Rumen-Protected Methionine Supplementation in the Diet Improved the Production Performance of Dairy Goats by Optimizing the Amino Acid Profile and Lipid Metabolism and Modulating the Colonic Microbiome
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Management
2.2. Experimental Procedures and Sample Collection
2.3. Laboratory Analyses
2.4. Fecal Microbiota 16S rRNA Gene Sequencing
2.5. Statistical Analyses
3. Results
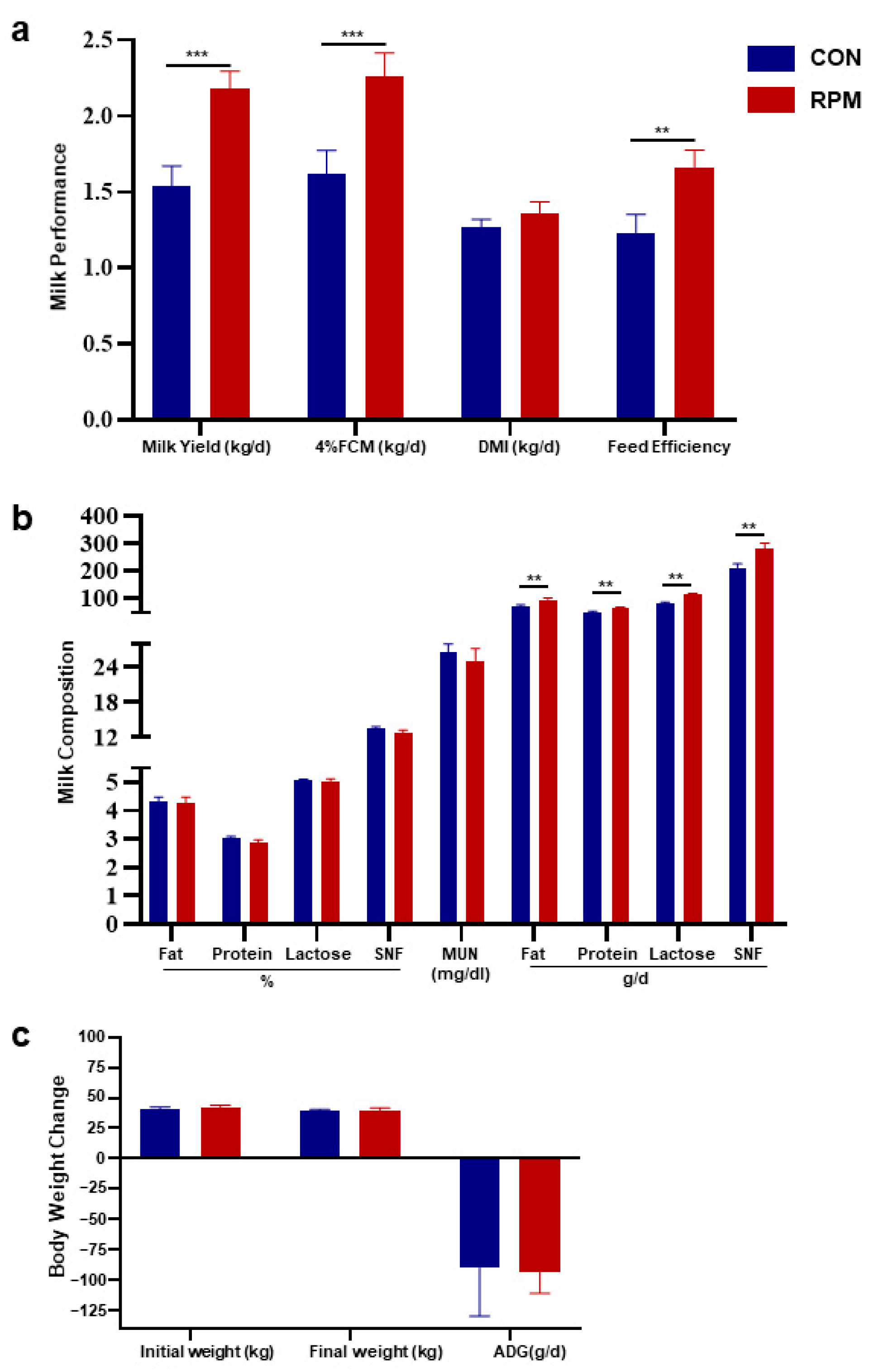
3.1. Production Performance
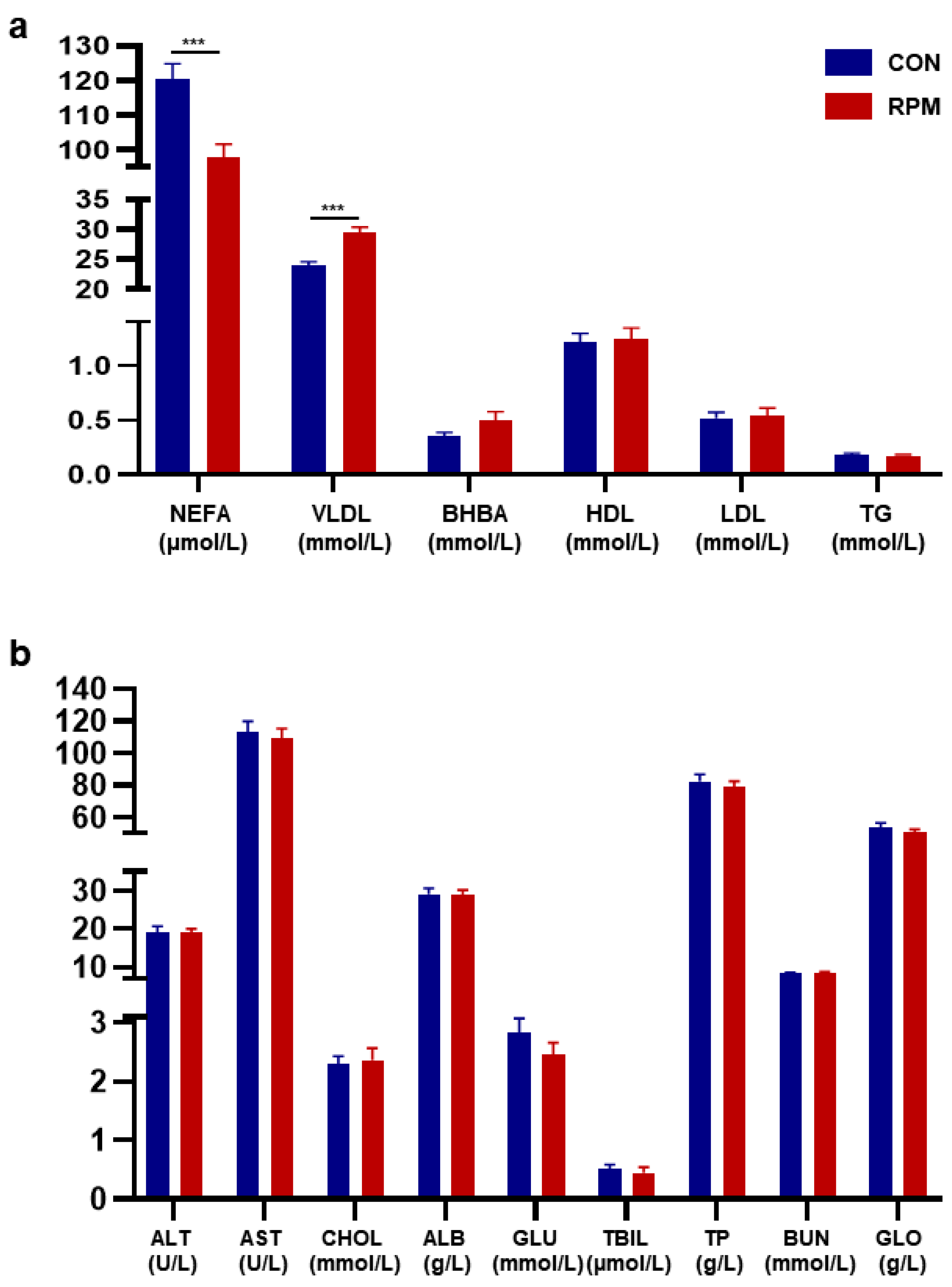
3.2. Blood Parameters
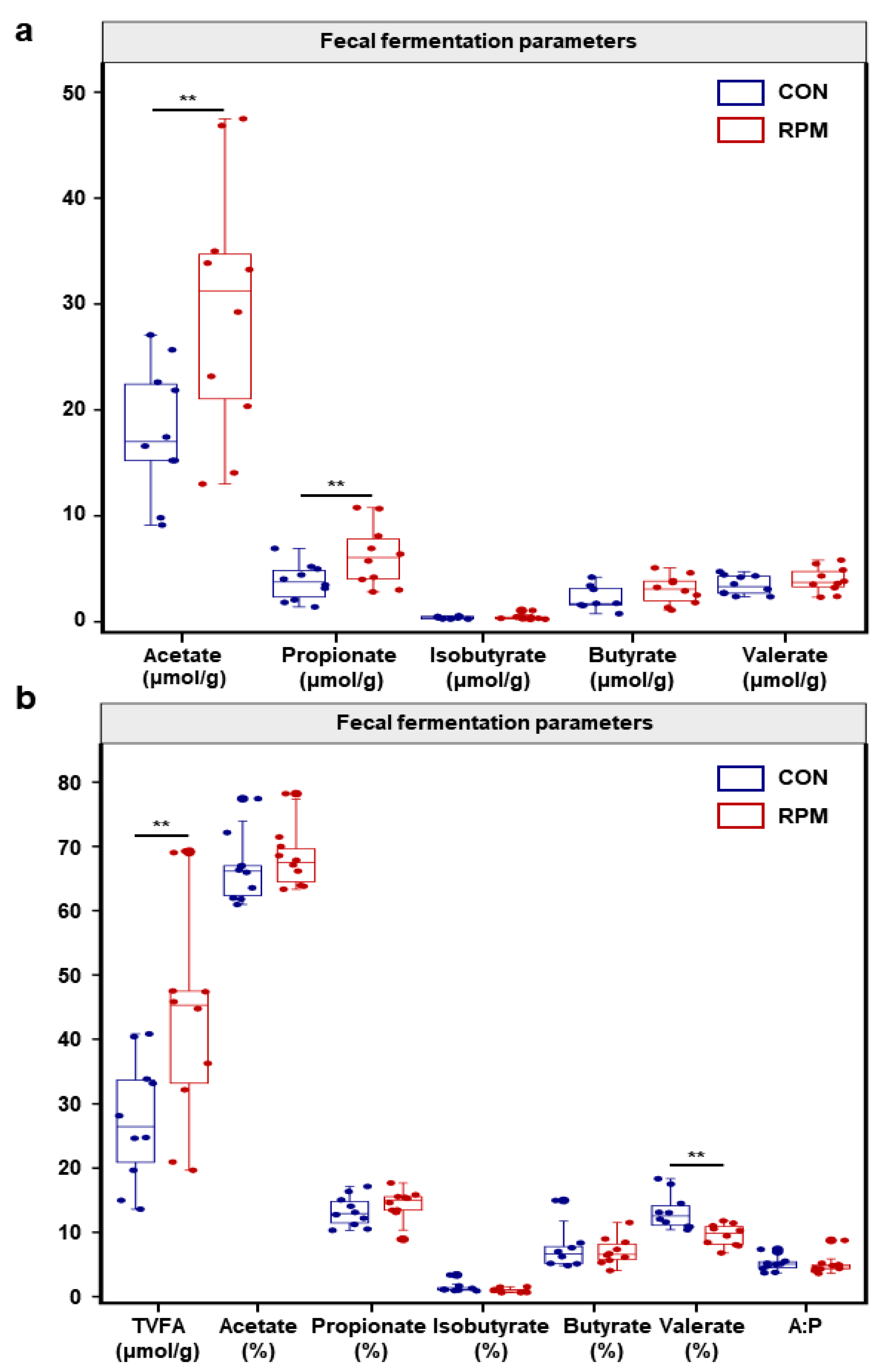
3.3. Fecal Fermentation Parameters
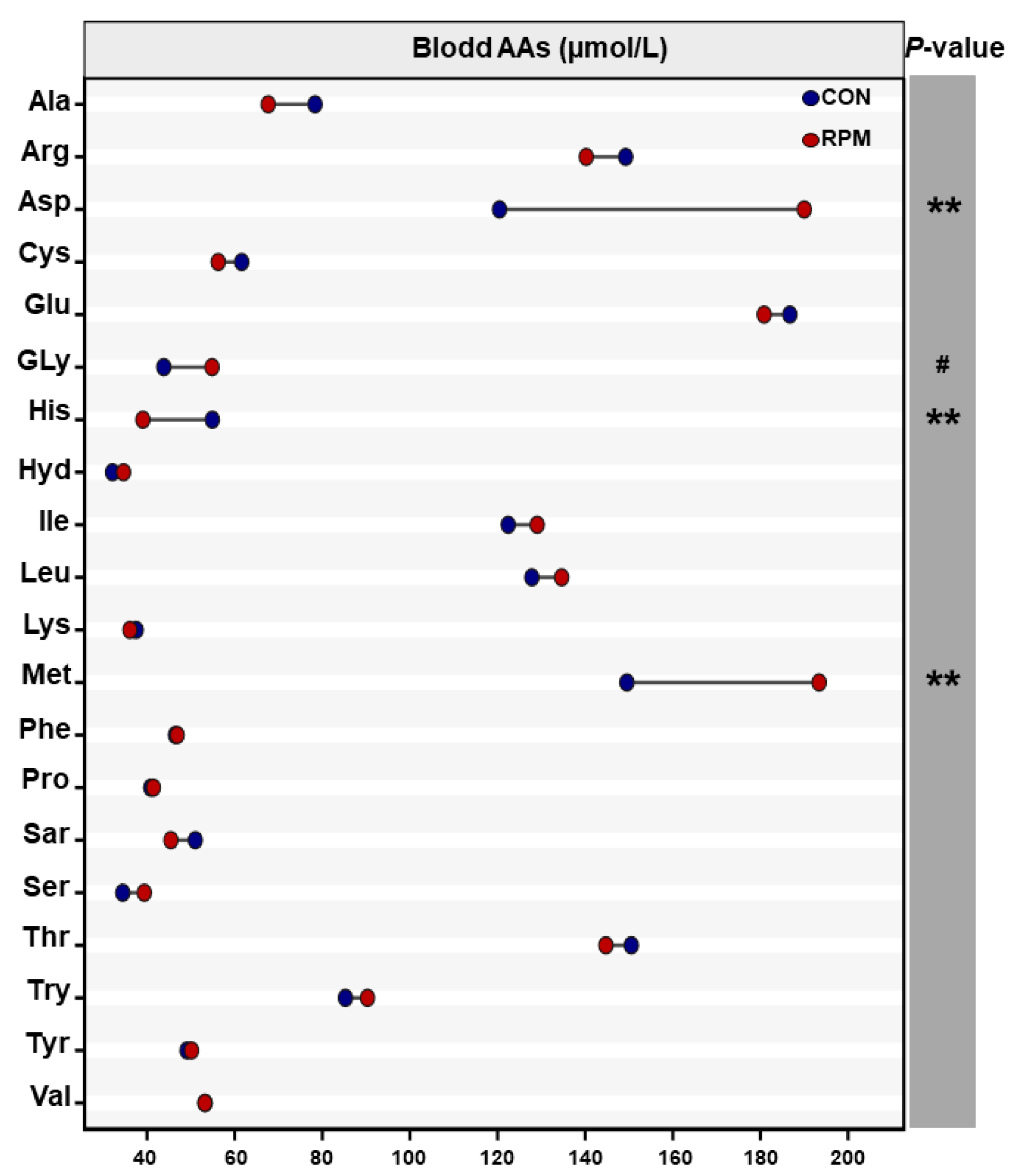
3.4. Blood Amino Acid Profile
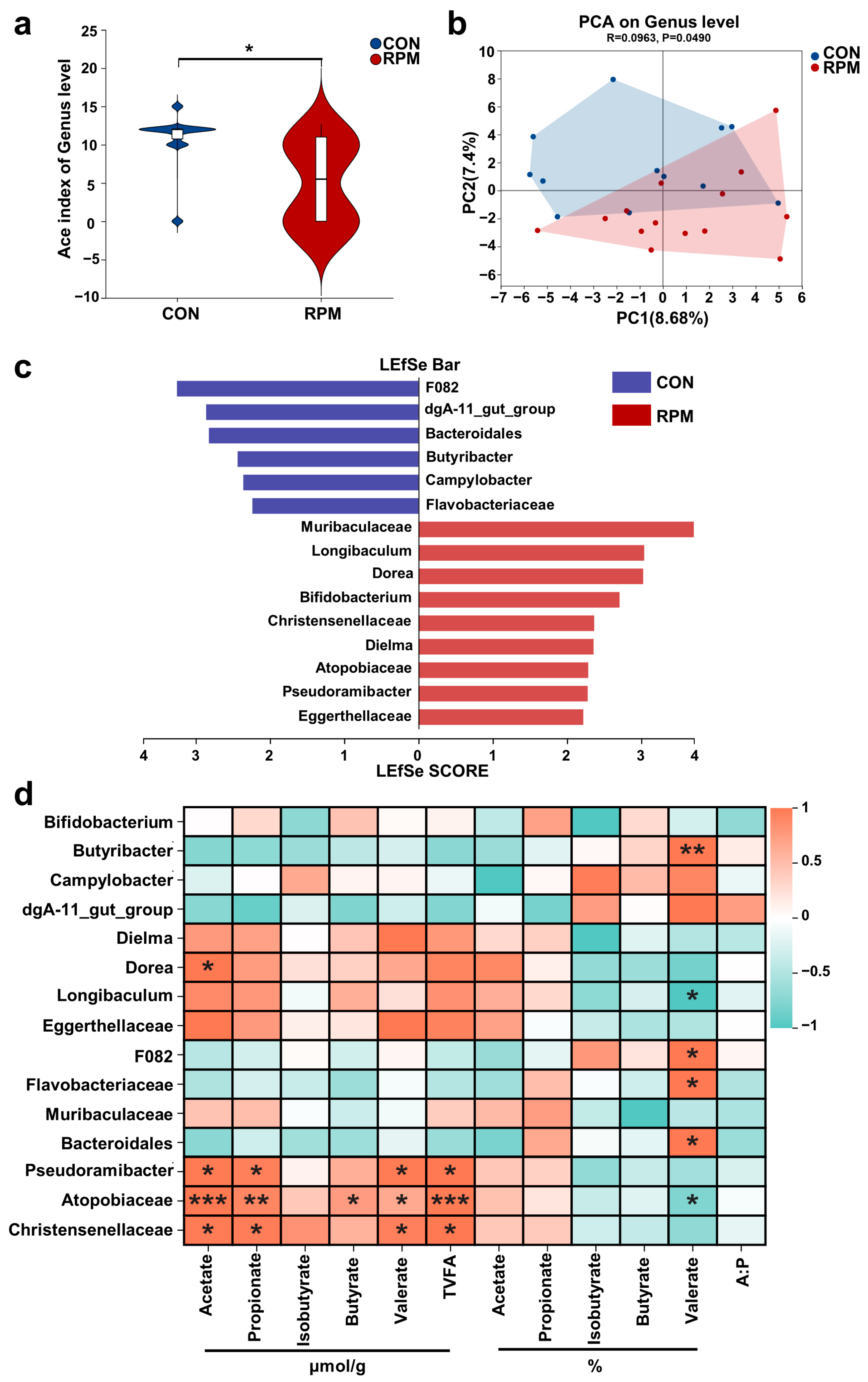
3.5. Fecal Microorganisms
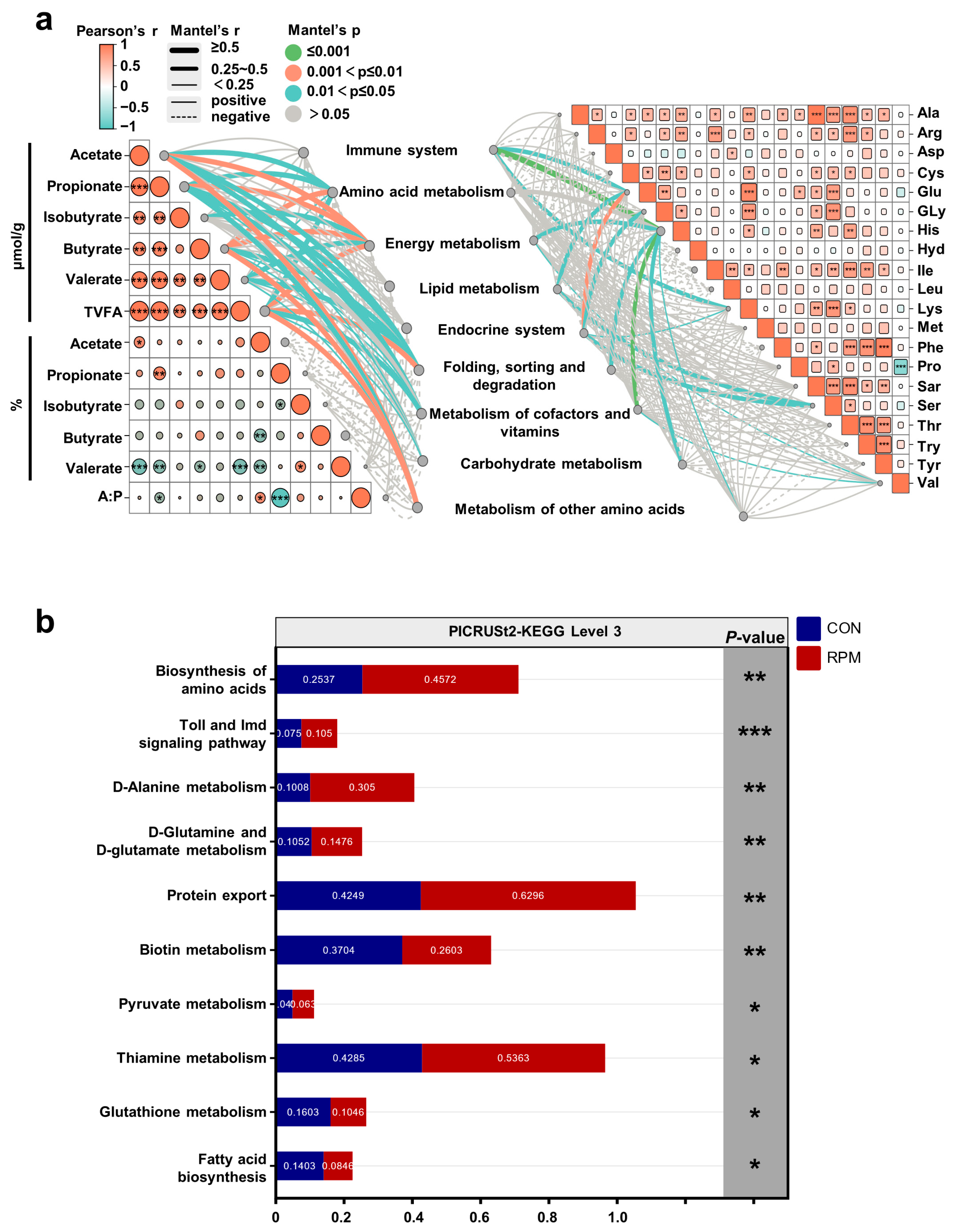
3.6. Relationships Between Fecal Microbial Function and VFAs, as Well as Blood Amino Acid Profiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Overton, T.R.; Waldron, M.R. Nutritional Management of Transition Dairy Cows: Strategies to Optimize Metabolic Health. J. Dairy Sci. 2004, 87, E105–E119. [Google Scholar] [CrossRef]
- Caixeta, L.S.; Omontese, B.O. Monitoring and Improving the Metabolic Health of Dairy Cows during the Transition Period. Animals 2021, 11, 352. [Google Scholar] [CrossRef]
- Gross, J.; van Dorland, H.A.; Schwarz, F.J.; Bruckmaier, R.M. Endocrine changes and liver mRNA abundance of somatotropic axis and insulin system constituents during negative energy balance at different stages of lactation in dairy cows. J. Dairy Sci. 2011, 94, 3484–3494. [Google Scholar] [CrossRef]
- Wang, Y.; Huo, P.; Sun, Y.; Zhang, Y. Effects of Body Condition Score Changes During Peripartum on the Postpartum Health and Production Performance of Primiparous Dairy Cows. Animals 2019, 9, 1159. [Google Scholar] [CrossRef]
- Leal Yepes, F.A.; Mann, S.; Overton, T.R.; Behling-Kelly, E.; Nydam, D.V.; Wakshlag, J.J. Hepatic effects of rumen-protected branched-chain amino acids with or without propylene glycol supplementation in dairy cows during early lactation. J. Dairy Sci. 2021, 104, 10324–10337. [Google Scholar] [CrossRef]
- Mann, S.; Leal Yepes, F.A.; Wakshlag, J.J.; Behling-Kelly, E.; McArt, J.A.A. The effect of different treatments for early-lactation hyperketonemia on liver triglycerides, glycogen, and expression of key metabolic enzymes in dairy cattle. J. Dairy Sci. 2018, 101, 1626–1637. [Google Scholar] [CrossRef]
- Newman, A.; Mann, S.; Nydam, D.V.; Overton, T.R.; Behling-Kelly, E. Impact of dietary plane of energy during the dry period on lipoprotein parameters in the transition period in dairy cattle. J. Anim. Physiol. Anim. Nutr. 2016, 100, 118–126. [Google Scholar] [CrossRef]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef] [PubMed]
