Effects of Replacing Fishmeal with Enzymatically Hydrolyzed Pork Bone Meal (EHPBM) on Growth, Antioxidant Capacity, and Nutritional Metabolism in Micropterus salmoides
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diet Preparation
2.2. Experimental Management
2.3. Sample Collection
2.4. Chemical Analysis
2.5. Histopathology of the Liver
2.6. Gene Expression Analysis
2.7. Data Analysis
3. Results
3.1. Growth Performance
3.2. Whole Body Composition
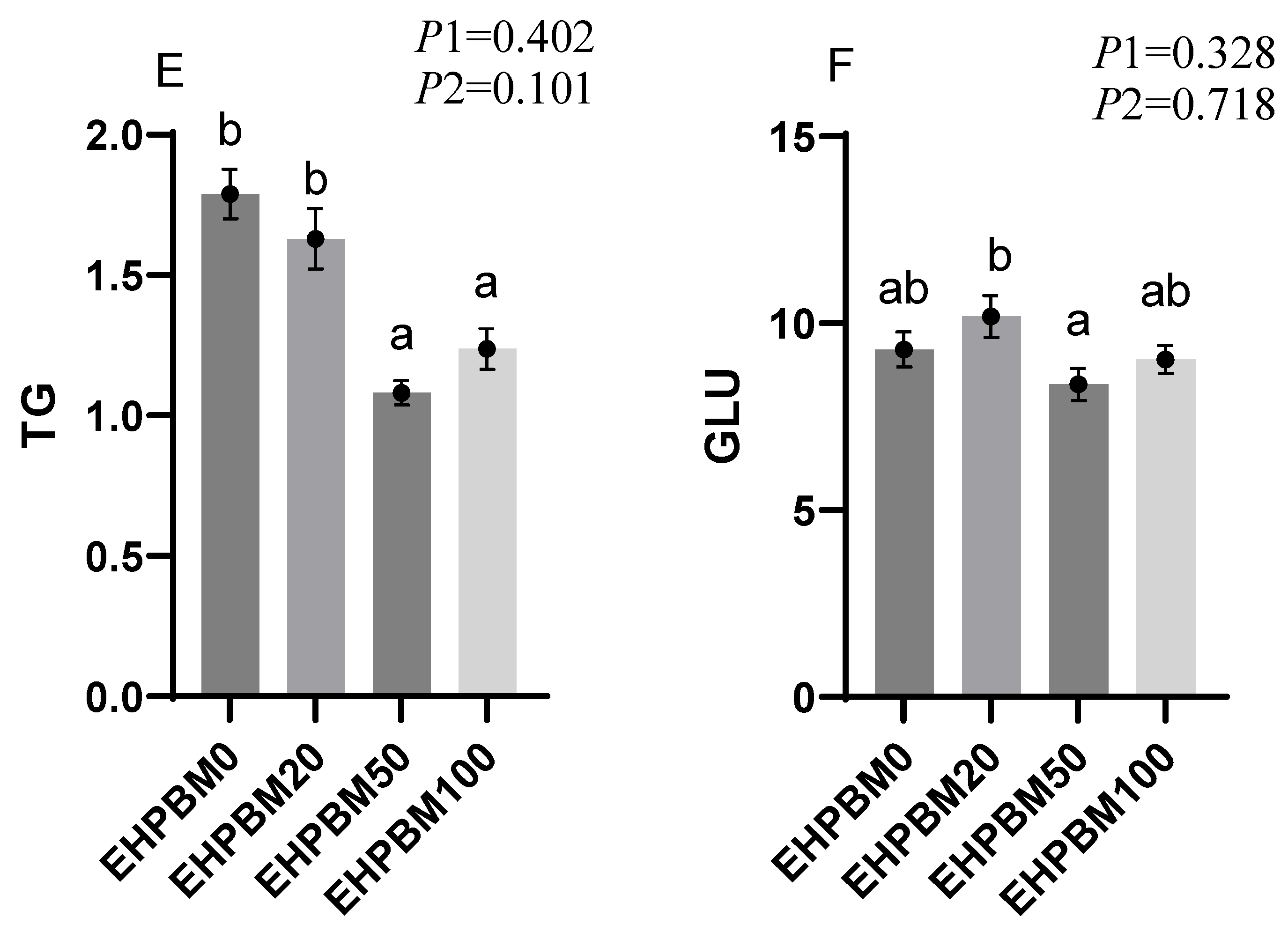
3.3. Plasma Biochemistry
3.4. Hepatic Enzymatic Indices
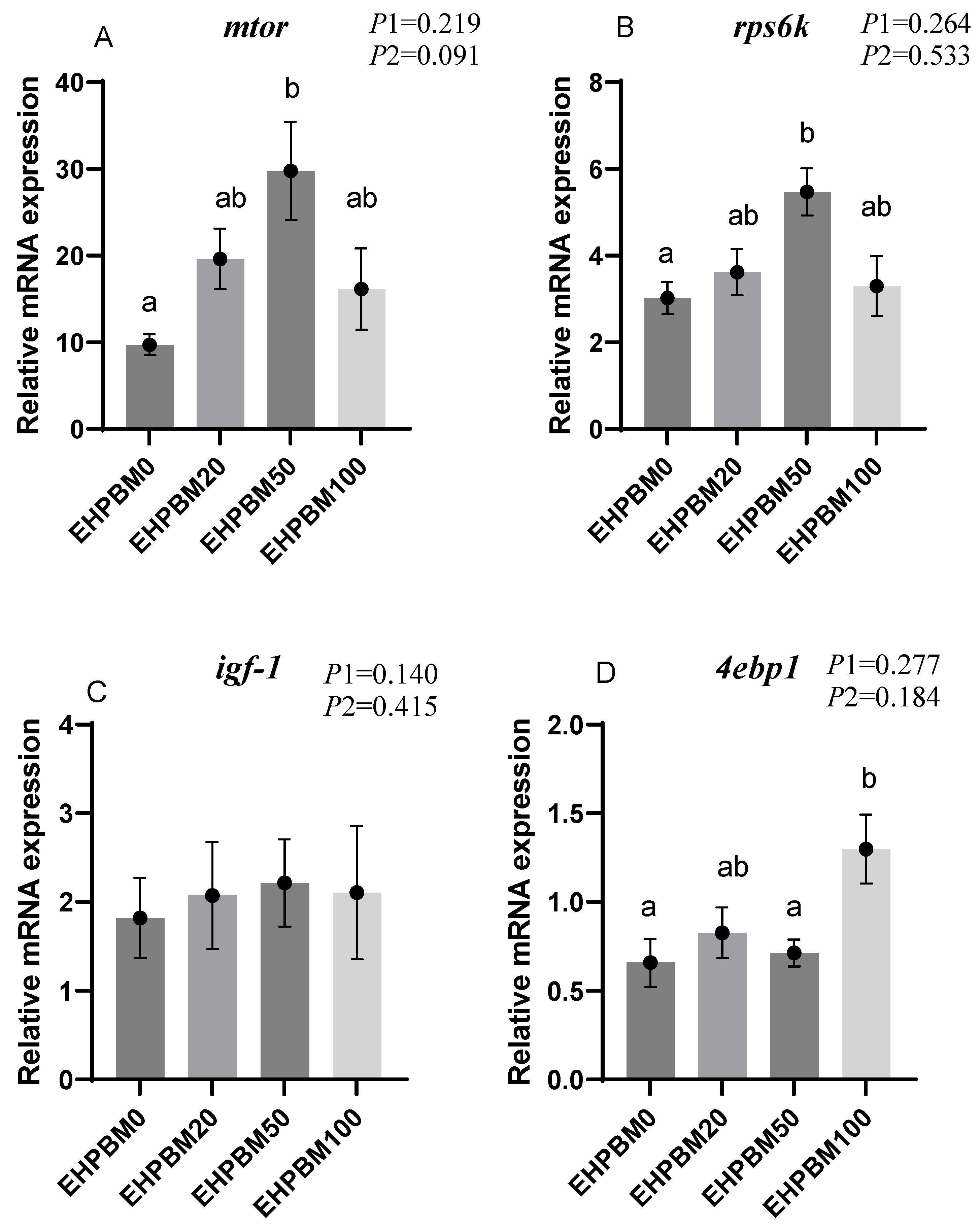
3.5. Protein Metabolism
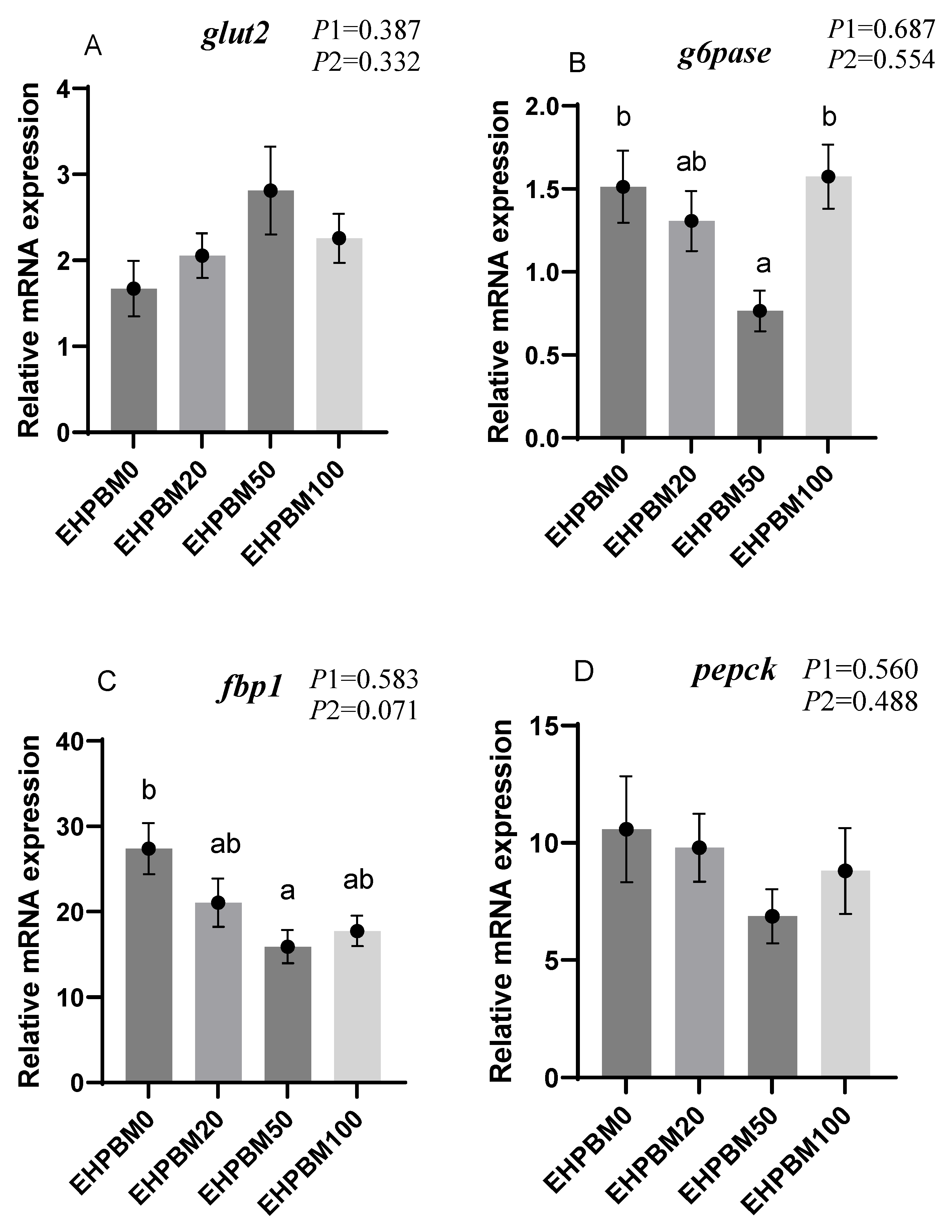
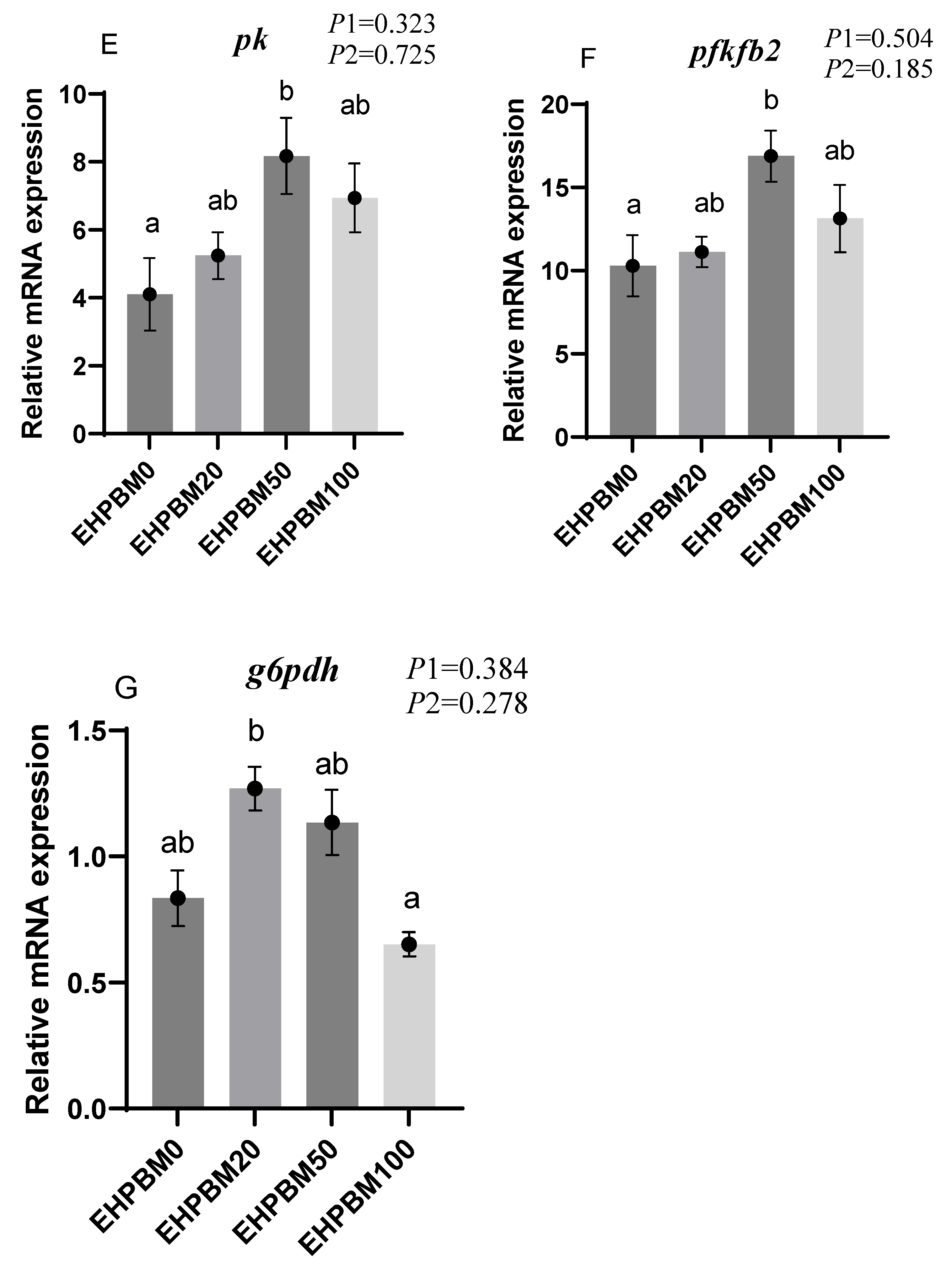
3.6. Glucose Metabolism
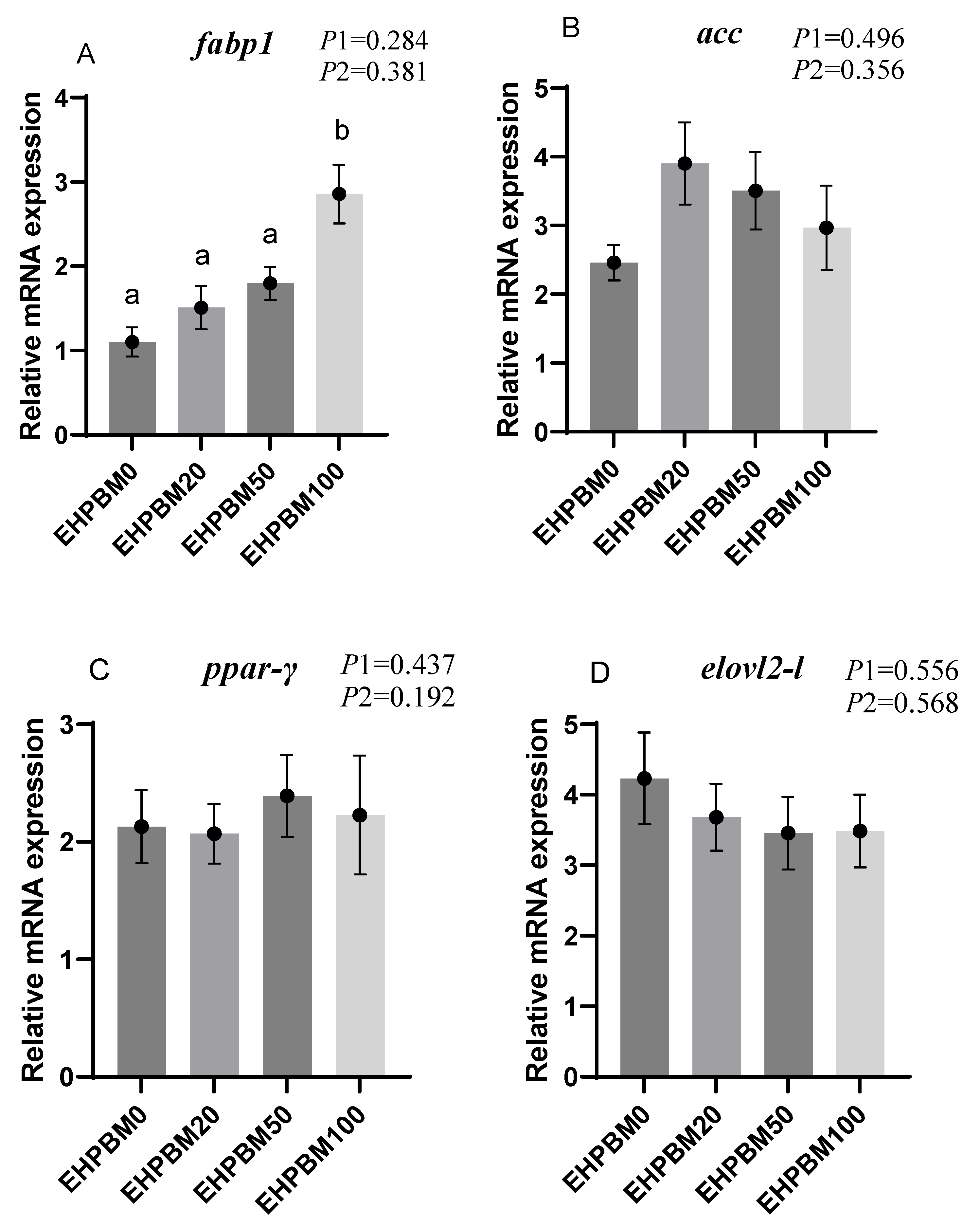
3.7. Lipid Metabolism
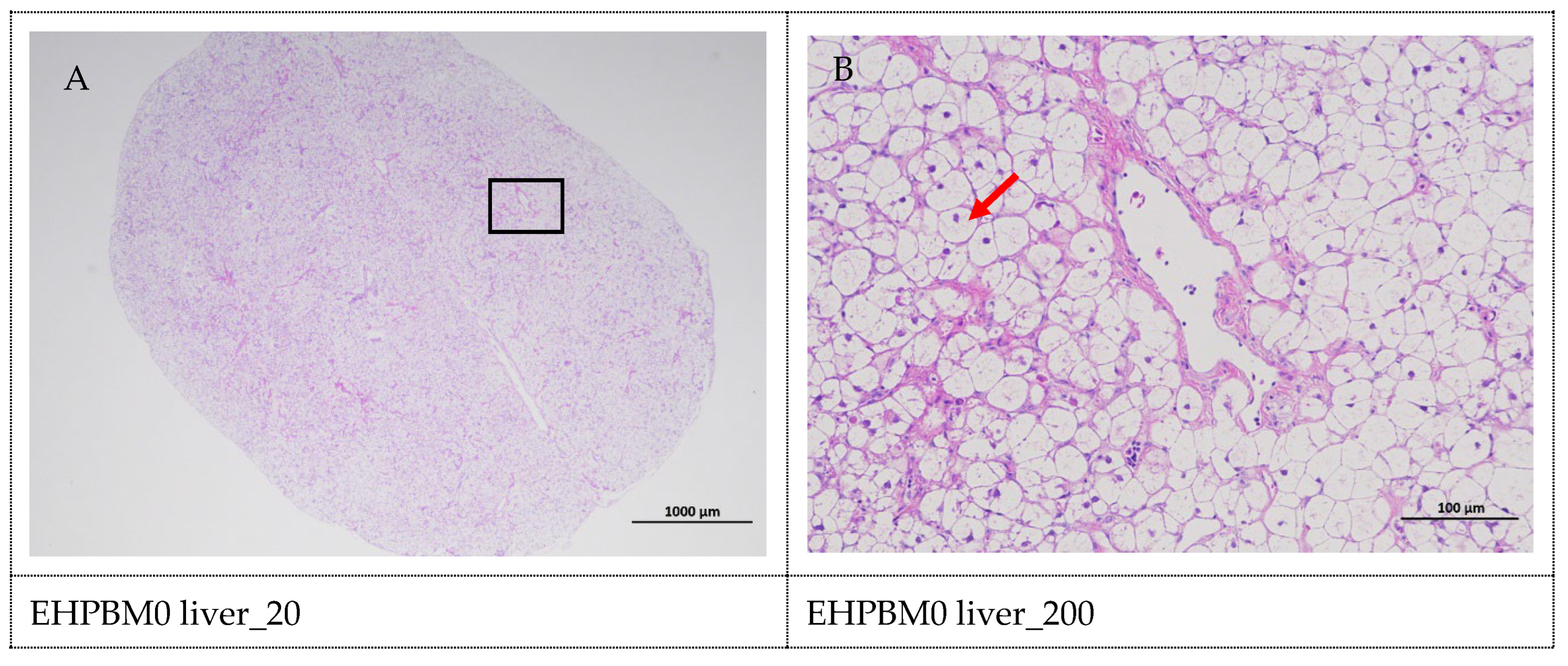
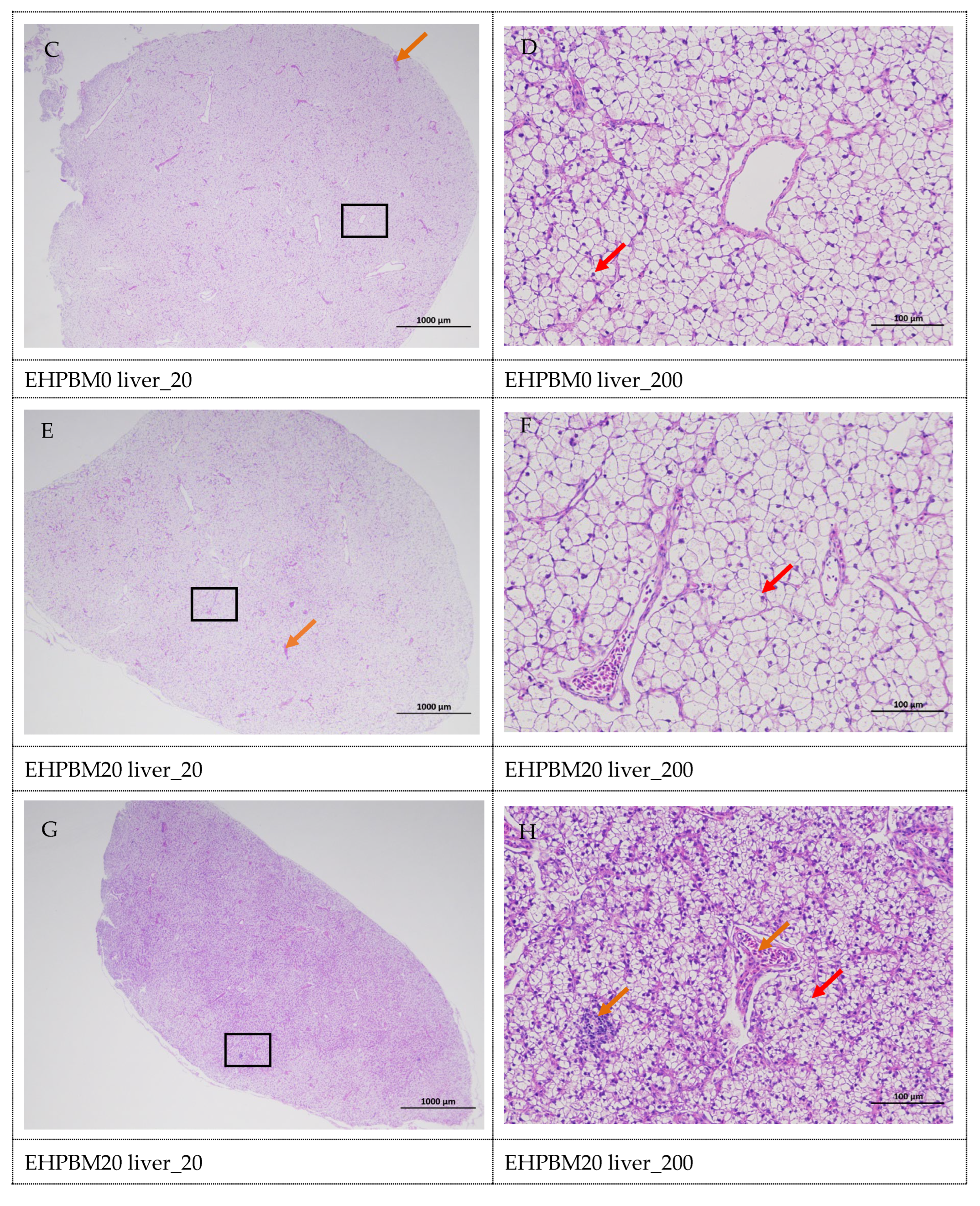
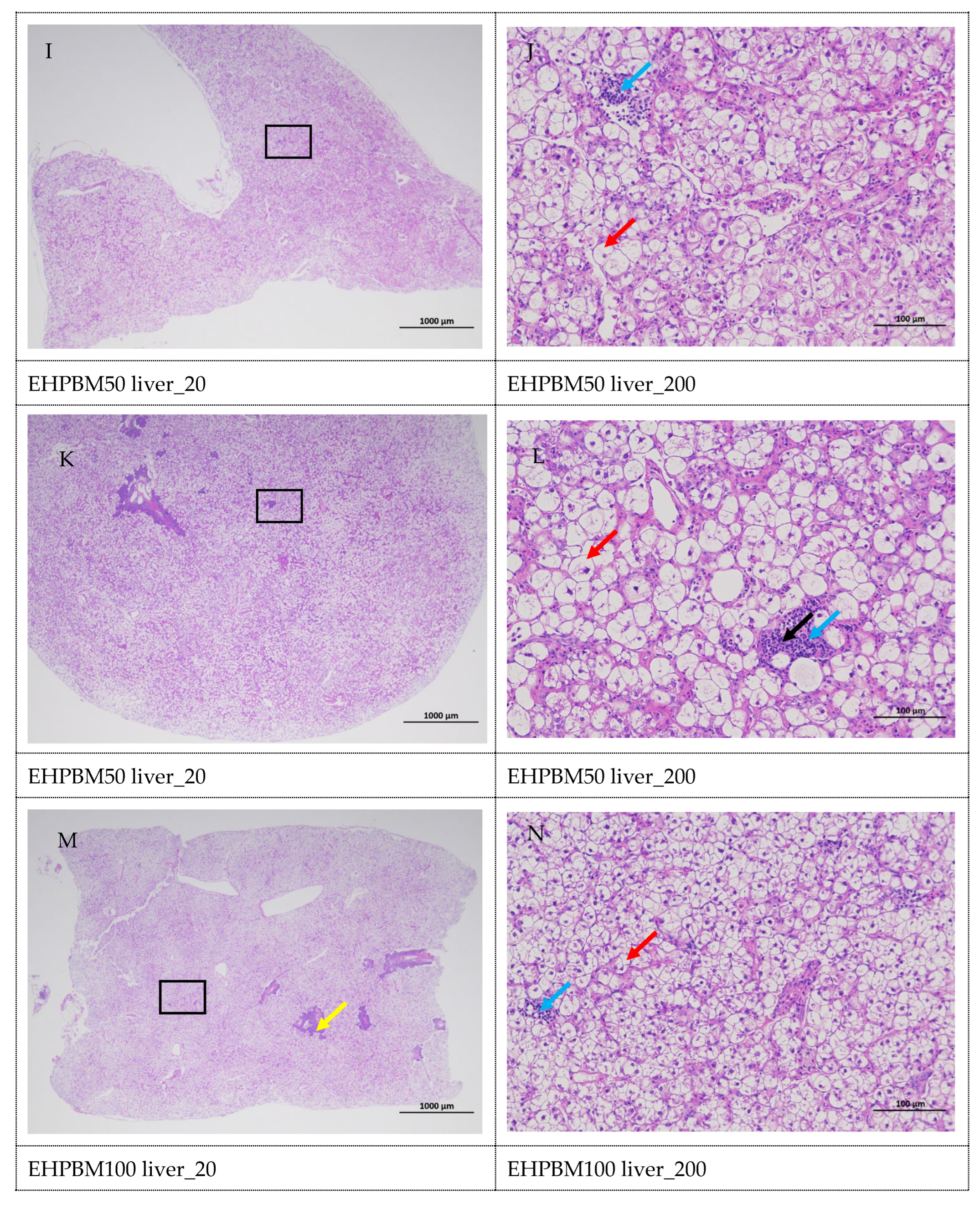
3.8. Histopathology of the Liver
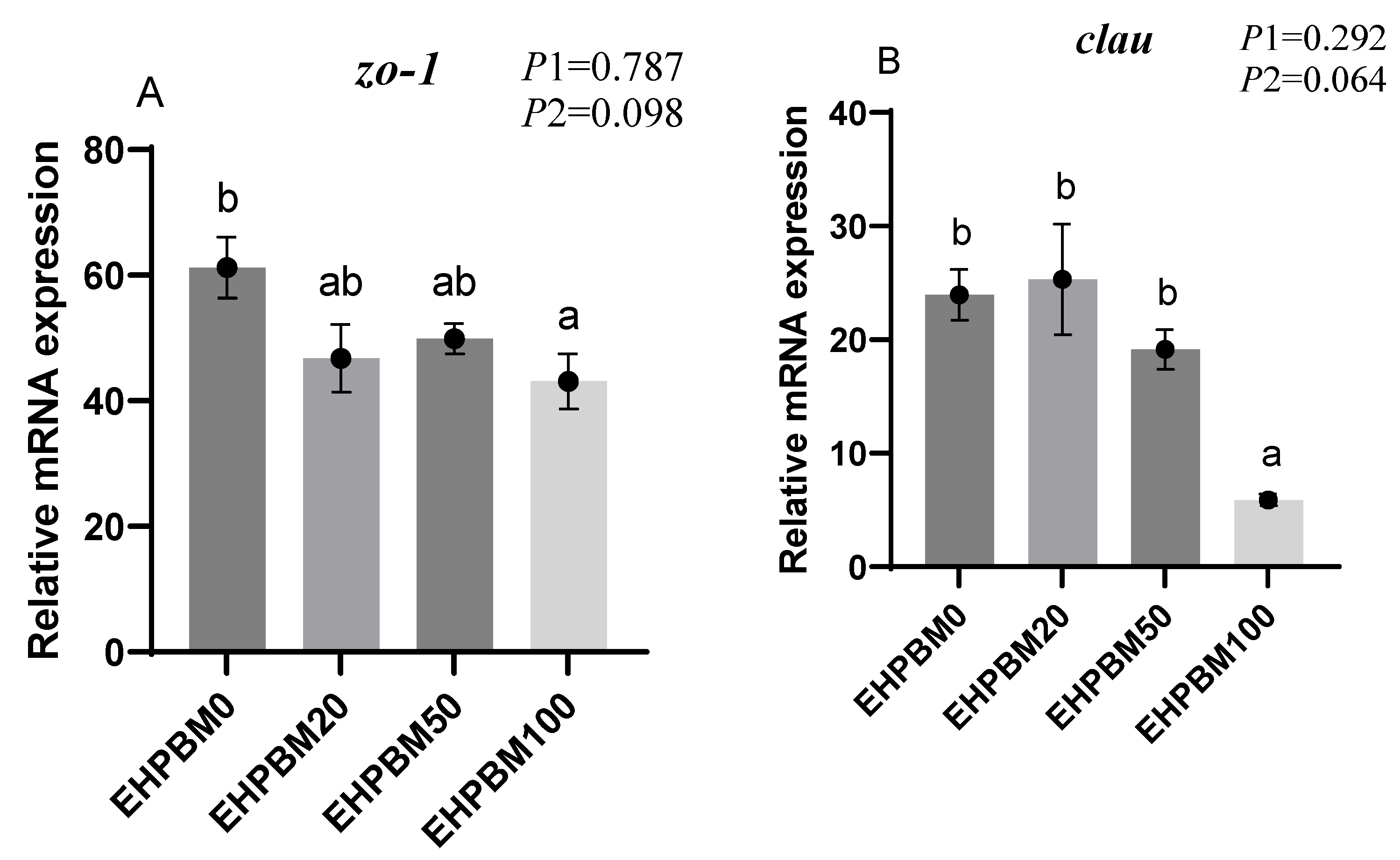
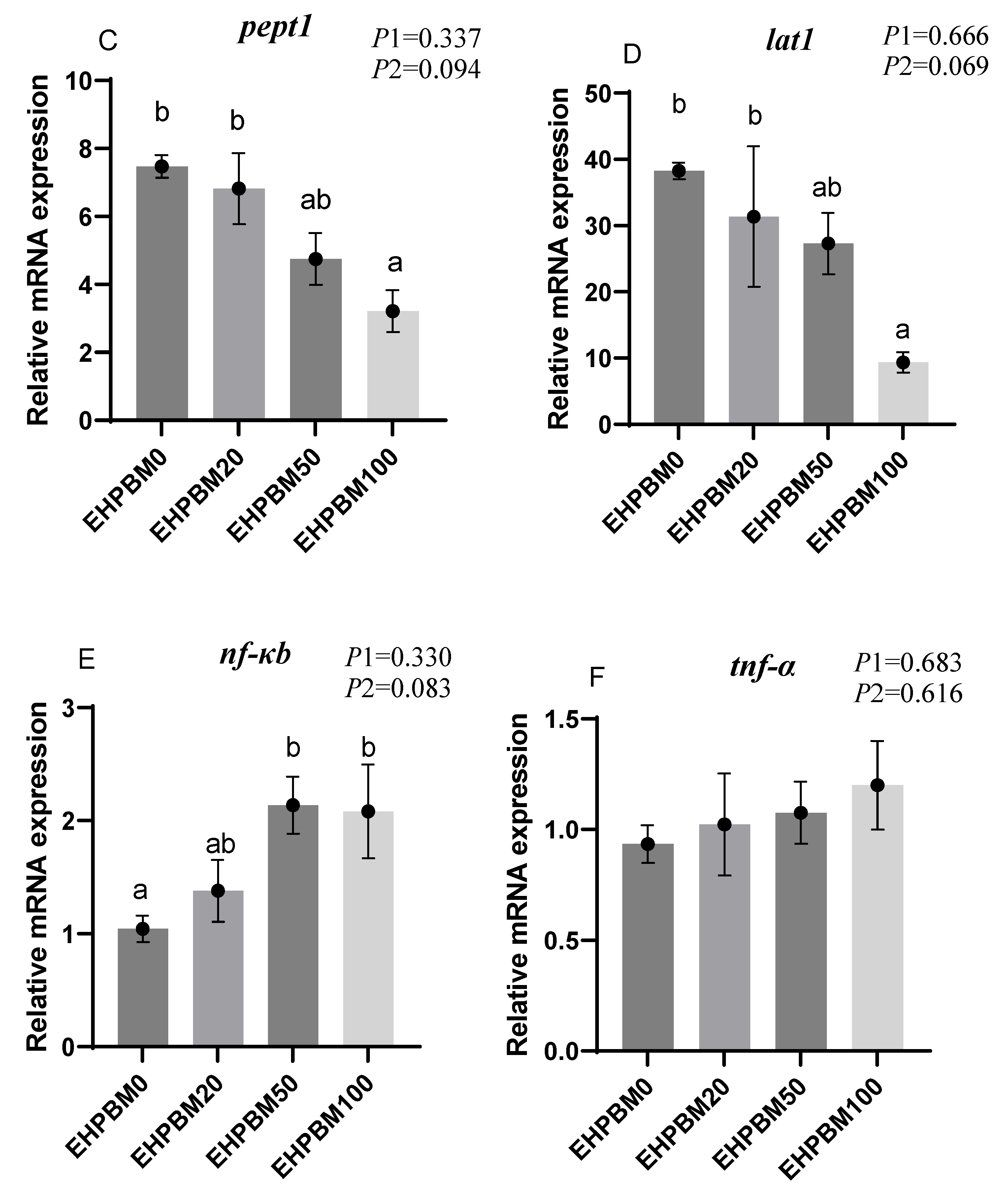
3.9. Intestinal Barrier and Transport Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gasco, L.; Gai, F.; Maricchiolo, G.; Genovese, L.; Ragonese, S.; Bottari, T.; Caruso, G. Fishmeal Alternative Protein Sources for Aquaculture Feeds. In Feeds for the Aquaculture Sector; SpringerBriefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–28. ISBN 978-3-319-77940-9. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2024; FAO: Roma, Italy, 2024; ISBN 978-92-5-138763-4. [Google Scholar]
- Liu, H.; Zhou, M.; Dong, X.; Tan, B.; Zhang, S.; Yang, Y.; Chi, S.; Liu, H.; Yan, X.; Li, Z. Transcriptomic Analysis of Liver in Silver Sillago, Sillago Sihama Fed with High-Level Low-Gossypol Cottonseed Meal in Replacement of Fishmeal Diet. Animals 2023, 13, 1194. [Google Scholar] [CrossRef]
