Prognostic Value of Body Weight-Independent Tricuspid Annular Plane Systolic Excursion to Systolic Pulmonary Arterial Pressure Ratio in Canine Precapillary Pulmonary Hypertension: A Retrospective Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Clinical Data
2.3. Echocardiography
2.4. Statistical Analysis
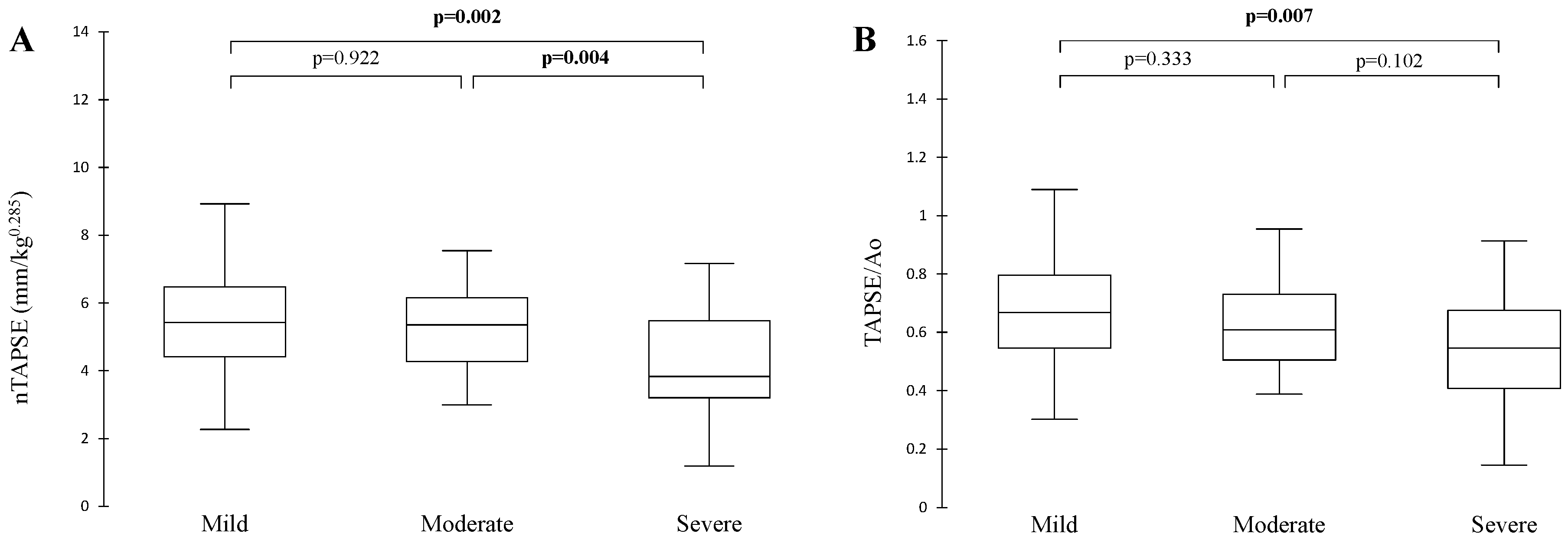
3. Results
3.1. Study Population
3.2. TAPSE/sPAP: Correlation with Different Echocardiographic Parameters and Association with HF
3.3. Short-Term Survival Analysis
3.4. Overall Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ao | Aortic root |
| ASE | American Society of Echocardiography |
| BW | Body weight |
| CO | Cardiac output |
| CPD | Cardio-pulmonary death |
| ECG | Electrocardiogram |
| EI | Eccentricity index |
| EId | Eccentricity index in diastole |
| EIs | Eccentricity index in systole |
| [Ees/Ea] | End-systolic/arterial elastance |
| ESC/ERS | European Society of Cardiology/European Respiratory Society |
| HF | Heart failure |
| HR | Hazard ratio |
| LA/Ao | Left atrium to aortic root ratio |
| LVFWd | Left ventricular free wall in diastole |
| MPA | Main pulmonary artery |
| nRAD | normalized right atrial diameter |
| nRVAd | normalized right ventricular end-diastolic area |
| nRVAs | normalized right ventricular end-systolic area |
| nTAPSE | normalized tricuspid annular plane systolic excursion |
| PCPH | Precapillary pulmonary hypertension |
| PH | Pulmonary hypertension |
| PV/PA | Pulmonary vein to pulmonary artery ratio |
| R-CHF | Right-sided congestive failure |
| RAD | Right atrial diameter |
| RV | Right ventricle/ventricular |
| RV-PA | Right ventricle to pulmonary artery |
| RVFAC | Right ventricular fractional area change |
| RVFWd | Right ventricular free wall in diastole |
| RVDd | Right ventricular internal diameter in diastole |
| sPAP | Systolic pulmonary artery pressure |
| TAPSE | Tricuspid annular plane systolic excursion |
| TAPSE/Ao | TAPSE normalized to aortic root |
| TAPSE/sPAP | TAPSE to sPAP ratio |
| TR | Tricuspid regurgitation |
| TRPG | Tricuspid regurgitation pressure gradient |
References
- Reinero, C.; Visser, L.C.; Kellihan, H.B.; Masseau, I.; Rozanski, E.; Clercx, C.; Williams, K.; Abbott, J.; Borgarelli, M.; Scansen, B.A. ACVIM consensus statement guidelines for the diagnosis, classification, treatment, and monitoring of pulmonary hypertension in dogs. J. Vet. Intern. Med. 2020, 34, 549–573. [Google Scholar] [CrossRef]
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U.; et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2019, 12, e009047. [Google Scholar] [CrossRef]
- Tello, K.; Axmann, J.; Ghofrani, H.A.; Naeije, R.; Narcin, N.; Rieth, A.; Seeger, W.; Gall, H.; Richter, M.J. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int. J. Cardiol. 2018, 266, 229–235. [Google Scholar] [CrossRef]
