Comparison of Virtual Non-Contrast Images Generated by Spectral Detector Computed Tomography and Conventional Computed Tomography Images of Histologically Confirmed Hepatic Pathologies in 28 Dogs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. CT Examinations
2.3. Imaging Features of Liver Lesions
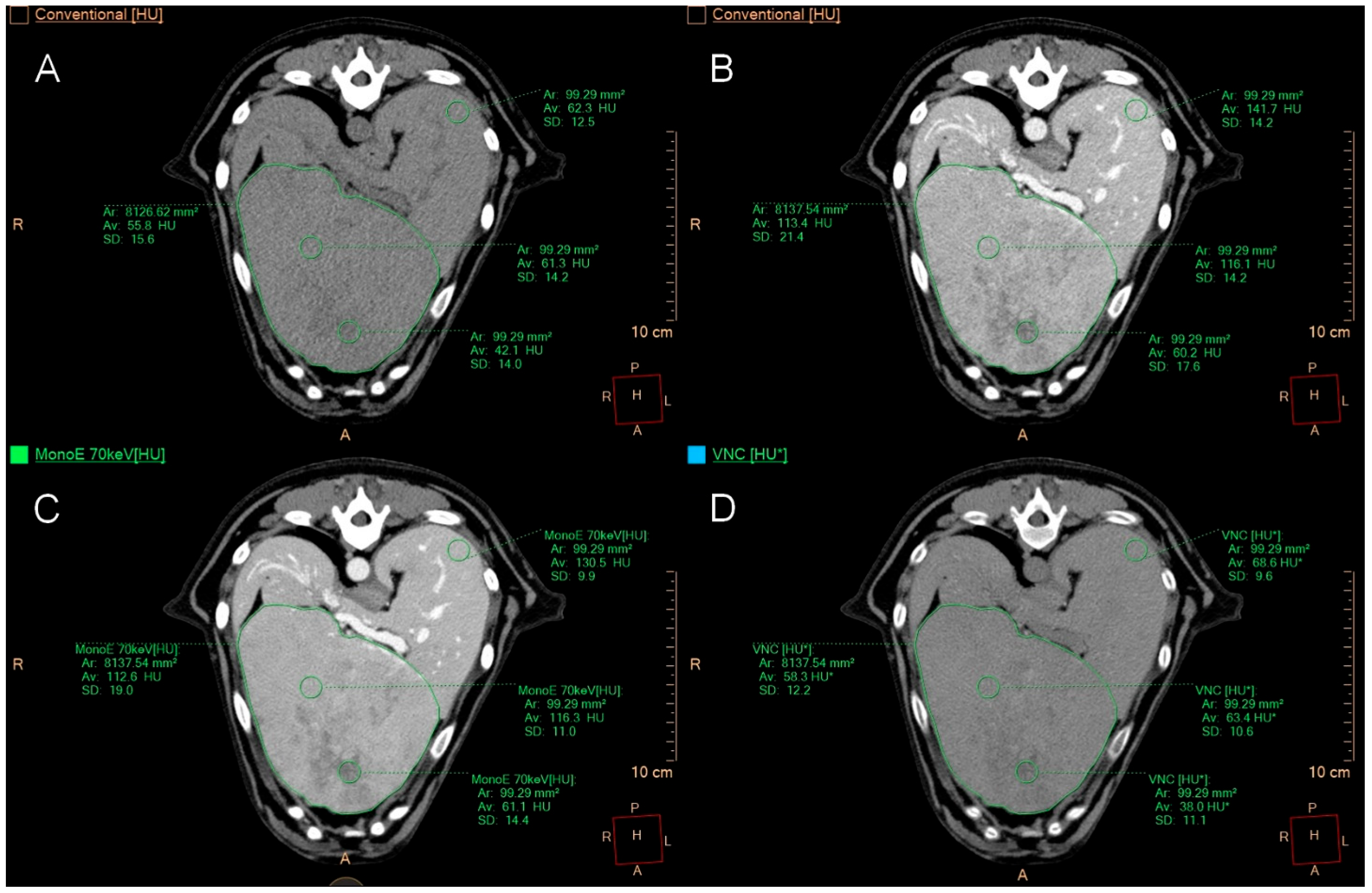
2.4. Quantitative Image Analysis
2.5. Qualitative Image Analysis
2.6. Histopathological Diagnosis
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Histopathological Findings
3.3. Imaging Features of Liver Lesions
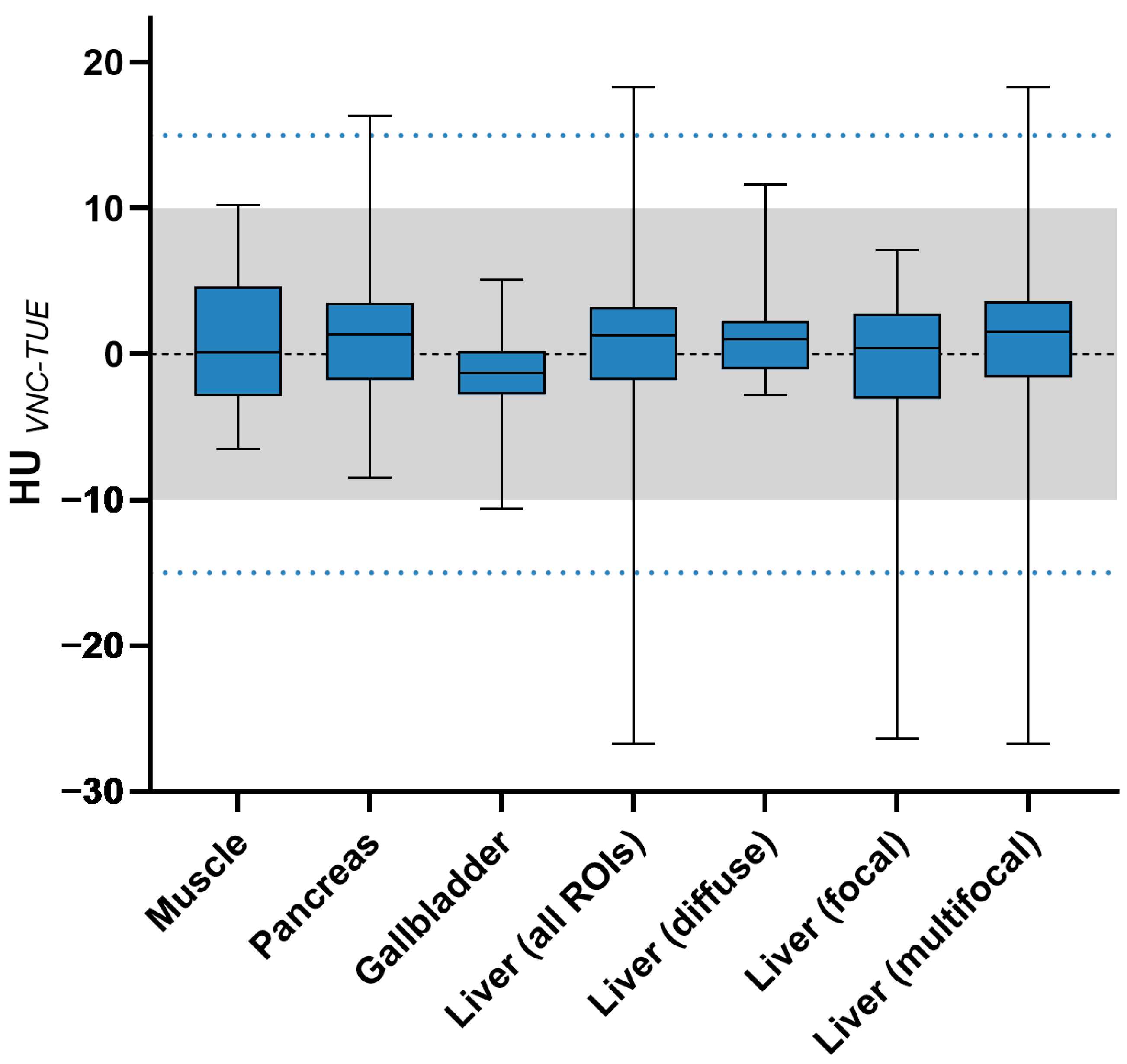
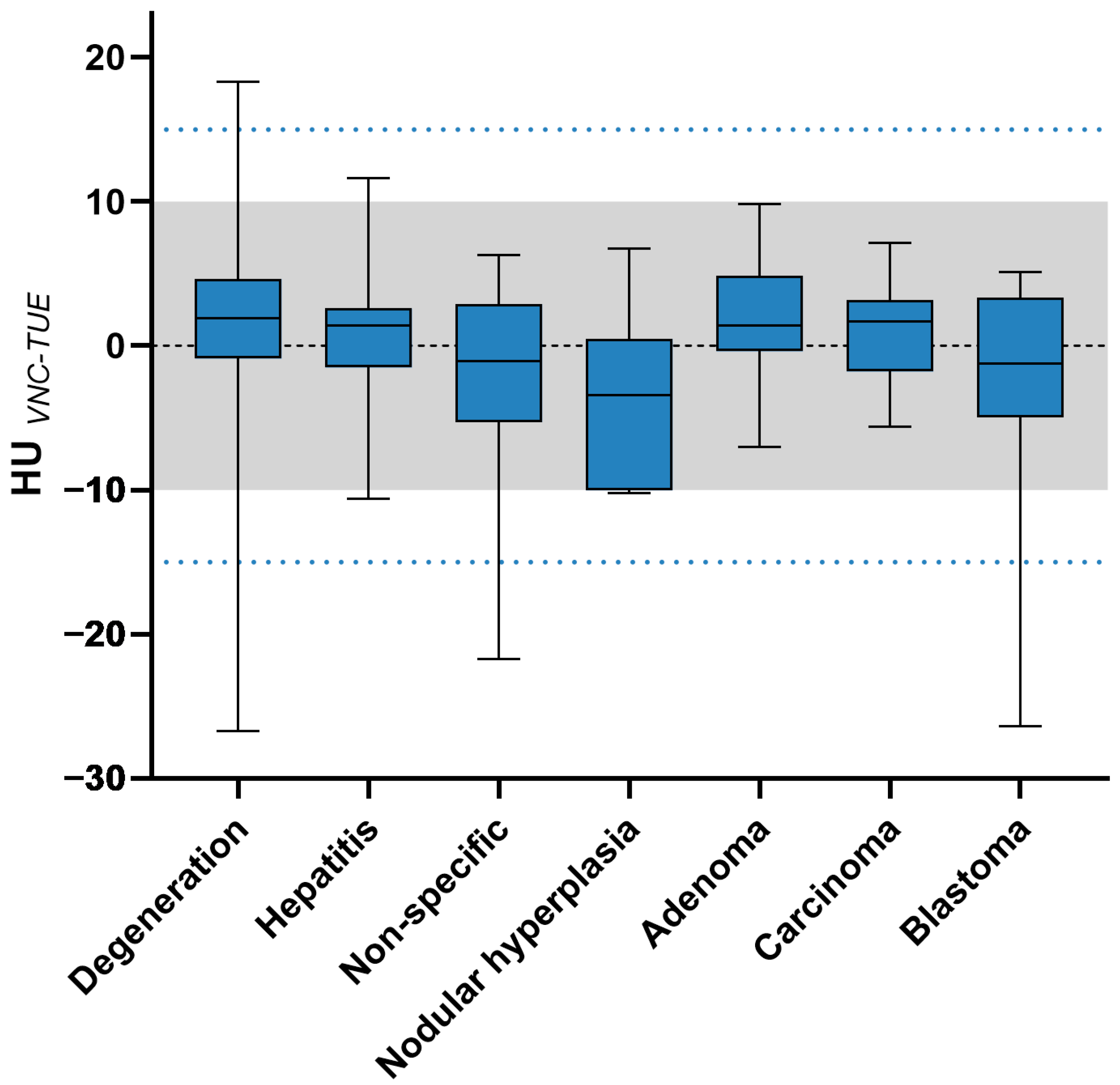
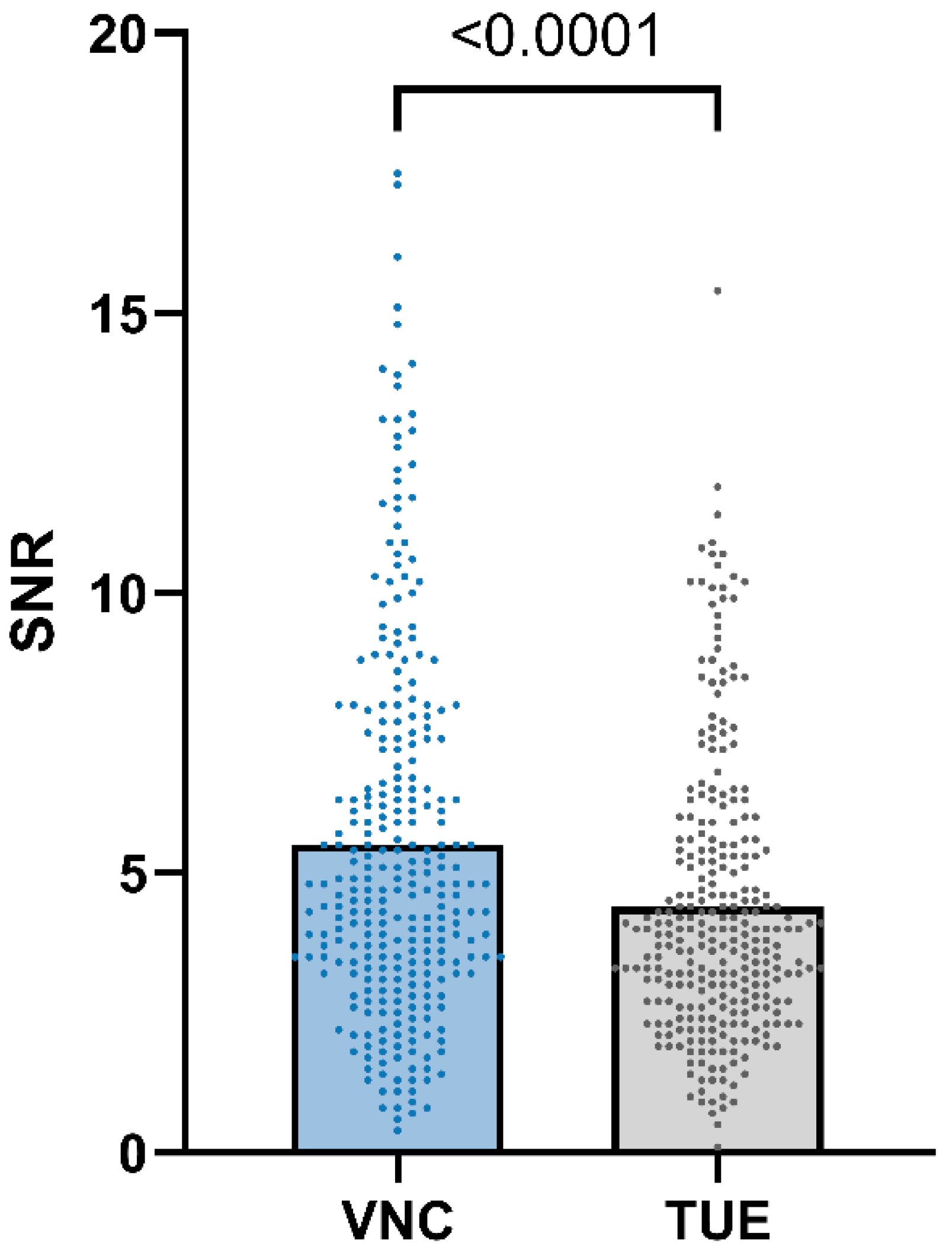
3.4. Quantitative Image Analysis
3.5. Qualitative Image Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | computed tomography |
| DECT | dual-energy computed tomography |
| HU | Hounsfield Unit |
| keV | kilo-electronvolts |
| KM | Kristina Merhof |
| LC | Lydia Claußen |
| PL | Philipp Lietz |
| ROI | region of interest |
| SBI | spectral-based images |
| SD | standard deviation |
| SDCT | spectral detector computed tomography |
| SNR | signal-to-noise ratio |
| TOST | two one-sided t-tests |
References
- Fukushima, K.; Kanemoto, H.; Ohno, K.; Takahashi, M.; Nakashima, K.; Fujino, Y.; Uchida, K.; Fujiwara, R.; Nishimura, R.; Tsujimoto, H. CT characteristics of primary hepatic mass lesions in dogs. Vet. Radiol. Ultrasound 2012, 53, 252–257. [Google Scholar] [CrossRef]
- Stehlík, L.; Di Tommaso, M.; Del Signore, F.; Paninárová, M.; Terragni, R.; Magni, T.; Pontonutti, L.; Carloni, A.; Alberti, M.; De Magistris, A.V.; et al. Triple-Phase Multidetector Computed Tomography in Distinguishing Canine Hepatic Lesions. Animals 2020, 11, 11. [Google Scholar] [CrossRef]
- Leela-Arporn, R.; Ohta, H.; Shimbo, G.; Hanazono, K.; Osuga, T.; Morishita, K.; Sasaki, N.; Takiguchi, M. Computed tomographic features for differentiating benign from malignant liver lesions in dogs. J. Vet. Med. Sci. 2019, 81, 1697–1704. [Google Scholar] [CrossRef]
- Lamb, C.R.; Steel, R.; Lipscomb, V.J. Determining the anatomical origin of canine hepatic masses by CT. J. Small Anim. Pract. 2018, 59, 752–757. [Google Scholar] [CrossRef]
- Burti, S.; Zotti, A.; Bonsembiante, F.; Contiero, B.; Banzato, T. Diagnostic Accuracy of Delayed Phase Post Contrast Computed Tomographic Images in the Diagnosis of Focal Liver Lesions in Dogs: 69 Cases. Front. Vet. Sci. 2021, 8, 611556. [Google Scholar] [CrossRef]
- Taniura, T.; Marukawa, K.; Yamada, K.; Hikasa, Y.; Ito, K. Differential diagnosis of hepatic tumor-like lesions in dog by using dynamic CT scanning. Hiroshima J. Med. Sci. 2009, 58, 17–24. [Google Scholar]
- Irausquin, R.A.; Scavelli, T.D.; Corti, L.; Stefanacci, J.D.; DeMarco, J.; Flood, S.; Rohrbach, B.W. Comparative evaluation of the liver in dogs with a splenic mass by using ultrasonography and contrast-enhanced computed tomography. Can. Vet. J. 2008, 49, 46–52. [Google Scholar]
- Kurokawa, S.; Tanaka, T.; Yamazaki, H.; Noguchi, S.; Wada, Y.; Nishida, H.; Akiyoshi, H. Comparing the CT and MRI findings for canine primary hepatocellular lesions. Vet. Rec. 2022, 190, e1083. [Google Scholar] [CrossRef]