- Montero, D.; Carvalho, M.; Terova, G.; Fontanillas, R.; Serradell, A.; Ginés, R.; Tuset, V.; Acosta, F.; Rimoldi, S.; Bajek, A.; et al. Nutritional Innovations in Superior European Sea Bass (Dicentrarchus labrax) Genotypes: Implications on Fish Performance and Feed Utilization. Aquaculture 2023, 572, 739486. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Bureau, D.P.; Chiu, A.; Elliott, M.; Farrell, A.P.; Forster, I.; Gatlin, D.M.; Goldburg, R.J.; Hua, K.; et al. Feeding Aquaculture in an Era of Finite Resources. Proc. Natl. Acad. Sci. USA 2009, 106, 15103–15110. [Google Scholar] [CrossRef]
- Dias, J.; Gomes, E.F.; Kaushik, S.J. Improvement of Feed Intake through Supplementation with an Attractant Mix in European Seabass Fed Plant-Protein Rich Diets. Aquat. Living Resour. 1997, 10, 385–389. [Google Scholar] [CrossRef]
- Lee, K.-J.; Powell, M.S.; Barrows, F.T.; Smiley, S.; Bechtel, P.; Hardy, R.W. Evaluation of Supplemental Fish Bone Meal Made from Alaska Seafood Processing Byproducts and Dicalcium Phosphate in Plant Protein Based Diets for Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2010, 302, 248–255. [Google Scholar] [CrossRef]
- Hussain, S.M.; Bano, A.A.; Ali, S.; Rizwan, M.; Adrees, M.; Zahoor, A.F.; Sarker, P.K.; Hussain, M.; Arsalan, M.Z.-H.; Yong, J.W.H.; et al. Substitution of Fishmeal: Highlights of Potential Plant Protein Sources for Aquaculture Sustainability. Heliyon 2024, 10, e26573. [Google Scholar] [CrossRef]
- Luthada-Raswiswi, R.; Mukaratirwa, S.; O’Brien, G. Animal Protein Sources as a Substitute for Fishmeal in Aquaculture Diets: A Systematic Review and Meta-Analysis. Appl. Sci. 2021, 11, 3854. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Composition of Amino Acids and Related Nitrogenous Nutrients in Feedstuffs for Animal Diets. Amino Acids 2020, 52, 523–542. [Google Scholar] [CrossRef]
- Hou, Y.; Yin, Y.; Wu, G. Dietary Essentiality of “Nutritionally Non-Essential Amino Acids” for Animals and Humans. Exp. Biol. Med. 2015, 240, 997–1007. [Google Scholar] [CrossRef]
- Li, P.; Wu, G. Roles of Dietary Glycine, Proline, and Hydroxyproline in Collagen Synthesis and Animal Growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Moutinho, S.; Martínez-Llorens, S.; Tomás-Vidal, A.; Jover-Cerdá, M.; Oliva-Teles, A.; Peres, H. Meat and Bone Meal as Partial Replacement for Fish Meal in Diets for Gilthead Seabream (Sparus aurata) Juveniles: Growth, Feed Efficiency, Amino Acid Utilization, and Economic Efficiency. Aquaculture 2017, 468, 271–277. [Google Scholar] [CrossRef]