- Visser, L.C.; Wood, J.E.; Johnson, L.R. Survival characteristics and prognostic importance of echocardiographic measurements of right heart size and function in dogs with pulmonary hypertension. J. Vet. Intern. Med. 2020, 34, 1379–1388. [Google Scholar] [CrossRef]
- Fauvel, C.; Raitiere, O.; Boucly, A.; De Groote, P.; Renard, S.; Bertona, J.; Lamblin, N.; Artaud-Macari, E.; Viacroze, C.; Schleifer, D.; et al. Interest of TAPSE/sPAP ratio for noninvasive pulmonary arterial hypertension risk assessment. J. Heart Lung Transplant. 2022, 41, 1761–1772. [Google Scholar] [CrossRef]
- Chan, I.P.; Weng, M.C.; Hsueh, T.; Lin, Y.C.; Lin, S.L. Prognostic value of right pulmonary artery distensibility in dogs with pulmonary hypertension. J. Vet. Sci. 2019, 20, e34. [Google Scholar] [CrossRef]
- Jaffey, J.A.; Wiggen, K.; Leach, S.B.; Masseau, I.; Girens, R.E.; Reinero, C.R. Pulmonary hypertension secondary to respiratory disease and/or hypoxia in dogs: Clinical features, diagnostic testing and survival. Vet. J. 2019, 251, 105347. [Google Scholar] [CrossRef]
- Pariaut, R.; Saelinger, C.; Strickland, K.N.; Beaufrere, H.; Reynolds, C.A.; Vila, J. Tricuspid annular plane systolic excursion (TAPSE) in dogs: Reference values and impact of pulmonary hypertension. J. Vet. Intern. Med. 2012, 26, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Vezzosi, T.; Domenech, O.; Costa, G.; Marchesotti, F.; Venco, L.; Zini, E.; Del Palacio, M.J.F.; Tognetti, R. Echocardiographic evaluation of the right ventricular dimension and systolic function in dogs with pulmonary hypertension. J. Vet. Intern. Med. 2018, 32, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Soydan, L.C.; Kellihan, H.B.; Bates, M.L.; Stepien, R.L.; Consigny, D.W.; Bellofiore, A.; Francois, C.J.; Chesler, N.C. Accuracy of Doppler echocardiographic estimates of pulmonary artery pressures in a canine model of pulmonary hypertension. J. Vet. Cardiol. 2015, 17, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Mukherjee, M.; Rudski, L.G.; Addetia, K.; Afilalo, J.; D’Alto, M.; Freed, B.H.; Friend, L.B.; Gargani, L.; Grapsa, J.; Hassoun, P.M.; et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults and Special Considerations in Pulmonary Hypertension: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2025, 38, 141–186. [Google Scholar] [CrossRef] [PubMed]
- Yuchi, Y.; Suzuki, R.; Teshima, T.; Matsumoto, H.; Koyama, H. Utility of tricuspid annular plane systolic excursion normalized by right ventricular size indices in dogs with postcapillary pulmonary hypertension. J. Vet. Intern. Med. 2021, 35, 107–119. [Google Scholar] [CrossRef]
- Poser, H.; Berlanda, M.; Monacolli, M.; Contiero, B.; Coltro, A.; Guglielmini, C. Tricuspid annular plane systolic excursion in dogs with myxomatous mitral valve disease with and without pulmonary hypertension. J. Vet. Cardiol. 2017, 19, 228–239. [Google Scholar] [CrossRef]