- Griebie, E.R.; David, F.H.; Ober, C.P.; Feeney, D.A.; Anderson, K.L.; Wuenschmann, A.; Jessen, C.R. Evaluation of canine hepatic masses by use of triphasic computed tomography and B-mode, color flow, power, and pulsed-wave Doppler ultrasonography and correlation with histopathologic classification. Am. J. Vet. Res. 2017, 78, 1273–1283. [Google Scholar] [CrossRef]
- Rutherford, R.A.; Pullan, B.R.; Isherwood, I. X-ray energies for effective atomic number determination. Neuroradiology 1976, 11, 23–28. [Google Scholar] [CrossRef]
- Millner, M.R.; McDavid, W.D.; Waggener, R.G.; Dennis, M.J.; Payne, W.H.; Sank, V.J. Extraction of information from CT scans at different energies. Med. Phys. 1979, 6, 70–71. [Google Scholar] [CrossRef]
- Heye, T.; Nelson, R.C.; Ho, L.M.; Marin, D.; Boll, D.T. Dual-energy CT applications in the abdomen. AJR Am. J. Roentgenol. 2012, 199, S64–S70. [Google Scholar] [CrossRef]
- Coursey, C.A.; Nelson, R.C.; Boll, D.T.; Paulson, E.K.; Ho, L.M.; Neville, A.M.; Marin, D.; Gupta, R.T.; Schindera, S.T. Dual-energy multidetector CT: How does it work, what can it tell us, and when can we use it in abdominopelvic imaging? Radiographics 2010, 30, 1037–1055. [Google Scholar] [CrossRef] [PubMed]
- Rassouli, N.; Etesami, M.; Dhanantwari, A.; Rajiah, P. Detector-based spectral CT with a novel dual-layer technology: Principles and applications. Insights Imaging 2017, 8, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.P.; Muenzel, D.; Dangelmaier, J.; Braren, R.; Pfeiffer, F.; Rummeny, E.J.; Noël, P.B.; Fingerle, A.A. Dual-layer spectral computed tomography: Virtual non-contrast in comparison to true non-contrast images. Eur. J. Radiol. 2018, 104, 108–114. [Google Scholar] [CrossRef]
- Lestra, T.; Mulé, S.; Millet, I.; Carsin-Vu, A.; Taourel, P.; Hoeffel, C. Applications of dual energy computed tomography in abdominal imaging. Diagn. Interv. Imaging 2016, 97, 593–603. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, G.; Qi, X.; Fan, X.; Wang, L. Quantitative analysis of the dual-energy CT virtual spectral curve for focal liver lesions characterization. Eur. J. Radiol. 2014, 83, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Laukamp, K.R.; Lennartz, S.; Hashmi, A.; Obmann, M.; Ho, V.; Große Hokamp, N.; Graner, F.P.; Gilkeson, R.; Persigehl, T.; Gupta, A.; et al. Iodine accumulation of the liver in patients treated with amiodarone can be unmasked using material decomposition from multiphase spectral-detector CT. Sci. Rep. 2020, 10, 6994. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Huang, X.; Zhang, Q.; Xie, Y.; Pang, L.; Bai, L.; Zhou, J. Clinical value of spectral CT imaging combined with AFP in identifying liver cancer and hepatic focal nodular hyperplasia. J. Buon 2019, 24, 1429–1434. [Google Scholar]
- Lv, P.; Lin, X.; Gao, J.; Chen, K. Spectral CT: Preliminary studies in the liver cirrhosis. Korean J. Radiol. 2012, 13, 434–442. [Google Scholar] [CrossRef]