- Li, M.H.; Bosworth, B.G.; Lucas, P.M. Evaluation of Porcine Meat and Bone Meal in Diets for Pond-Raised Hybrid Catfish. N. Am. J. Aquac. 2018, 80, 69–73. [Google Scholar] [CrossRef]
- Piazza, G.J.; Garcia, R.A. Meat & Bone Meal Extract and Gelatin as Renewable Flocculants. Bioresour. Technol. 2010, 101, 781–787. [Google Scholar] [CrossRef]
- National Research Council and Subcommittee on Poultry Nutrition. Nutrient Requirements of Poultry, 9th ed.; National Academies Press: Washington, DC, USA, 1994; ISBN 978-0-309-04892-7. [Google Scholar]
- Ravindran, V.; Hendriks, W.H.; Camden, B.J.; Thomas, D.V.; Morel, P.C.H.; Butts, C.A. Amino Acid Digestibility of Meat and Bone Meals for Broiler Chickens. Aust. J. Agric. Res. 2002, 53, 1257. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, C.; Pei, Z.; Zhang, W.; Wei, Z.; Chen, H.; Huang, Y. Retrospect of Fishmeal Substitution in Largemouth Bass (Micropterus salmoides): A Review. Fish Physiol. Biochem. 2025, 51, 21. [Google Scholar] [CrossRef]
- Mohammady, E.Y.; Soaudy, M.R.; Elashry, M.A.; Hassaan, M.S. Assessment of the Nutritional Impact of Substituting Fishmeal with Enzymatically Hydrolyzed Jojoba Meal (Simmondsia chinensis) in the Diets of Nile Tilapia, Oreochromis Niloticus. Aquaculture 2025, 596, 741888. [Google Scholar] [CrossRef]
- Macelline, S.P.; McQuade, L.R.; Mclnerney, B.V.; Moss, A.F.; Selle, P.H.; Liu, S.Y. Protein Digestive Dynamics of Meat and Bone Meals in Broiler Chickens. Anim. Nutr. 2020, 6, 521–528. [Google Scholar] [CrossRef]
- Masagounder, K.; Firman, J.D.; Hayward, R.S.; Sun, S.; Brown, P.B. Apparent Digestibilities of Common Feedstuffs for Bluegill Lepomis macrochirus and Largemouth Bass Micropterus salmoides Using Individual Test Ingredients. Aquac. Nutr. 2009, 15, 29–37. [Google Scholar] [CrossRef]