- Cornell, C.C.; Kittleson, M.D.; Della Torre, P.; Haggstrom, J.; Lombard, C.W.; Pedersen, H.D.; Vollmar, A.; Wey, A. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J. Vet. Intern. Med. 2004, 18, 311–321. [Google Scholar] [CrossRef]
- Rishniw, M.; Erb, H.N. Evaluation of four 2-dimensional echocardiographic methods of assessing left atrial size in dogs. J. Vet. Intern. Med. 2000, 14, 429–435. [Google Scholar] [CrossRef]
- Thomas, W.P.; Gaber, C.E.; Jacobs, G.J.; Kaplan, P.M.; Lombard, C.W.; Moise, N.S.; Moses, B.L. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J. Vet. Intern. Med. 1993, 7, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Kellihan, H.B.; Stepien, R.L. Pulmonary hypertension in dogs: Diagnosis and therapy. Vet. Clin. North. Am. Small Anim. Pract. 2010, 40, 623–641. [Google Scholar] [CrossRef]
- Lancellotti, P.; Moura, L.; Pierard, L.A.; Agricola, E.; Popescu, B.A.; Tribouilloy, C.; Hagendorff, A.; Monin, J.L.; Badano, L.; Zamorano, J.L.; et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur. J. Echocardiogr. 2010, 11, 307–332. [Google Scholar] [CrossRef] [PubMed]
- Oyama, M.A.; Sisson, D.D. Assessment of cardiac chamber size using anatomic M-mode. Vet. Radiol. Ultrasound 2005, 46, 331–336. [Google Scholar] [CrossRef]
- Feldhutter, E.K.; Domenech, O.; Vezzosi, T.; Tognetti, R.; Sauter, N.; Bauer, A.; Eberhard, J.; Friederich, J.; Wess, G. Echocardiographic reference intervals for right ventricular indices, including 3-dimensional volume and 2-dimensional strain measurements in healthy dogs. J. Vet. Intern. Med. 2022, 36, 8–19. [Google Scholar] [CrossRef]
- Caivano, D.; Dickson, D.; Pariaut, R.; Stillman, M.; Rishniw, M. Tricuspid annular plane systolic excursion-to-aortic ratio provides a bodyweight-independent measure of right ventricular systolic function in dogs. J. Vet. Cardiol. 2018, 20, 79–91. [Google Scholar] [CrossRef]
- Gentile-Solomon, J.M.; Abbott, J.A. Conventional echocardiographic assessment of the canine right heart: Reference intervals and repeatability. J. Vet. Cardiol. 2016, 18, 234–247. [Google Scholar] [CrossRef]
- Lekane, M.; Burnotte, P.; Gommeren, K.; Mc Entee, K.; Merveille, A.C. Left ventricular eccentricity index to assess precapillary pulmonary hypertension in dogs. J. Vet. Cardiol. 2024, 51, 220–231. [Google Scholar] [CrossRef]