- Jorritsma, R.; Jorritsma, H.; Schukken, Y.H.; Wentink, G.H. Relationships between fatty liver and fertility and some periparturient diseases in commercial Dutch dairy herds. Theriogenology 2000, 54, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.G.; Broderick, G.A. A 100-Year Review: Protein and amino acid nutrition in dairy cows. J. Dairy Sci. 2017, 100, 10094–10112. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lei, J.; Hancock, S.; Scanlan, V.; Broomfield, S.; Currie, A.; Thompson, A. Lamb survival, glutathione redox state and immune function of neonates and lambs from periparturient Merino ewes supplemented with rumen-protected methionine. Arch. Anim. Nutr. 2016, 70, 389–401. [Google Scholar] [CrossRef]
- Xu, T.; Alharthi, A.S.M.; Batistel, F.; Helmbrecht, A.; Parys, C.; Trevisi, E.; Shen, X.; Loor, J.J. Hepatic phosphorylation status of serine/threonine kinase 1, mammalian target of rapamycin signaling proteins, and growth rate in Holstein heifer calves in response to maternal supply of methionine. J. Dairy Sci. 2018, 101, 8476–8491. [Google Scholar] [CrossRef]
- Zhou, Z.; Ferdous, F.; Montagner, P.; Luchini, D.N.; Corrêa, M.N.; Loor, J.J. Methionine and choline supply during the peripartal period alter polymorphonuclear leukocyte immune response and immunometabolic gene expression in Holstein cows. J. Dairy Sci. 2018, 101, 10374–10382. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Cao, Y.; Cai, C.; Li, S.; Yu, C.; Yao, J. Regulation of Nutritional Metabolism in Transition Dairy Cows: Energy Homeostasis and Health in Response to Post-Ruminal Choline and Methionine. PLoS ONE 2016, 11, e0160659. [Google Scholar] [CrossRef] [PubMed]
- Jacometo, C.B.; Zhou, Z.; Luchini, D.; Trevisi, E.; Corrêa, M.N.; Loor, J.J. Maternal rumen-protected methionine supplementation and its effect on blood and liver biomarkers of energy metabolism, inflammation, and oxidative stress in neonatal Holstein calves. J. Dairy Sci. 2016, 99, 6753–6763. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Wang, X.; Wu, G.; Feng, C.; Zhou, H.; Li, D.; Wang, J. Improving amino acid nutrition to prevent intrauterine growth restriction in mammals. Amino Acids 2014, 46, 1605–1623. [Google Scholar] [CrossRef]
- Martinov, M.V.; Vitvitsky, V.M.; Banerjee, R.; Ataullakhanov, F.I. The logic of the hepatic methionine metabolic cycle. Biochim. Et. Biophys. Acta 2010, 1804, 89–96. [Google Scholar] [CrossRef]
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef]
- Osorio, J.S.; Ji, P.; Drackley, J.K.; Luchini, D.; Loor, J.J. Supplemental Smartamine M or MetaSmart during the transition period benefits postpartal cow performance and blood neutrophil function. J. Dairy Sci. 2013, 96, 6248–6263. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, H.G. Amino Acids Supplementation for the Milk and Milk Protein Production of Dairy Cows. Animals 2021, 11, 2118. [Google Scholar] [CrossRef]