- Graser, A.; Johnson, T.R.; Chandarana, H.; Macari, M. Dual energy CT: Preliminary observations and potential clinical applications in the abdomen. Eur. Radiol. 2009, 19, 13–23. [Google Scholar] [CrossRef]
- Laukamp, K.R.; Ho, V.; Obmann, V.C.; Herrmann, K.; Gupta, A.; Borggrefe, J.; Lennartz, S.; Große Hokamp, N.; Ramaiya, N. Virtual non-contrast for evaluation of liver parenchyma and vessels: Results from 25 patients using multi-phase spectral-detector CT. Acta Radiol. 2020, 61, 1143–1152. [Google Scholar] [CrossRef]
- Kaufmann, S.; Sauter, A.; Spira, D.; Gatidis, S.; Ketelsen, D.; Heuschmid, M.; Claussen, C.D.; Thomas, C. Tin-filter enhanced dual-energy-CT: Image quality and accuracy of CT numbers in virtual noncontrast imaging. Acad. Radiol. 2013, 20, 596–603. [Google Scholar] [CrossRef]
- Jamali, S.; Michoux, N.; Coche, E.; Dragean, C.A. Virtual unenhanced phase with spectral dual-energy CT: Is it an alternative to conventional true unenhanced phase for abdominal tissues? Diagn. Interv. Imaging 2019, 100, 503–511. [Google Scholar] [CrossRef]
- Ananthakrishnan, L.; Rajiah, P.; Ahn, R.; Rassouli, N.; Xi, Y.; Soesbe, T.C.; Lewis, M.A.; Lenkinski, R.E.; Leyendecker, J.R.; Abbara, S. Spectral detector CT-derived virtual non-contrast images: Comparison of attenuation values with unenhanced CT. Abdom. Radiol. 2017, 42, 702–709. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, R.; Deng, W.; An, S.; Li, Y.; Sheng, M.; Chen, X.; Qian, Y.; Yu, Y.; Mu, D.; et al. Feasibility and accuracy of coronary artery calcium score on virtual non-contrast images derived from a dual-layer spectral detector CT: A retrospective multicenter study. Front. Cardiovasc. Med. 2023, 10, 1114058. [Google Scholar] [CrossRef]
- Bucolo, G.M.; Ascenti, V.; Barbera, S.; Fontana, F.; Aricò, F.M.; Piacentino, F.; Coppola, A.; Cicero, G.; Marino, M.A.; Booz, C.; et al. Virtual Non-Contrast Spectral CT in Renal Masses: Is It Time to Discard Conventional Unenhanced Phase? J. Clin. Med. 2023, 12, 4718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, G.; Xu, L.; Bai, X.; Lu, X.; Yu, S.; Sun, H.; Jin, Z. Utilisation of virtual non-contrast images and virtual mono-energetic images acquired from dual-layer spectral CT for renal cell carcinoma: Image quality and radiation dose. Insights Imaging 2022, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.F.; Liu, A.L.; Wang, H.Q.; Liu, J.H.; Sun, M.Y.; Liu, Y.J. Virtual non-contrast computer tomography (CT) with spectral CT as an alternative to conventional unenhanced CT in the assessment of gastric cancer. Asian Pac. J. Cancer Prev. 2015, 16, 2521–2526. [Google Scholar] [CrossRef]
- Steinhardt, M.; Marka, A.W.; Ziegelmayer, S.; Makowski, M.; Braren, R.; Graf, M.; Gawlitza, J. Comparison of Virtual Non-Contrast and True Non-Contrast CT Images Obtained by Dual-Layer Spectral CT in COPD Patients. Bioengineering 2024, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.; Ansems, J.; Ommen, W.V.; Linden, D.V.; Looijmans, F.; Tesselaar, E. Comparison of true non-contrast and virtual non-contrast images in the characterization of renal lesions using detector-based spectral CT. Br. J. Radiol. 2023, 96, 20220157. [Google Scholar] [CrossRef]