- Visser, L.C.; Scansen, B.A.; Schober, K.E.; Bonagura, J.D. Echocardiographic assessment of right ventricular systolic function in conscious healthy dogs: Repeatability and reference intervals. J. Vet. Cardiol. 2015, 17, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Tognetti, R.; Domenech, O.; Marchesotti, F.; Patata, V.; Vezzosi, T. Echocardiographic reference intervals of the dimensions of the main pulmonary artery and the right pulmonary artery: A prospective study in 269 healthy dogs. J. Vet. Cardiol. 2023, 50, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Roels, E.; Merveille, A.C.; Moyse, E.; Gomart, S.; Clercx, C.; Mc Entee, K. Diagnostic value of the pulmonary vein-to-right pulmonary artery ratio in dogs with pulmonary hypertension of precapillary origin. J. Vet. Cardiol. 2019, 24, 85–94. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Kultursay, B.; Keskin, B.; Tanyeri, S.; Kulahcioglu, S.; Hakgor, A.; Mutlu, D.; Bulus, C.; Tokgoz, H.C.; Yucel, E.; Sekban, A.; et al. Prognostic Impact of the Tricuspid Annular Plane Systolic Excursion/Pulmonary Arterial Systolic Pressure Ratio in Acute Pulmonary Embolism. Anatol. J. Cardiol. 2024, 28, 479–485. [Google Scholar] [CrossRef]
- Kjaergaard, J.; Iversen, K.K.; Akkan, D.; Moller, J.E.; Kober, L.V.; Torp-Pedersen, C.; Hassager, C. Predictors of right ventricular function as measured by tricuspid annular plane systolic excursion in heart failure. Cardiovasc. Ultrasound 2009, 7, 51. [Google Scholar] [CrossRef]
- Kazimierczyk, R.; Kazimierczyk, E.; Knapp, M.; Sobkowicz, B.; Malek, L.A.; Blaszczak, P.; Ptaszynska-Kopczynska, K.; Grzywna, R.; Kaminski, K.A. Echocardiographic Assessment of Right Ventricular-Arterial Coupling in Predicting Prognosis of Pulmonary Arterial Hypertension Patients. J. Clin. Med. 2021, 10, 2995. [Google Scholar] [CrossRef]
- Feldhutter, E.K.; Domenech, O.; Vezzosi, T.; Tognetti, R.; Eberhard, J.; Friederich, J.; Wess, G. Right ventricular size and function evaluated by various echocardiographic indices in dogs with pulmonary hypertension. J. Vet. Intern. Med. 2022, 36, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Legris, V.; Thibault, B.; Dupuis, J.; White, M.; Asgar, A.W.; Fortier, A.; Pitre, C.; Bouabdallaoui, N.; Henri, C.; O’Meara, E.; et al. Right ventricular function and its coupling to pulmonary circulation predicts exercise tolerance in systolic heart failure. ESC Heart Fail. 2022, 9, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Guazzi, M.; Scardovi, A.B.; Klersy, C.; Clemenza, F.; Carluccio, E.; Temporelli, P.L.; Rossi, A.; Faggiano, P.; Traversi, E.; et al. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur. J. Heart Fail. 2017, 19, 873–879. [Google Scholar] [CrossRef]
- de Pinto, M.; Coppi, F.; Spinella, A.; Pagnoni, G.; Morgante, V.; Macripo, P.; Boschini, M.; Guerra, A.F.; Tampieri, F.; Secchi, O.; et al. The predictive role of the TAPSE/sPAP ratio for cardiovascular events and mortality in systemic sclerosis with pulmonary hypertension. Front. Cardiovasc. Med. 2024, 11, 1430903. [Google Scholar] [CrossRef]