- Patton, R.A. Effect of rumen-protected methionine on feed intake, milk production, true milk protein concentration, and true milk protein yield, and the factors that influence these effects: A meta-analysis. J. Dairy Sci. 2010, 93, 2105–2118. [Google Scholar] [CrossRef]
- Zanton, G.I.; Bowman, G.R.; Vázquez-Añón, M.; Rode, L.M. Meta-analysis of lactation performance in dairy cows receiving supplemental dietary methionine sources or postruminal infusion of methionine. J. Dairy Sci. 2014, 97, 7085–7101. [Google Scholar] [CrossRef]
- Flores, A.; Mendoza, G.; Pinos-Rodriguez, J.M.; Plata, F.; Vega, S.; Bárcena, R. Effects of rumen-protected methionine on milk production of dairy goats. Ital. J. Anim. Sci. 2009, 8, 271–275. [Google Scholar] [CrossRef]
- Alonso-Mélendez, E.; Mendoza, G.D.; Castrejón-Pineda, F.A.; Ducoing-Watty, A.E. Milk production in dairy goats supplemented with different levels of ruminally protected methionine. J. Dairy Res. 2016, 83, 148–150. [Google Scholar] [CrossRef]
- Schmidely, P.; Bahloul, L. Milk performance and oxidative status responses to rumen protected methionine supplementation in genotyped α-S1 casein lactating dairy goats fed two levels of metabolizable protein diets. Small Rumin. Res. 2022, 209, 106638. [Google Scholar] [CrossRef]
- Boutinaud, M.; Chanat, E.; Leduc, A.; Wiart, S.; Poton, P.; Balhoul, L.; Lemosquet, S. Methionine supplementation impacts casein gene expression and cell death in the mammary tissue of lactating dairy goats fed low and adequate net energy supplies. In Proceedings of the 2020 American Dairy Science Association (ADSA) Annual Meeting 2020, West Palm Beach, FL, USA, 21–24 June 2020; p. 22. [Google Scholar]
- Hristov, A.N.; Bannink, A.; Crompton, L.A.; Huhtanen, P.; Kreuzer, M.; McGee, M.; Nozière, P.; Reynolds, C.K.; Bayat, A.R.; Yáñez-Ruiz, D.R.; et al. Invited review: Nitrogen in ruminant nutrition: A review of measurement techniques. J. Dairy Sci. 2019, 102, 5811–5852. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen metabolism in the rumen. J. Dairy Sci. 2005, 88 (Suppl. 1), E9–E21. [Google Scholar] [CrossRef]
- Jiao, J.; Wu, J.; Zhou, C.; He, Z.; Tan, Z.; Wang, M. Ecological niches and assembly dynamics of diverse microbial consortia in the gastrointestine of goat kids. ISME J. 2024, 18, wrae002. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Wang, M.; Zhou, C.; Jiao, J.; Tan, Z. Enhancing Metabolic Efficiency through Optimizing Metabolizable Protein Profile in a Time Progressive Manner with Weaned Goats as a Model: Involvement of Gut Microbiota. Microbiol. Spectr. 2022, 10, e0254521. [Google Scholar] [CrossRef]