- Tidwell, J.H.; Coyle, S.D.; Bright, L.A.; Yasharian, D. Evaluation of Plant and Animal Source Proteins for Replacement of Fish Meal in Practical Diets for the Largemouth Bass Micropterus Salmoides. J. World Aquac. Soc. 2005, 36, 454–463. [Google Scholar] [CrossRef]
- Forster, I.P.; Dominy, W.; Obaldo, L.; Tacon, A.G.J. Rendered Meat and Bone Meals as Ingredients of Diets for Shrimp Litopenaeus vannamei (Boone, 1931). Aquaculture 2003, 219, 655–670. [Google Scholar] [CrossRef]
- Avramenko, N.A.; Low, N.H.; Nickerson, M.T. The Effects of Limited Enzymatic Hydrolysis on the Physicochemical and Emulsifying Properties of a Lentil Protein Isolate. Food Res. Int. 2013, 51, 162–169. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Shivhare, U.S.; Gill, B.S. Physico-Chemical and Functional Properties of Native and Hydrolyzed Kidney Bean (Phaseolus vulgaris L.) Protein Isolates. Food Res. Int. 2015, 76, 11–18. [Google Scholar] [CrossRef]
- Davies, S.J.; Laporte, J.; Gouveia, A.; Salim, H.S.; Woodgate, S.M.; Hassaan, M.S.; El-Haroun, E.R. Validation of Processed Animal Proteins (Mono-PAPS) in Experimental Diets for Juvenile Gilthead Sea Bream (Sparus aurata L.) as Primary Fish Meal Replacers within a European Perspective: XXXX. Aquac. Nutr. 2019, 25, 225–238. [Google Scholar] [CrossRef]
- Kotzamanis, Y.P.; Gisbert, E.; Gatesoupe, F.J.; Zambonino Infante, J.; Cahu, C. Effects of Different Dietary Levels of Fish Protein Hydrolysates on Growth, Digestive Enzymes, Gut Microbiota, and Resistance to Vibrio Anguillarum in European Sea Bass (Dicentrarchus labrax) Larvae. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 147, 205–214. [Google Scholar] [CrossRef]
- Ospina-Salazar, G.H.; Ríos-Durán, M.G.; Toledo-Cuevas, E.M.; Martínez-Palacios, C.A. The Effects of Fish Hydrolysate and Soy Protein Isolate on the Growth Performance, Body Composition and Digestibility of Juvenile Pike Silverside, Chirostoma estor. Anim. Feed Sci. Technol. 2016, 220, 168–179. [Google Scholar] [CrossRef]