- Anastasiou, V.; Papazoglou, A.S.; Moysidis, D.V.; Daios, S.; Barmpagiannos, K.; Gossios, T.; Efthimiadis, G.K.; Karamitsos, T.; Ziakas, A.; Kamperidis, V. The prognostic impact of right ventricular-pulmonary arterial coupling in heart failure: A systematic review and meta-analysis. Heart Fail. Rev. 2024, 29, 13–26. [Google Scholar] [CrossRef]
- Vicenzi, M.; Caravita, S.; Rota, I.; Casella, R.; Deboeck, G.; Beretta, L.; Lombi, A.; Vachiery, J.L. The added value of right ventricular function normalized for afterload to improve risk stratification of patients with pulmonary arterial hypertension. PLoS ONE 2022, 17, e0265059. [Google Scholar] [CrossRef]
- Sert, S.; Selcuk, N.; Yildirimturk, O.; Orhan, G. Prognostic value of TAPSE/PASP ratio in right ventricular failure after left ventricular assist device implantation: Experience from a tertiary center. Turk. Gogus Kalp Damar Cerrahisi Derg. 2022, 30, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Ozpelit, E.; Akdeniz, B.; Ozpelit, E.M.; Tas, S.; Alpaslan, E.; Bozkurt, S.; Arslan, A.; Badak, O. Impact of Severe Tricuspid Regurgitation on Accuracy of Echocardiographic Pulmonary Artery Systolic Pressure Estimation. Echocardiography 2015, 32, 1483–1490. [Google Scholar] [CrossRef]
- Fei, B.; Fan, T.; Zhao, L.; Pei, X.; Shu, X.; Fang, X.; Cheng, L. Impact of severe tricuspid regurgitation on accuracy of systolic pulmonary arterial pressure measured by Doppler echocardiography: Analysis in an unselected patient population. Echocardiography 2017, 34, 1082–1088. [Google Scholar] [CrossRef]



| Parameter (Abbreviation) | Full Name | Measurement Type |
|---|---|---|
| sPAP (or TRPG) | Systolic pulmonary artery pressure (or tricuspid regurgitation pressure gradient) | Right ventricular pressure overload |
| nTAPSE | Tricuspid annular plane systolic excursion normalized to body weight | Right ventricular systolic function |
| TAPSE/Ao | Tricuspid annular plane systolic excursion normalized to aortic root ratio | Right ventricular systolic function |
| nRAD | Normalized right atrial diameter to body weight | Right atrial size |
| RVD/Ao | Right ventricular diameter to aortic root ratio | Right ventricular size |
| nRVAd | Normalized right ventricular area in diastole to body weight | Right ventricular size |
| nRVAs | Normalized right ventricular area in systole to body weight | Right ventricular systolic function |
| RVFAC | Right ventricular fractional area change | Right ventricular systolic function |
| RVFWd/LVFWd | Right ventricular free wall to left ventricular free wall ratio in diastole | Right ventricular free wall thickness |
| EI | Eccentricity index | Interventricular septal flattening |
| MPA/Ao | Mean pulmonary artery to aortic root ratio | Size of pulmonary artery |
| PV/PA | Pulmonary vein to pulmonary artery ratio | Size of pulmonary artery |
| Severity of PCPH | ||||
|---|---|---|---|---|