- Xu, S.Y.; Feng, X.R.; Zhao, W.; Bi, Y.L.; Diao, Q.Y.; Tu, Y. Rumen and hindgut microbiome regulate average daily gain of preweaning Holstein heifer calves in different ways. Microbiome 2024, 12, 131. [Google Scholar] [CrossRef]
- Luo, Z.; Ou, H.; Tan, Z.; Jiao, J. Rumen-protected methionine and lysine supplementation to the low protein diet improves animal growth through modulating colonic microbiome in lambs. J. Anim. Sci. Biotechnol. 2025, 16, 46. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Li, F.; Yang, X.J.; Cao, Y.C.; Li, S.X.; Yao, J.H.; Li, Z.J.; Sun, F.F. Effects of dietary effective fiber to rumen degradable starch ratios on the risk of sub-acute ruminal acidosis and rumen content fatty acids composition in dairy goat. Anim. Feed. Sci. Technol. 2014, 189, 54–62. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Lee, C.; Lobos, N.E.; Weiss, W.P. Effects of supplementing rumen-protected lysine and methionine during prepartum and postpartum periods on performance of dairy cows. J. Dairy Sci. 2019, 102, 11026–11039. [Google Scholar] [CrossRef]
- Potts, S.B.; Brady, K.M.; Scholte, C.M.; Moyes, K.M.; Sunny, N.E.; Erdman, R.A. Rumen-protected choline and methionine during the periparturient period affect choline metabolites, amino acids, and hepatic expression of genes associated with one-carbon and lipid metabolism. J. Dairy Sci. 2023, 106, 4559–4579. [Google Scholar] [CrossRef]
- Wei, C.; He, T.; Wan, X.; Liu, S.; Dong, Y.; Qu, Y. Meta-Analysis of Rumen-Protected Methionine in Milk Production and Composition of Dairy Cows. Animals 2022, 12, 1505. [Google Scholar] [CrossRef] [PubMed]
- Cannas, A.; Pes, A.; Mancuso, R.; Vodret, B.; Nudda, A. Effect of dietary energy and protein concentration on the concentration of milk urea nitrogen in dairy ewes. J. Dairy Sci. 1998, 81, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Awawdeh, M.S. Effects of supplemental lysine and methionine on performance of nursing Awassi ewes fed two levels of dietary protein. Trop. Anim. Health Prod. 2022, 54, 61. [Google Scholar] [CrossRef]
- Sauvant, D.; Hijar, G.; Noziere, P. Actualisation des besoins protéiques des ruminants et application à la détermination des réponses des femelles laitières aux apports de protéines digestibles dans l’intestin (PDI). INRAE Prod. Anim. 2014, 28, 347–368. [Google Scholar] [CrossRef]
- Noziere, P.; Sauvant, D.; Delaby, L. INRA Feeding System for Ruminants; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018. [Google Scholar]
- Park, J.K.; Yeo, J.M.; Bae, G.S.; Kim, E.J.; Kim, C.H. Effects of supplementing limiting amino acids on milk production in dairy cows consuming a corn grain and soybean meal-based diet. J. Anim. Sci. Technol. 2020, 62, 485–494. [Google Scholar] [CrossRef]