- Qian, J.; Chen, D.; Zhang, Y.; Gao, X.; Xu, L.; Guan, G.; Wang, F. Ultrasound-Assisted Enzymatic Protein Hydrolysis in Food Processing: Mechanism and Parameters. Foods 2023, 12, 4027. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Z.; Yang, G.; Zhang, D.; Qin, H.; Xia, B.; Liu, S.; Chen, J. Supplementation of Enzymatic Hydrolysate in Low-Fishmeal and Low-Crop Diet Improves Growth, Antioxidant Capacity, and Immunity of Juvenile Sea Cucumber Apostichopus japonicus (Selenka). Fishes 2025, 10, 42. [Google Scholar] [CrossRef]
- Xu, F.-M.; Hou, S.-W.; Wang, G.-X.; Gong, J.-Y.; Zhou, L.; Huang, Y.-H.; Huang, X.-D.; Liu, L. Effects of Zymolytic Black Soldier Fly (Hermetia illucens) Pulp as Dietary Supplementation in Largemouth Bass (Micropterus salmoides). Aquac. Rep. 2021, 21, 100823. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Q.; Huang, D.; Zhang, L.; Chen, X.; Wang, Y.; Liang, H.; Ren, M. Effects of Partial Substitution of Enzymatic Hydrolysate of Poultry By-Product Meal for Fishmeal on the Growth Performance, Hepatic Health, Antioxidant Capacity, and Immunity of Juvenile Largemouth Bass (Micropterus salmoides). Aquac. Rep. 2024, 35, 101990. [Google Scholar] [CrossRef]
- Maryam; Shah, S.Z.H.; Fatima, M.; Hussain, S.M.; Nadeem, H.; Hussain, M. The Effectiveness of Protease Supplemented Poultry By-product Meal-based Diet on Growth, Nutrient Digestibility and Digestive Enzyme Activities of Rohu (Labeo rohita). Aquac. Res. 2022, 53, 3841–3852. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Felix, N.; Prabu, E. Effects of Dietary Protein Substitution of Fish Meal with Bioprocessed Poultry By-product Meal on Growth Performances, Nutrient Utilization, Whole-body Composition and Haemato-biochemical Responses of GIFT Tilapia Reared in Floating Cages. Aquac. Res. 2021, 52, 5407–5418. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Q.; Cao, H.; Tong, T.; Huang, G.; Li, W. Replacement of Fish Meal by Meat and Bone Meal in Diets for Juvenile Snakehead Ophiocephalus Argus. Fish. Sci. 2015, 81, 723–729. [Google Scholar] [CrossRef]
- Bharadwaj, A.S.; Brignon, W.R.; Gould, N.L.; Brown, P.B.; Wu, Y.V. Evaluation of Meat and Bone Meal in Practical Diets Fed to Juvenile Hybrid Striped Bass Morone chrysops × M. saxatilis. J. World Aquac. Soc. 2002, 33, 448–457. [Google Scholar] [CrossRef]