| Mild | Moderate | Severe | p-Value | |
| Age (years) | 10.5 ± 3.0 | 10.6 ± 3.5 | 8.8 ± 4.4 | 0.10 (a) |
| BW (kg) | 13.6 ± 12.7 | 8.8 ± 5.6 | 11.2 ± 8.7 | 0.22 (a) |
| Clinical signs of low CO (n) | 1 | 7 | 31 | <0.001 (b) |
| Clinical signs of R-CHF (n) | 2 | 2 | 23 | 0.007 (b) |
| R-CHF and/or low CO (n) | 2 | 7 | 39 | <0.0001 (b) |
| On cardiac treatment at presentation (n) | 5 | 10 | 25 | 0.65 (b) |
| Death (yes:no) (n) | 6:4 | 21:10 | 37:17 | |
| Long-term CPD: short-term CPD (n) | 3:1 | 9:6 | 16:16 | |
| TR severity (n) (mild:moderate:severe) | 8:2:0 | 16:12:3 | 9:15:30 | <0.0001 (b) |
| Severity of PCPH | |||
|---|---|---|---|
| Mild | Moderate | Severe | |
| Total number of dogs | 10 | 31 | 54 |
| Group 1 (pulmonary arterial hypertension) | 0 | 0 | 1 |
| Group 3 (pulmonary causes) | 4 | 11 | 5 |
| Group 4 (pulmonary thromboembolism) | 1 | 3 | 11 |
| Group 5 (parasitic) | 0 | 3 | 17 |
| Group 6 (multifactorial/unclear) | 5 | 14 | 4 |
| Severity of PCPH | ||||
|---|---|---|---|---|
| Mild (n = 10) | Moderate (n = 34) | Severe (n = 54) | p-Value | |
| sPAP (mmHg) based on TRPG | 44.9 ± 5.52 | 61.2 ± 7.54 | 110.6 ± 28 | <0.0001 |
| nTAPSE/(sPAP (m/s)) | 1.8 ± 0.46 | 1.5 ± 0.53 | 0.84 ± 0.35 | <0.0001 |
| (TAPSE/Ao)/(sPAP (m/s)) × 10 | 2.1 ± 0.60 | 1.8 ± 0.58 | 1.1 ± 0.40 | <0.0001 |
| nTAPSE/(sPAP(mmHg)) × 10 | 1.3 ± 0.43 | 0.96 ± 0.34 | 0.44 ± 0.19 | <0.0001 |
| (TAPSE/Ao)/(sPAP(mmHg)) × 100 | 1.6 ± 0.55 | 1.2 ± 0.40 | 0.55 ± 0.24 | <0.0001 |
| nTAPSE/(sPAP(m/s)) | (TAPSE/Ao)/(sPAP(m/s)) × 10 | nTAPSE/(sPAP(mmHg)) × 10 | (TAPSE/Ao)/(sPAP(mmHg)) × 100 | p-Value | |
|---|---|---|---|---|---|
| sPAP based on TRPG (mmHg) | −0.74 | −0.76 | −0.86 | −0.86 | <0.001 |
| nRAD (mm/kg0.30) | −0.58 | −0.59 | −0.68 | −0.66 | <0.001 |
| RVDd/Ao | −0.60 | −0.57 | −0.71 | −0.63 | <0.001 |
| nRVAd (cm2/kg0.665) | −0.48 | −0.51 | −0.55 | −0.57 | <0.001 |
| RVFWd/LVFWd | −0.50 | −0.47 | −0.55 | −0.51 | <0.001 |
| EId | −0.61 | −0.62 | −0.71 | −0.69 | <0.001 |
| EIs | −0.74 | −0.75 | −0.82 | −0.80 | <0.001 |
| LA/Ao | 0.25 | 0.33 | 0.23 | 0.27 | <0.023 |
| MPA/Ao | −0.53 | −0.46 | −0.60 | −0.52 | <0.001 |
| PV/PA | 0.66 | 0.67 | 0.69 | 0.68 | <0.001 |
| nRVAs (cm2/kg0.695) | −0.65 | −0.67 | −0.68 | −0.69 | <0.001 |
| RVFAC | 0.61 | 0.64 | 0.59 | 0.60 | <0.001 |
| nTAPSE (mm/kg0.285) | 0.77 | 0.81 | 0.72 | 0.70 | <0.001 |
| TAPSE/Ao | 0.71 | 0.81 | 0.65 | 0.68 | <0.001 |
| Simple Cox Regression Models | Multiple Cox Regression Model 1 | Multiple Cox Regression Model 2 | ||||
|---|---|---|---|---|---|---|
| HR (95%CI) | p-Value | Adj. HR (95%CI) | p-Value | Adj. HR (95%CI) | p-Value | |
| Age (years) | 1.0 (0.99–1.1) | 0.99 | - | - | - | - |
| Clinical signs of HF | 1.5 (0.69–3.3) | 0.30 | - | - | - | - |
| Etiology of PCPH (ref. = group 6) | ||||||
| Group 1 | - | - | - | - | - | - |