- Si-Mohamed, S.; Dupuis, N.; Tatard-Leitman, V.; Rotzinger, D.; Boccalini, S.; Dion, M.; Vlassenbroek, A.; Coulon, P.; Yagil, Y.; Shapira, N.; et al. Virtual versus true non-contrast dual-energy CT imaging for the diagnosis of aortic intramural hematoma. Eur. Radiol. 2019, 29, 6762–6771. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.J.; McInnes, M.D.F.; El-Khodary, M.; McGrath, T.A.; Schieda, N. Diagnostic accuracy of virtual non-contrast enhanced dual-energy CT for diagnosis of adrenal adenoma: A systematic review and meta-analysis. Eur. Radiol. 2017, 27, 4324–4335. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Han, D.; Dang, S.; Yu, N.; Yang, Q.; Yang, C.; Jin, C.; Dou, Y. Replacing true unenhanced imaging in renal carcinoma with virtual unenhanced images in dual-energy spectral CT: A feasibility study. Clin. Radiol. 2021, 76, 81.e21–81.e27. [Google Scholar] [CrossRef]
- Lietz, P.; Brüntgens, M.; Wang-Leandro, A.; Volk, H.A.; Meller, S.; Merhof, K. Virtual non-contrast images of detector-based spectral computed tomography in dogs: A promising alternative to true non-contrast images in veterinary medicine. Front. Vet. Sci. 2023, 10, 1251535. [Google Scholar] [CrossRef] [PubMed]
- van Gemmeren, A.M.; Claußen, L.K.; Lietz, P.; Meller, S.; Wang-Leandro, A.; Beineke, A.; Nerschbach, V.; Volk, H.A.; Merhof, K. Spectral detector computed tomography imaging of histologically confirmed splenic pathologies in 30 canine patients: A comparison of virtual non-contrast images and true unenhanced images. Front. Vet. Sci. 2025, 12, 1645439. [Google Scholar] [CrossRef]
- Mikić, M.; Lietz, P.; Dierig, J.A.; Meller, S.; Pees, M.; Merhof, K. Evaluation of virtual non-contrast detector-based spectral CT images in comparison to true unenhanced images in 20 rabbits. Front. Vet. Sci. 2025, 12, 1521986. [Google Scholar] [CrossRef]
- Szablics, F.; Bérczi, Á.; Csőre, J.; Borzsák, S.; Szentiványi, A.; Kiss, M.; Juhász, G.; Papp, D.; Suhai, F.I.; Csobay-Novák, C. Virtual Non-Contrast Reconstructions Derived from Dual-Energy CTA Scans in Peripheral Arterial Disease: Comparison with True Non-Contrast Images and Impact on Radiation Dose. J. Clin. Med. 2025, 14, 5571. [Google Scholar] [CrossRef]
- Dunning, C.A.S.; Rajendran, K.; Inoue, A.; Rajiah, P.; Weber, N.; Fletcher, J.G.; McCollough, C.H.; Leng, S. Optimal Virtual Monoenergetic Photon Energy (keV) for Photon-Counting-Detector Computed Tomography Angiography. J. Comput. Assist. Tomogr. 2023, 47, 569–575. [Google Scholar] [CrossRef]
- D’Angelo, T.; Cicero, G.; Mazziotti, S.; Ascenti, G.; Albrecht, M.H.; Martin, S.S.; Othman, A.E.; Vogl, T.J.; Wichmann, J.L. Dual energy computed tomography virtual monoenergetic imaging: Technique and clinical applications. Br. J. Radiol. 2019, 92, 20180546. [Google Scholar] [CrossRef]
- D’Angelo, T.; Mastrodicasa, D.; Lanzafame, L.R.M.; Yel, I.; Koch, V.; Gruenewald, L.D.; Sharma, S.P.; Ascenti, V.; Micari, A.; Blandino, A.; et al. Optimization of window settings for coronary arteries assessment using spectral CT-derived virtual monoenergetic imaging. Radiol. Med. 2024, 129, 999–1007. [Google Scholar] [CrossRef]