- Robaina, L.; Moyano, F.J.; Izquierdo, M.S.; Socorro, J.; Vergara, J.M.; Montero, D. Corn Gluten and Meat and Bone Meals as Protein Sources in Diets for Gilthead Seabream (Sparus aurata): Nutritional and Histological Implications. Aquaculture 1997, 157, 347–359. [Google Scholar] [CrossRef]
- De La Higuera, M.; Akharbach, H.; Hidalgo, M.C.; Peragón, J.; Lupiáñez, J.A.; García-Gallego, M. Liver and white muscle protein turnover rates in the European eel (Anguilla anguilla): Effects of dietary protein quality. Aquaculture 1999, 179, 203–216. [Google Scholar] [CrossRef]
- Huang, D.; Wu, Y.; Lin, Y.; Chen, J.; Karrow, N.; Ren, X.; Wang, Y. Dietary Protein and Lipid Requirements for Juvenile Largemouth Bass, Micropterus salmoides. J. World Aquac. Soc. 2017, 48, 782–790. [Google Scholar] [CrossRef]
- Lin, S.; Chen, Y.; Zhou, W.; Xue, Y.; Zhai, X. Nutritional Regulation Strategies for High-Quality Development of Largemouth Bass. Feed Ind. 2022, 43, 12–17. [Google Scholar] [CrossRef]
- The Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC International: Rockville, MD, USA, 1990. [Google Scholar]
- Yi, C.; Liang, H.; Huang, D.; Yu, H.; Xue, C.; Gu, J.; Chen, X.; Wang, Y.; Ren, M.; Zhang, L. Phenylalanine Plays Important Roles in Regulating the Capacity of Intestinal Immunity, Antioxidants and Apoptosis in Largemouth Bass (Micropterus salmoides). Animals 2023, 13, 2980. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, D.; Ren, M.; Gu, J.; Liang, H. Influence of Eucommia Ulmoides Extract on the Growth, Glucose Metabolism, and Antioxidant Capacity of Largemouth Bass (Micropterus salmoides). Fishes 2025, 10, 269. [Google Scholar] [CrossRef]
- Zhao, F.; Xu, P.; Xu, G.; Huang, D.; Zhang, L.; Ren, M.; Liang, H. Dietary Valine Affects Growth Performance, Intestinal Immune and Antioxidant Capacity in Juvenile Largemouth Bass (Micropterus salmoides). Anim. Feed Sci. Technol. 2023, 295, 115541. [Google Scholar] [CrossRef]