| Group 3 | 1.1 (0.38–3.4) | 0.83 | 1.5 (0.48–4.7) | 0.49 | 1.6 (0.50–5.1) | 0.43 |
| Group 4 | 2.4 (0.90–6.6) | 0.08 | 2.3 (0.87–6.3) | 0.09 | 2.5 (0.93–6.9) | 0.07 |
| Group 5 | 1.2 (0.41–3.7) | 0.71 | 0.93 (0.31–2.8) | 0.90 | 0.92 (0.31–2.8) | 0.88 |
| Severity of PCPH (ref.= mild) | ||||||
| moderate | 1.1 (0.23–5.4) | 0.89 | - | - | - | - |
| severe | 1.7 (0.39–7.3) | 0.48 | - | - | - | - |
| nTAPSE/(sPAP(m/s)) | 0.49 (0.23–11.1) | 0.08 | 0.44 (0.19–1.0) | 0.07 | - | - |
| (TAPSE/Ao)/(sPAP(m/s)) × 10 | 0.46 (0.22–0.98) | 0.04 | - | - | 0.39 (0.16–0.91) | 0.03 |
| nTAPSE/(sPAP(mmHg)) × 10 | 0.42 (0.14–1.3) | 0.12 | - | - | - | - |
| (TAPSE/Ao)/(sPAP(mmHg)) × 100 | 0.50 (0.20–1.2) | 0.13 | - | - | - | - |
| Simple Cox Regression Models | Multiple Cox Regression Model 1 | Multiple Cox Regression Model 2 | Multiple Cox Regression Model 3 | Multiple Cox Regression Model 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p-Value | Adj. HR (95%CI) | p-Value | Adj. HR (95%CI) | p-Value | Adj. HR (95%CI) | p-Value | Adj. HR (95%CI) | p-Value | |
| Age (years) | 0.99 (0.89–1.1) | 0.82 | - | - | - | - | - | - | - | - |
| Clinical signs of HF | 1.7 (0.74–3.9) | 0.21 | - | - | - | - | - | - | - | - |
| Etiology of PCPH (ref. = group 6) | ||||||||||
| Group1 | - | - | - | - | - | - | - | - | - | - |
| Group 3 | 1.2 (0.34–4.0) | 0.82 | 1.7 (0.45–6.0) | 0.45 | 1.8 (0.48–6.6) | 0.39 | 1.7 (0.46–6.3) | 0.42 | 1.6 (0.43–5.9) | 0.48 |
| Group 4 | 3.1 (1.1–8.9) | 0.03 | 3.0 (1.05–8.6) | 0.041 | 3.3 (1.1–9.6) | 0.027 | 3.2 (1.1–9.2) | 0.032 | 3.1 (1.1–8.8) | 0.037 |
| Group 5 | 1.6 (0.50–5.0) | 0.44 | 1.2 (0.37–3.7) | 0.80 | 1.2 (0.37–3.7) | 0.79 | 1.1 (0.36–3.6) | 0.83 | 1.1 (0.36–3.6) | 0.83 |
| Severity of PCPH (ref. = mild) | ||||||||||
| moderate | 1.9 (0.23–16.0) | 0.54 | - | - | - | - | - | - | - | - |
| sever | 3.2 (0.42–24.1) | 0.26 | - | - | - | - | - | - | - | - |
| nTAPSE/sPAP(m/s): | 0.41 (0.17–0.96) | 0.039 | 0.38 (0.15–0.97) | 0.044 | - | - | - | - | - | - |
| (TAPSE/Ao)/(sPAP(m/s)) × 10 | 0.40 (0.18–0.92) | 0.031 | - | - | 0.33 (0.12–0.87) | 0.025 | - | - | - | - |
| nTAPSE/(sPAP(mmHg)) × 10 | 0.30 (0.083–1.1) | 0.061 | - | - | - | - | 0.23 (0.049–1.04) | 0.056 | - | - |
| (TAPSE/Ao)/(sPAP(mmHg × 100 | 0.40 (0.14–1.1) | 0.082 | - | - | - | - | - | - | 0.33 (0.097–1.1) | 0.081 |
| Simple Cox Regression Models | Multiple Cox Regression Model | |||
|---|---|---|---|---|
| HR (95%CI) | p-Value | Adj. HR (95%CI) | p-Value | |
| Age (years) | 1.1 (1.03–1.2) | 0.004 | 1.1 (0.99–1.1) | 0.058 |
| Clinical signs of HF | 1.4 (0.84–2.3) | 0.20 | - | - |
| Etiology of PCPH (ref. = group 6) | ||||
| Group 1 | - | - | - | - |
| Group 3 | 0.93 (0.48–1.8) | 0.83 | 0.89 (0.46–1.7) | 0.74 |
| Group 4 | 2.3 (1.2–4.4) | 0.01 | 2.0 (1.05–3.8) | 0.03 |
| Group 5 | 0.50 (0.23–1.1) | 0.09 | 0.61 (0.27–1.4) | 0.24 |