- Rassouli, N.; Chalian, H.; Rajiah, P.; Dhanantwari, A.; Landeras, L. Assessment of 70-keV virtual monoenergetic spectral images in abdominal CT imaging: A comparison study to conventional polychromatic 120-kVp images. Abdom. Radiol. 2017, 42, 2579–2586. [Google Scholar] [CrossRef]
- Malik, P.; Vidyarthi, A. Stacked deep model-based classification of the multiclass brain hemorrhages in CT scans. Int. J. Imaging Syst. Technol. 2024, 34, e22955. [Google Scholar] [CrossRef]
- Rothuizen, J.; Bunch, S.E.; Charles, J.A.; Cullen, J.M.; Desmet, V.J.; Szatmári, V.; Twedt, D.C.; van den Ingh, T.S.; van Winkle, T.; Washabau, R.J. WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases; Saunders Limited: Uckfield, UK, 2006. [Google Scholar]
- Zopfs, D.; Graffe, J.; Reimer, R.P.; Schäfer, S.; Persigehl, T.; Maintz, D.; Borggrefe, J.; Haneder, S.; Lennartz, S.; Große Hokamp, N. Quantitative distribution of iodinated contrast media in body computed tomography: Data from a large reference cohort. Eur. Radiol. 2021, 31, 2340–2348. [Google Scholar] [CrossRef]
- Durieux, P.; Gevenois, P.A.; Muylem, A.V.; Howarth, N.; Keyzer, C. Abdominal Attenuation Values on Virtual and True Unenhanced Images Obtained With Third-Generation Dual-Source Dual-Energy CT. AJR Am. J. Roentgenol. 2018, 210, 1042–1058. [Google Scholar] [CrossRef] [PubMed]
- So, A.; Nicolaou, S. Spectral Computed Tomography: Fundamental Principles and Recent Developments. Korean J. Radiol. 2021, 22, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Mangold, S.; Thomas, C.; Fenchel, M.; Vuust, M.; Krauss, B.; Ketelsen, D.; Tsiflikas, I.; Claussen, C.D.; Heuschmid, M. Virtual nonenhanced dual-energy CT urography with tin-filter technology: Determinants of detection of urinary calculi in the renal collecting system. Radiology 2012, 264, 119–125. [Google Scholar] [CrossRef]
- Wen, D.; Pu, Q.; Peng, P.; Yue, X.; Ming, Y.; Yang, H.; Yang, J.; Zhang, X.; Liu, H.; Yang, L.; et al. Comparison of virtual and true non-contrast images from dual-layer spectral detector computed tomography (CT) in patients with colorectal cancer. Quant. Imaging Med. Surg. 2024, 14, 6260–6272. [Google Scholar] [CrossRef]
- Lehti, L.; Söderberg, M.; Höglund, P.; Wassélius, J. Comparing Arterial- and Venous-Phase Acquisition for Optimization of Virtual Noncontrast Images From Dual-Energy Computed Tomography Angiography. J. Comput. Assist. Tomogr. 2019, 43, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Laukamp, K.R.; Kessner, R.; Halliburton, S.; Zopfs, D.; Gupta, A.; Große Hokamp, N. Virtual Noncontrast Images From Portal Venous Phase Spectral-Detector CT Acquisitions for Adrenal Lesion Characterization. J. Comput. Assist. Tomogr. 2021, 45, 24–28. [Google Scholar] [CrossRef]
- Ding, Y.; Richter, A.; Stiller, W.; Kauczor, H.U.; Weber, T.F. Association between true non-contrast and virtual non-contrast vertebral bone CT attenuation values determined using dual-layer spectral detector CT. Eur. J. Radiol. 2019, 121, 108740. [Google Scholar] [CrossRef] [PubMed]
