Animals 2023, 13, 3624. [Google Scholar] [CrossRef]
- Peters, A.H.; Plug, A.W.; van Vugt, M.J.; de Boer, P. A Drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res. 1997, 5, 66–68. [Google Scholar] [CrossRef]
- Howell, W.M.; Black, D.A. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: A 1-step method. Experientia 1980, 36, 1014–1015. [Google Scholar] [CrossRef]
- Anderson, L.K.; Reeves, A.; Webb, L.M.; Ashley, T. Distribution of crossing over on mouse synaptonemal complexes using immunofluorescent localization of MLH1 protein. Genetics 1999, 151, 1569–1579. [Google Scholar] [CrossRef]
- Liehr, T.; Kreskowski, K.; Ziegler, M.; Piaszinski, K.; Rittscher, K. The standard FISH procedure. In Fluorescence In Situ Hybridization (FISH): Application Guide; Liehr, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 109–118. [Google Scholar]
- Reeves, A. MicroMeasure: A new computer program for the collection and analysis of cytogenetic data. Genome 2001, 44, 439–443. [Google Scholar] [CrossRef]
- Bonaminio, G.; Fechheimer, N. The gonadal histology of triploid chicken (Gallus Domesticus) Embryos. Genet. Sel. Evol. 1993, 25, 205–210. [Google Scholar] [CrossRef]
- Forstmeier, W.; Ellegren, H. Trisomy and triploidy are sources of embryo mortality in the zebra finch. Proc. R. Soc. B Biol. Sci. 2010, 277, 2655–2660. [Google Scholar] [CrossRef]
- Thorne, M.; Collins, R.; Sheldon, B. Triploidy and other chromosomal abnormalities in a selected line of chickens. Genet. Sel. Evol. 1991, 23, 212–216. [Google Scholar] [CrossRef]
- Tiersch, T.R.; Beck, M.L.; Douglass, M. ZZW autotriploidy in a blue-and-yellow macaw. Genetica 1991, 84, 209–212. [Google Scholar] [CrossRef]
- Küpper, C.; Augustin, J.; Edwards, S.; Székely, T.; Kosztolányi, A.; Burke, T.; Janes, D.E. Triploid plover female provides support for a role of the W chromosome in avian sex determination. Biol. Lett. 2012, 8, 787–789. [Google Scholar] [CrossRef]
- Solari, A.J.; Moses, M.J. Synaptonemal complexes in a tetraploid mouse spermatocyte. Exp. Cell Res. 1977, 108, 464–467. [Google Scholar] [CrossRef]
- Codina-Pascual, M.; Navarro, J.; Egozcue, J.; Benet, J. A human tetraploid pachytene spermatocyte as the possible origin of diploid sperm: A Case Report. Hum. Reprod. 2006, 21, 1795–1797. [Google Scholar] [CrossRef]
- Virabyan, L.; Petrosyan, R.; Ayvazyan, I.; Khachatrian, E.R.; Dombrovskaya, Y.; Solovyeva, V.; Spangenberg, V. Polyploidy in germ cell of brown bear Ursus arctos (Ursidae, Mammalia): A Case Report. Reprod. Biol. 2025, 25, 101024. [Google Scholar] [CrossRef]
- Matveevsky, S.; Kolomiets, O.; Bogdanov, A.; Hakhverdyan, M.; Bakloushinskaya, I. Chromosomal Evolution in Mole Voles Ellobius (Cricetidae, Rodentia): Bizarre Sex Chromosomes, Variable Autosomes and Meiosis. Genes 2017, 8, 306. [Google Scholar] [CrossRef]
- Fechheimer, N.S. Poikiloploidy among spermatogenic cells of Mus musculus. Reproduction 1961, 2, 68–79. [Google Scholar] [CrossRef]
- Ford, C.E.; Evans, E.P. Origin of apparent polyploid spermatocytes in the mouse. Nature 1971, 230, 389–390. [Google Scholar] [CrossRef]
- Pearson, P.L.; Madan, K. True polyploid meiosis in the human male. Genet. Mol. Biol. 2018, 41, 410–413. [Google Scholar] [CrossRef]