- Papadomichelakis, G.; Koutsotolis, K.; Zabeli, G.; Zervas, G. The effect of lactating dairy ewes’ diet supplementation with ALIMET (liquid methionine) on milk yield and milk composition. Ital. J. Anim. Sci. 2002, 1, 301–305. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, Y.; Wang, G.; Zheng, X.; Hao, H. Gut Microbial Metabolites of Aromatic Amino Acids as Signals in Host-Microbe Interplay. Trends Endocrinol. Metab. TEM 2020, 31, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Liu, S.; Ma, Y.; Ma, M.; Ullah, Q.; Khan, I.M.; Wang, J.; Xiao, J.; Chen, T.; Khan, A.; et al. Overview of the effect of rumen-protected limiting amino acids (methionine and lysine) and choline on the immunity, antioxidative, and inflammatory status of periparturient ruminants. Front. Immunol. 2022, 13, 1042895. [Google Scholar] [CrossRef]
- Li, Y.; Bi, Y.; Diao, Q.; Piao, M.; Wang, B.; Kong, F.; Hu, F.; Tang, M.; Sun, Y.; Tu, Y. The Limiting Sequence and Appropriate Amino Acid Ratio of Lysine, Methionine, and Threonine for Seven- to Nine-Month-Old Holstein Heifers Fed Corn-Soybean M-Based Diet. Animals 2019, 9, 750. [Google Scholar] [CrossRef]
- Antongiovanni, M.; Secchiari, P.; Mele, M.; Buccioni, A.; Serra, A.; Ferruzzi, G.; Rapaccini, S.; Pistoia, A. Olive oil calcium soaps and rumen protected methionine in the diet of lactating ewes: Effect on milk quality. Ital. J. Anim. Sci. 2002, 1, 55–63. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Mavrommatis, A.; Kalogeropoulos, T.; Chatzikonstantinou, M.; Koutsouli, P.; Sotirakoglou, K.; Labrou, N.; Zervas, G. The effect of dietary supplementation with rumen-protected methionine alone or in combination with rumen-protected choline and betaine on sheep milk and antioxidant capacity. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Grummer, R.R. Nutritional and management strategies for the prevention of fatty liver in dairy cattle. Vet. J. 2008, 176, 10–20. [Google Scholar] [CrossRef]
- Batistel, F.; Arroyo, J.M.; Garces, C.I.M.; Trevisi, E.; Parys, C.; Ballou, M.A.; Cardoso, F.C.; Loor, J.J. Ethyl-cellulose rumen-protected methionine alleviates inflammation and oxidative stress and improves neutrophil function during the periparturient period and early lactation in Holstein dairy cows. J. Dairy Sci. 2018, 101, 480–490. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, W.; Lin, X.Y.; Hu, Z.Y.; Yan, Z.G.; Wang, Y.; Shi, K.R.; Liu, G.M.; Wang, Z.H. Effects of rumen-protected methionine and other essential amino acid supplementation on milk and milk component yields in lactating Holstein cows. J. Dairy Sci. 2019, 102, 7936–7947. [Google Scholar] [CrossRef]