- Hojjati, M.; Van Hedent, S.; Rassouli, N.; Tatsuoka, C.; Jordan, D.; Dhanantwari, A.; Rajiah, P. Quality of routine diagnostic abdominal images generated from a novel detector-based spectral CT scanner: A technical report on a phantom and clinical study. Abdom. Radiol. 2017, 42, 2752–2759. [Google Scholar] [CrossRef] [PubMed]
- Greffier, J.; Villani, N.; Defez, D.; Dabli, D.; Si-Mohamed, S. Spectral CT imaging: Technical principles of dual-energy CT and multi-energy photon-counting CT. Diagn. Interv. Imaging 2023, 104, 167–177. [Google Scholar] [CrossRef] [PubMed]


| Evaluation Criteria | Classifications |
|---|---|
| Peritoneal fluid | None, mild, moderate, severe |
| Surface of the liver | Smooth, irregular |
| Contours of the liver (apart from the lesion) | Sharp angulation, rounded borders |
| Lesion type | Diffuse, focal, multifocal |
| Number of lesions | 1, 2–5, 5–10, >10 |
| Localisation of lesions | Lobus hepatis sinister lateralis/medialis, Lobus quadratus, Lobus hepatis dexter lateralis/medialis, Lobus caudatus, all lobes |
| Shape of lesions | Round, oval, amorphous, and different types of shapes of multifocal lesions |
| Border of lesions | Irregular, regular |
| Margination of lesions | Well-defined, ill-defined, different for different lesions |
| Extent of lesions | Intraparenchymal, extending over the hepatic border, both types of lesions present |
| Size of lesions | Maximum extension in cm (applies to the largest lesion, measured in all three planes) |
| Capsule formation | No, yes |
| Cavitation | No, one lesion, several lesions |
| Attenuation pre-/post-contrast compared to the surrounding parenchyma | Hypoattenuating, isoattenuating, hyperattenuating, and different attenuations within the same lesion or several lesions |
| Enhancement pattern | Homogeneous, heterogeneous, mainly peripheral, circular peripheral (target lesions), different types of enhancement in different lesions |
| Degree of enhancement | Mild, moderate, severe, different within the same lesion or in different lesions |
| Mineralisations | None, mild, moderate, severe |
| Size of portal lymph nodes | Normal, mild/moderate/severe enlargement |
| Structure of portal lymph nodes | Homogeneous, heterogeneous |
| Enhancement pattern of portal lymph nodes | Homogeneous, heterogeneous |
| Lesion Type Based on Imaging Characteristics | ROI Placement |
|---|---|
| Diffuse | Left lateral lobe (1 ROI) Left medial lobe (1 ROI) Right lateral lobe (1 ROI) Right medial lobe (1 ROI) Central (2 ROIs) |
| Focal | Entire lesion (1 polygonal ROI) Periphery of the lesion (2 ROIs) Centre of the lesion (2 ROIs) Normal Parenchyma (2 ROIs) |
| Multifocal (main lesion and additional lesions) | Main lesion: -Entire lesion (1 polygonal ROI) -Periphery of the lesion (2 ROIs) -Centre of the lesion (2 ROIs) Additional (mineralised) lesion: -Entire lesion (1 polygonal ROI) -Periphery of the lesion (2 ROIs) -Centre of the lesion (2 ROIs) Normal appearing parenchyma (2 ROIs) |
| Multifocal (only multiple small lesions) | 1 ROI per lesion (4 ROIs) Normal appearing parenchyma (2 ROIs) |
| Image Noise and Image Quality: SBI Reconstructions vs. Conventional CT Images | |