- Pigozzi, M.I.; Solari, A.J. The germ-line-restricted chromosome in the zebra finch: Recombination in females and elimination in males. Chromosoma 2005, 114, 403–409. [Google Scholar] [CrossRef]
- Malinovskaya, L.P.; Tishakova, K.; Shnaider, E.P.; Borodin, P.M.; Torgasheva, A.A. Heterochiasmy and sexual dimorphism: The case of the barn swallow (Hirundo Rustica, Hirundinidae, Aves). Genes 2020, 11, 1119. [Google Scholar] [CrossRef]
- Torgasheva, A.; Malinovskaya, L.; Zadesenets, K.; Shnaider, E.; Rubtsov, N.; Borodin, P. Germline-restricted chromosome (GRC) in female and male meiosis of the great tit (Parus Major, Linnaeus, 1758). Front. Genet. 2021, 12, 768056. [Google Scholar] [CrossRef]
- Janssen, A.; Colmenares, S.U.; Karpen, G.H. Heterochromatin: Guardian of the genome. Annu. Rev. Cell Dev. Biol. 2018, 34, 265–288. [Google Scholar] [CrossRef]
- Mitrentsi, I.; Lou, J.; Kerjouan, A.; Verigos, J.; Reina-San-Martin, B.; Hinde, E.; Soutoglou, E. Heterochromatic repeat clustering imposes a physical barrier on homologous recombination to prevent chromosomal translocations. Mol. Cell 2022, 82, 2132–2147.e6. [Google Scholar] [CrossRef]
| Antibody | Host | Supplier (Catalog no.) | Dilution | Reaction Type |
|---|---|---|---|---|
| Anti-SYCP3 | Rabbit | Abcam (ab15093) | 1:300 | Unconjugated |
| Anti-centromere antibodies | Human | Antibodies Inc. (15-234) | 1:70 | Unconjugated |
| Anti-H3K9me3 | Rabbit | Abcam (ab8898) | 1:100 | Unconjugated |
| Anti-MLH1 | Mouse | Abcam (ab14206) | 1:30 | Unconjugated |
| Anti-mouse | Goat | Jackson ImmunoResearch (115-095-003) | 1:30 | FITC |
| Anti-rabbit | Goat | Jackson ImmunoResearch (111-165-144) | 1:250 | Cy3 |
| Anti-human | Donkey | Jackson ImmunoResearch (709-155-149) | 1:65 | AMCA |
| Anti-rabbit | Donkey | Jackson ImmunoResearch (711-095-152) | 1:100 | FITC |
| Anti-rabbit | Goat | Jackson ImmunoResearch (111-175-144) | 1:100 | Cy5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grishko, E.; Malinovskaya, L.; Tishakova, K.; Borodin, P. Germline-Restricted Chromosome (GRC) in Diploid and Polyploid Spermatocytes of the Eurasian Bullfinch, Pyrrhula pyrrhula (Fringillidae, Passeriformes, Aves). Animals 2025, 15, 3394. https://doi.org/10.3390/ani15233394
Grishko E, Malinovskaya L, Tishakova K, Borodin P. Germline-Restricted Chromosome (GRC) in Diploid and Polyploid Spermatocytes of the Eurasian Bullfinch, Pyrrhula pyrrhula (Fringillidae, Passeriformes, Aves). Animals. 2025; 15(23):3394. https://doi.org/10.3390/ani15233394
Chicago/Turabian StyleGrishko, Ekaterina, Lyubov Malinovskaya, Katerina Tishakova, and Pavel Borodin. 2025. "Germline-Restricted Chromosome (GRC) in Diploid and Polyploid Spermatocytes of the Eurasian Bullfinch, Pyrrhula pyrrhula (Fringillidae, Passeriformes, Aves)" Animals 15, no. 23: 3394. https://doi.org/10.3390/ani15233394
APA StyleGrishko, E., Malinovskaya, L., Tishakova, K., & Borodin, P. (2025). Germline-Restricted Chromosome (GRC) in Diploid and Polyploid Spermatocytes of the Eurasian Bullfinch, Pyrrhula pyrrhula (Fringillidae, Passeriformes, Aves). Animals, 15(23), 3394. https://doi.org/10.3390/ani15233394