- Zenobi, M.G.; Gardinal, R.; Zuniga, J.E.; Dias, A.L.G.; Nelson, C.D.; Driver, J.P.; Barton, B.A.; Santos, J.E.P.; Staples, C.R. Effects of supplementation with ruminally protected choline on performance of multiparous Holstein cows did not depend upon prepartum caloric intake. J. Dairy Sci. 2018, 101, 1088–1110. [Google Scholar] [CrossRef]
- Zhou, Z.; Vailati-Riboni, M.; Trevisi, E.; Drackley, J.K.; Luchini, D.N.; Loor, J.J. Better postpartal performance in dairy cows supplemented with rumen-protected methionine compared with choline during the peripartal period. J. Dairy Sci. 2016, 99, 8716–8732. [Google Scholar] [CrossRef]
- Batistel, F.; Arroyo, J.M.; Bellingeri, A.; Wang, L.; Saremi, B.; Parys, C.; Trevisi, E.; Cardoso, F.C.; Loor, J.J. Ethyl-cellulose rumen-protected methionine enhances performance during the periparturient period and early lactation in Holstein dairy cows. J. Dairy Sci. 2017, 100, 7455–7467. [Google Scholar] [CrossRef] [PubMed]
- Preynat, A.; Lapierre, H.; Thivierge, M.C.; Palin, M.F.; Cardinault, N.; Matte, J.J.; Desrochers, A.; Girard, C.L. Effects of supplementary folic acid and vitamin B(12) on hepatic metabolism of dairy cows according to methionine supply. J. Dairy Sci. 2010, 93, 2130–2142. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, P.S.; Lingrell, S.; Yao, Z.; Vance, D.E. Phosphatidylcholine biosynthesis is required for secretion of truncated apolipoprotein Bs from McArdle RH7777 cells only when a neutral lipid core is formed. J. Lipid Res. 1997, 38, 447–458. [Google Scholar] [CrossRef]
- Yao, Z.M.; Vance, D.E. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J. Biol. Chem. 1988, 263, 2998–3004. [Google Scholar] [CrossRef]
- Bernabucci, U.; Ronchi, B.; Basiricò, L.; Pirazzi, D.; Rueca, F.; Lacetera, N.; Nardone, A. Abundance of mRNA of apolipoprotein b100, apolipoprotein e, and microsomal triglyceride transfer protein in liver from periparturient dairy cows. J. Dairy Sci. 2004, 87, 2881–2888. [Google Scholar] [CrossRef]
- Elhadi, A.; Calsamiglia, S.; Rodríguez-Prado, M.E.; Belaid, M.A.; Such, X.; Bahloul, L.; Caja, G. Lactational and digestive responses of reducing dietary protein level and supplementing rumen-protected methionine in early-lactation dairy ewes. J. Dairy Sci. 2025, 108, 7074–7090. [Google Scholar] [CrossRef]
- Soergel, H.; Loosli, F.; Muhle-Goll, C. Strain-Specific Liver Metabolite Profiles in Medaka. Metabolites 2021, 11, 744. [Google Scholar] [CrossRef]
- Zhou, Z.; Vailati-Riboni, M.; Luchini, D.N.; Loor, J.J. Methionine and Choline Supply during the Periparturient Period Alter Plasma Amino Acid and One-Carbon Metabolism Profiles to Various Extents: Potential Role in Hepatic Metabolism and Antioxidant Status. Nutrients 2016, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Mitchell, M.D.; Walker, C.G.; Roche, J.R.; Verkerk, G.A. Amino acid concentrations in uterine fluid during early pregnancy