|---|---|
| 1 | SBI reconstructions markedly worse than conventional CT images |
| 2 | SBI reconstructions mildly worse than conventional CT images |
| 3 | SBI reconstructions equivalent to conventional CT images |
| 4 | SBI reconstructions mildly better than conventional CT images |
| 5 | SBI reconstructions markedly better than conventional CT images |
| Parenchymal Iodine Subtraction in VNC | |
|---|---|
| 1 | Insufficient subtraction of contrast medium |
| 2 | Partly sufficient removal of contrast medium with larger, incomplete areas |
| 3 | Moderate removal of contrast medium with incomplete areas in parts of the parenchyma |
| 4 | Almost complete removal of contrast medium |
| 5 | Complete removal of contrast medium |
| Region of Interest (All ROIs) | ≤5 | ≤10 | ≤15 | |||
|---|---|---|---|---|---|---|
| Localisation | ||||||
| Muscle | 20/28 | 71.43% | 27/28 | 96.43% | 28/28 | 100% |
| Pancreas | 24/28 | 85.71% | 27/28 | 96.43% | 27/28 | 96.43% |
| Gallbladder | 26/28 | 92.86% | 27/28 | 96.43% | 28/28 | 100% |
| Liver | 154/203 | 75.86% | 188/203 | 92.61% | 198/203 | 97.54% |
| Imaging characteristics | ||||||
| Diffuse | 21/24 | 87.5% | 22/24 | 91.67% | 24/24 | 100% |
| Focal | 35/40 | 87.5% | 39/40 | 97.5% | 39/40 | 97.5% |
| Multifocal | 98/139 | 70.5% | 127/139 | 91.37% | 135/139 | 97.12% |
| Cavitary lesions | 34/40 | 85% | 40/40 | 100% | 40/40 | 100% |
| Periphery of malignant neoplasia | 8/12 | 66.67% | 12/12 | 100% | 12/12 | 100% |
| Histopathological diagnosis | ||||||
| Degeneration | 51/73 | 69.86% | 65/73 | 89.04% | 70/73 | 95.89% |
| Hepatitis | 42/49 | 85.71% | 46/49 | 93.88% | 49/49 | 100% |
| Non-specific | 11/17 | 64.7% | 15/17 | 88.24% | 16/17 | 94.12% |
| Nodular hyperplasia | 3/6 | 50% | 5/6 | 83.33% | 6/6 | 100% |
| Adenoma | 12/16 | 75% | 16/16 | 100% | 16/16 | 100% |
| Carcinoma | 25/28 | 89.29% | 28/28 | 100% | 28/28 | 100% |
| Blastoma | 10/14 | 71.43% | 13/14 | 92.86% | 13/14 | 92.86% |
| Categories (All ROIs) | ≤5 HUs | ≤10 HUs | ≤15 HUs |
|---|---|---|---|
| Muscle (n = 28) | 0.0035 | <0.0001 | <0.0001 |
| Pancreas (n = 28) | 0.0077 | <0.0001 | <0.0001 |
| Gall bladder (n = 28) | <0.0001 | <0.0001 | <0.0001 |
| Liver (n = 203) | 0.0002 | <0.0001 | <0.0001 |
| —diffuse (n = 37) | 0.0533 | <0.0001 | <0.0001 |
| —one lesion (n = 40) | 0.0118 | <0.0001 | <0.0001 |
| —multifocal (n = 126) | 0.0122 | <0.0001 | <0.0001 |
| —degenerative (n = 73) | 0.232 | <0.0001 | <0.0001 |
| —hepatitis (n = 49) | <0.0001 | <0.0001 | <0.0001 |
| —non-specific (n = 17) | 0.4852 | 0.0005 | <0.0001 |
| —hyperplasia (n = 6) | 0.7105 | 0.0213 | 0.0009 |
| —adenoma (n = 16) | 0.0121 | <0.0001 | <0.0001 |
| —carcinoma (n = 28) | <0.0001 | <0.0001 | <0.0001 |
| —blastoma (n = 14) | 0.5845 | 0.0098 | <0.0001 |
| All ROIs (n = 287) | <0.0001 | <0.0001 | <0.0001 |
| Patient Nr. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| Location | Score: Iodine Subtraction | Average Score (Location) | |||||||||||||
| Liver (overall) | 3 | 3 | 5 | 3 | 5 | 4 | 4 | 3 | 4 | 3 | 3 | 4 | 4 | 4 | 3.71 |
| Liver (main lesion) | 4 | 5 | 4 | 5 | 4 | 3 | 5 | 4.29 | |||||||