- Yi, C.; Huang, D.; Yu, H.; Gu, J.; Liang, H.; Ren, M. Enzymatically Hydrolyzed Poultry By-Product Supplementation, Instead of Fishmeal, Alone Improves the Quality of Largemouth Bass (Micropterus salmoides) Back Muscle without Compromising Growth. Foods 2023, 12, 3485. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, S.; Wei, H.; Zheng, L.; Liu, Z.; Fang, H.; Xie, J.; Liao, S.; Tian, L.; Liu, Y.; et al. High Dietary Starch Impaired Growth Performance, Liver Histology and Hepatic Glucose Metabolism of Juvenile Largemouth Bass, Micropterus salmoides. Aquac. Nutr. 2020, 26, 1083–1095. [Google Scholar] [CrossRef]
- Li, S.; Sang, C.; Wang, A.; Zhang, J.; Chen, N. Effects of Dietary Carbohydrate Sources on Growth Performance, Glycogen Accumulation, Insulin Signaling Pathway and Hepatic Glucose Metabolism in Largemouth Bass, Micropterus salmoides. Aquaculture 2019, 513, 734391. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Bureau, D.P. Inclusion of Rendered Animal Ingredients as Fishmeal Substitutes in Practical Diets for Cuneate Drum, Nibea miichthioides (Chu, Lo et Wu). Aquac. Nutr. 2007, 13, 81–87. [Google Scholar] [CrossRef]
- Yan, Q.; Zhu, X.; Yang, Y.; Han, D.; Xie, S. Feasibility of Partial Replacement of Fishmeal with Proteins from Different Sources in Diets of Korean Rockfish (Sebastes schlegeli). J. Ocean Univ. China 2014, 13, 1054–1060. [Google Scholar] [CrossRef]
- Lee, J.; Choi, I.C.; Kim, K.T.; Cho, S.H.; Yoo, J.Y. Response of Dietary Substitution of Fishmeal with Various Protein Sources on Growth, Body Composition and Blood Chemistry of Olive Flounder (Paralichthys olivaceus, Temminck & Schlegel, 1846). Fish Physiol. Biochem. 2012, 38, 735–744. [Google Scholar] [CrossRef]
- Song, F.; Xu, D.; Mai, K.; Zhou, H.; Xu, W.; He, G. Comparative Study on the Cellular and Systemic Nutrient Sensing and Intermediary Metabolism after Partial Replacement of Fishmeal by Meat and Bone Meal in the Diet of Turbot (Scophthalmus maximus L.). PLoS ONE 2016, 11, e0165708. [Google Scholar] [CrossRef]
- Nguyen, M.C.; Fotedar, R.; Pham, H.D. Can Shrimp Hydrolysate Improve the Efficacy of Meat and Bone Meal Diet in Juvenile Giant Trevally Caranx Ignobilis? Aquac. Int. 2024, 32, 1909–1926. [Google Scholar] [CrossRef]