differ in fertile and subfertile dairy cow strains. J. Dairy Sci. 2014, 97, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.K.; Cammell, S.B.; Humphries, D.J.; Beever, D.E.; Sutton, J.D.; Newbold, J.R. Effects of postrumen starch infusion on milk production and energy metabolism in dairy cows. J. Dairy Sci. 2001, 84, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, Z.; Lu, J.; Zhang, X.; Chen, Z.; Wan, Z.; Cai, Y.; Wang, F.; Zhang, Y. Effects of maternal rumen-protected methionine supplementation on ewe colostrum composition, lamb growth performance, rumen development and microbiome. Anim. Feed. Sci. Technol. 2024, 318, 116131. [Google Scholar] [CrossRef]
- Miyake, S.; Ding, Y.; Soh, M.; Low, A.; Seedorf, H. Cultivation and description of Duncaniella dubosii sp. nov., Duncaniella freteri sp. nov. and emended description of the species Duncaniella muris. Int. J. Syst. Evol. Microbiol. 2020, 70, 3105–3110. [Google Scholar] [CrossRef]
- Miyake, S.; Ding, Y.; Soh, M.; Seedorf, H. Complete Genome Sequence of Duncaniella muris Strain B8, Isolated from the Feces of C57/BL6 Mice. Microbiol. Resour. Announc. 2019, 8, 10–1128. [Google Scholar] [CrossRef]
- Smith, B.J.; Miller, R.A.; Schmidt, T.M. Muribaculaceae Genomes Assembled from Metagenomes Suggest Genetic Drivers of Differential Response to Acarbose Treatment in Mice. mSphere 2021, 6, e0085121. [Google Scholar] [CrossRef]
- Ormerod, K.L.; Wood, D.L.; Lachner, N.; Gellatly, S.L.; Daly, J.N.; Parsons, J.D.; Dal’Molin, C.G.; Palfreyman, R.W.; Nielsen, L.K.; Cooper, M.A.; et al. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 2016, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, B.; Zhang, X.; Akbar, M.T.; Wu, T.; Zhang, Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function. Nutrients 2024, 16, 2660. [Google Scholar] [CrossRef]
- Chung, Y.W.; Gwak, H.J.; Moon, S.; Rho, M.; Ryu, J.H. Functional dynamics of bacterial species in the mouse gut microbiome revealed by metagenomic and metatranscriptomic analyses. PLoS ONE 2020, 15, e0227886. [Google Scholar] [CrossRef]
- Singh, R.P. Glycan utilisation system in Bacteroides and Bifidobacteria and their roles in gut stability and health. Appl. Microbiol. Biotechnol. 2019, 103, 7287–7315. [Google Scholar] [CrossRef]
- Wang, C.; Lin, J.; Duan, M.; He, J.; Halizere, S.; Chen, N.; Chen, X.; Jiao, Y.; He, W.; Dyar, K.A.; et al. Multi-omics reveals different signatures of obesity-prone and obesity-resistant mice. iMetaOmics 2025, 2, e59. [Google Scholar] [CrossRef]
- Abdugheni, R.; Wang, W.Z.; Wang, Y.J.; Du, M.X.; Liu, F.L.; Zhou, N.; Jiang, C.Y.; Wang, C.Y.; Wu, L.; Ma, J.; et al. Metabolite profiling of human-originated Lachnospiraceae at the strain level. iMeta 2022, 1, e58. [Google Scholar] [CrossRef]