| Spleen | 3 | 3 | 5 | 3 | 5 | 4 | 5 | 4 | 3 | 2 | 4 | 5 | 3 | 3.77 | |
| Pancreas | 4 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 4 | 4.71 |
| Gallbladder | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 4.86 |
| Muscle | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Average Score (Patient) | 4 | 4 | 5 | 4 | 5 | 4.6 | 4.83 | 4.33 | 4.33 | 4 | 4.2 | 4.5 | 4.8 | 4.2 | 4.4 |
| Score: Image Quality/Image Noise | Average Score (Quality) | ||||||||||||||
| 4 | 4 | 4 | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 5 | 4 | 4 | 4 | 4 | |
| Patient Nr. | |||||||||||||||
| 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | ||
| Location | Score: Iodine Subtraction | Average Score (Location) | |||||||||||||
| Liver (overall) | 4 | 4 | 4 | 3 | 5 | 4 | 4 | 4 | 4 | 3 | 4 | 4 | 5 | 4 | 4 |
| Liver (main lesion) | 4 | 4 | 5 | 3 | 4 | 4 | 4 | 5 | 5 | 4.22 | |||||
| Spleen | 4 | 5 | 5 | 3 | 5 | 5 | 3 | 3 | 4 | 5 | 4 | 5 | 4 | 4.23 | |
| Pancreas | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 4.86 |
| Gallbladder | 4 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 4.79 |
| Muscle | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Average Score (Patient) | 4.4 | 4.67 | 4.67 | 4 | 4.83 | 4.8 | 4.17 | 4.2 | 4.5 | 4.33 | 4.6 | 4.67 | 4.83 | 4.8 | 4.52 |
| Score: Image Quality/Image Noise | Average Score (Quality) | ||||||||||||||
| 4 | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3.93 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claußen, L.K.; van Gemmeren, A.M.; Lietz, P.; Meller, S.; Wang-Leandro, A.; Beineke, A.; Nerschbach, V.; Volk, H.A.; Merhof, K. Comparison of Virtual Non-Contrast Images Generated by Spectral Detector Computed Tomography and Conventional Computed Tomography Images of Histologically Confirmed Hepatic Pathologies in 28 Dogs. Animals 2025, 15, 3366. https://doi.org/10.3390/ani15233366
Claußen LK, van Gemmeren AM, Lietz P, Meller S, Wang-Leandro A, Beineke A, Nerschbach V, Volk HA, Merhof K. Comparison of Virtual Non-Contrast Images Generated by Spectral Detector Computed Tomography and Conventional Computed Tomography Images of Histologically Confirmed Hepatic Pathologies in 28 Dogs. Animals. 2025; 15(23):3366. https://doi.org/10.3390/ani15233366
Chicago/Turabian StyleClaußen, Lydia K., Alkje M. van Gemmeren, Philipp Lietz, Sebastian Meller, Adriano Wang-Leandro, Andreas Beineke, Verena Nerschbach, Holger A. Volk, and Kristina Merhof. 2025. "Comparison of Virtual Non-Contrast Images Generated by Spectral Detector Computed Tomography and Conventional Computed Tomography Images of Histologically Confirmed Hepatic Pathologies in 28 Dogs" Animals 15, no. 23: 3366. https://doi.org/10.3390/ani15233366
APA StyleClaußen, L. K., van Gemmeren, A. M., Lietz, P., Meller, S., Wang-Leandro, A., Beineke, A., Nerschbach, V., Volk, H. A., & Merhof, K. (2025). Comparison of Virtual Non-Contrast Images Generated by Spectral Detector Computed Tomography and Conventional Computed Tomography Images of Histologically Confirmed Hepatic Pathologies in 28 Dogs. Animals, 15(23), 3366. https://doi.org/10.3390/ani15233366