| Severity of PCPH (ref. = mild) | ||||
| moderate | 1.6 (0.63–4.0) | 0.32 | - | - |
| severe | 1.4 (0.59–3.3) | 0.45 | - | - |
| nTAPSE/(sPAP(m/s)) | 0.99 (0.62–1.6) | 0.95 | - | - |
| (TAPSE/Ao)/(sPAP(m/s)) × 10 | 0.95 (0.62–1.5) | 0.80 | - | - |
| nTAPSE/(sPAP(mmHg)) × 10 | 0.96 (0.51–1.8) | 0.89 | - | - |
| (TAPSE/Ao)/(sPAP(mmHg)) × 100 | 0.99 (0.59–1.7) | 0.98 | - | - |
| Simple Cox Regression Models | Multiple Cox Regression Model | |||
|---|---|---|---|---|
| HR (95%CI) | p-Value | Adj. HR (95%CI) | p-Value | |
| Age (years) | 1.1 (1.0–1.2) | 0.05 | 1.1 (0.98–1.1) | 0.15 |
| Clinical signs of HF | 1.9 (1.1–3.4) | 0.03 | 2.0 (1.1–3.9) | 0.03 |
| Etiology of PCPH (ref. = group 6) | ||||
| Group 1 | - | - | - | - |
| Group 3 | 0.95 (0.44–2.0) | 0.90 | 1.1 (0.50–2.4) | 0.80 |
| Group 4 | 2.5 (1.2–5.1) | 0.01 | 1.8 (0.86–3.8) | 0.12 |
| Group 5 | 0.68 (0.29–1.6) | 0.36 | 0.62 (0.25–1.5) | 0.30 |
| Severity of PCPH (ref. = mild) | ||||
| moderate | 1.7 (0.54–5.0) | 0.38 | - | - |
| severe | 1.8 (0.64–5.2) | 0.26 | - | - |
| nTAPSE/(sPAP(m/s)) | 0.71 (0.42–1.2) | 0.20 | - | - |
| (TAPSE/Ao)/(sPAP(m/s)) × 10 | 0.73 (0.44–1.2) | 0.23 | - | - |
| nTAPSE/(sPAP(mmHg)) × 10 | 0.61 (0.29–1.3) | 0.20 | - | - |
| (TAPSE/Ao)/(sPAP(mmHg)) × 100 | 0.73 (0.40–1.4) | 0.32 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Renterghem, E.; Legrand, M.; Lekane, M.; Roels, E.; Gommeren, K.; Merveille, A.-C. Prognostic Value of Body Weight-Independent Tricuspid Annular Plane Systolic Excursion to Systolic Pulmonary Arterial Pressure Ratio in Canine Precapillary Pulmonary Hypertension: A Retrospective Study. Animals 2025, 15, 3365. https://doi.org/10.3390/ani15233365
Van Renterghem E, Legrand M, Lekane M, Roels E, Gommeren K, Merveille A-C. Prognostic Value of Body Weight-Independent Tricuspid Annular Plane Systolic Excursion to Systolic Pulmonary Arterial Pressure Ratio in Canine Precapillary Pulmonary Hypertension: A Retrospective Study. Animals. 2025; 15(23):3365. https://doi.org/10.3390/ani15233365
Chicago/Turabian StyleVan Renterghem, Emilie, Margaux Legrand, Marine Lekane, Elodie Roels, Kris Gommeren, and Anne-Christine Merveille. 2025. "Prognostic Value of Body Weight-Independent Tricuspid Annular Plane Systolic Excursion to Systolic Pulmonary Arterial Pressure Ratio in Canine Precapillary Pulmonary Hypertension: A Retrospective Study" Animals 15, no. 23: 3365. https://doi.org/10.3390/ani15233365
APA StyleVan Renterghem, E., Legrand, M., Lekane, M., Roels, E., Gommeren, K., & Merveille, A.-C. (2025). Prognostic Value of Body Weight-Independent Tricuspid Annular Plane Systolic Excursion to Systolic Pulmonary Arterial Pressure Ratio in Canine Precapillary Pulmonary Hypertension: A Retrospective Study. Animals, 15(23), 3365. https://doi.org/10.3390/ani15233365