- Chen, J.; Lei, X.J.; Wang, L.; Zhang, Y.L.; Wang, D.D.; Zhao, L.C.; Liu, T.; Yang, Y.T.; Yao, J.H. Effects of rumen-protected leucine on production performance and starch digestion in the small intestine of lactating goats. Anim. Feed. Sci. Technol. 2022, 287, 115270. [Google Scholar] [CrossRef]
- Jimenez, N.R.; Mancilla, V.; Łaniewski, P.; Herbst-Kralovetz, M.M. Immunometabolic Contributions of Atopobiaceae Family Members in Human Papillomavirus Infection, Cervical Dysplasia and Cancer. J. Infect. Dis. 2024, 232, 767–778. [Google Scholar] [CrossRef]
- Wang, C.; Nakakoji, S.; Ng, T.C.A.; Zhu, P.; Tsukada, R.; Tatara, M.; Ng, H.Y. Acclimatizing waste activated sludge in a thermophilic anaerobic fixed-bed biofilm reactor to maximize biogas production for food waste treatment at high organic loading rates. Water Res. 2023, 242, 120299. [Google Scholar] [CrossRef]
| Items | Basal Diets |
|---|---|
| Ingredient | |
| Alfalfa hay | 33.33 |
| Oat hay | 26.67 |
| Ground corn | 20.96 |
| Soybean meal | 7.88 |
| Wheat bran | 6.16 |
| Soybean flour | 2.20 |
| Premix 2 | 2.20 |
| NaCl | 0.16 |
| NaHCO3 | 0.44 |
| Nutrient | |
| Starch | 15.96 |
| CP | 16.71 |
| NDF | 40.06 |
| ADF | 27.23 |
| Metabolizable AA 3 | |
| Arg | 6.18 |
| His | 2.45 |
| Ile | 4.79 |
| Leu | 8.06 |
| Lys | 6.10 |
| Met | 1.65 |
| Phe | 4.99 |
| Thr | 4.66 |
| Try | 1.64 |
| Val | 5.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Wang, J.; Zhang, Y.; Li, J.; Liu, H.; Wu, S.; Yao, J. Rumen-Protected Methionine Supplementation in the Diet Improved the Production Performance of Dairy Goats by Optimizing the Amino Acid Profile and Lipid Metabolism and Modulating the Colonic Microbiome. Animals 2025, 15, 3386. https://doi.org/10.3390/ani15233386
Jiang X, Wang J, Zhang Y, Li J, Liu H, Wu S, Yao J. Rumen-Protected Methionine Supplementation in the Diet Improved the Production Performance of Dairy Goats by Optimizing the Amino Acid Profile and Lipid Metabolism and Modulating the Colonic Microbiome. Animals. 2025; 15(23):3386. https://doi.org/10.3390/ani15233386
Chicago/Turabian StyleJiang, Xingwei, Jiarui Wang, Yuhao Zhang, Jing Li, Huifeng Liu, Shengru Wu, and Junhu Yao. 2025. "Rumen-Protected Methionine Supplementation in the Diet Improved the Production Performance of Dairy Goats by Optimizing the Amino Acid Profile and Lipid Metabolism and Modulating the Colonic Microbiome" Animals 15, no. 23: 3386. https://doi.org/10.3390/ani15233386
APA StyleJiang, X., Wang, J., Zhang, Y., Li, J., Liu, H., Wu, S., & Yao, J. (2025). Rumen-Protected Methionine Supplementation in the Diet Improved the Production Performance of Dairy Goats by Optimizing the Amino Acid Profile and Lipid Metabolism and Modulating the Colonic Microbiome. Animals, 15(23), 3386. https://doi.org/10.3390/ani15233386