- Hossain, M.S.; Kader, M.A.; Dey, T.; Sony, N.M.; Bulbul, M.; Koshio, S. Effect of High Inclusion of Rendered Animal By-Product Ingredients on Growth, Digestibility and Economic Performances in Climbing Perch Anabas testudineus. Aquac. Res. 2017, 48, 931–940. [Google Scholar] [CrossRef]
- Gilbert, E.R.; Wong, E.A.; Webb, K.E. BOARD-INVITED REVIEW: Peptide Absorption and Utilization: Implications for Animal Nutrition and Health. J. Anim. Sci. 2008, 86, 2135–2155. [Google Scholar] [CrossRef]
- Zambonino Infante, J.L.; Cahu, C.L.; Peres, A. Partial Substitution of Di- and Tripeptides for Native Proteins in Sea Bass Diet Improves Dicentrarchus labrax Larval Development. J. Nutr. 1997, 127, 608–614. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, R.; Xue, M.; Xie, S.; Wu, X.; Guo, L. Fishmeal Can Be Completely Replaced by Soy Protein Concentrate by Increasing Feeding Frequency in Nile Tilapia (Oreochromis niloticus GIFT Strain) Less than 2 g: Complete Replacement of Fishmeal in the Feed for Nile Tilapia Fry. Aquac. Nutr. 2010, 16, 648–653. [Google Scholar] [CrossRef]
- Bureau, D.P.; Harris, A.M.; Cho, C.Y. Apparent digestibility of rendered animal protein ingredients for rainbow trout (Oncorhynchus mykiss). Aquaculture 1999, 180, 345–358. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, D.; Gu, J.; Liang, H.; Ren, M. Evaluation of Dietary Vitamin B6 Requirement of Juvenile Largemouth Bass (Micropterus salmoides) on the Basis of Growth Performance, Transaminase Activity, and Nutrient Metabolism. Aquac. Rep. 2025, 42, 102743. [Google Scholar] [CrossRef]
- Li, J.; Xu, W.; Lai, W.; Kong, A.; Zhang, Z.; Pang, Y.; Wang, Z.; Shentu, J.; Wu, X.; Mai, K.; et al. Effect of Dietary Methionine on Growth Performance, Lipid Metabolism and Antioxidant Capacity of Large Yellow Croaker (Larimichthys crocea) Fed with High Lipid Diets. Aquaculture 2021, 536, 736388. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Bakke-McKellep, A.M.; Baeverfjord, G. Effects of Graded Levels of Standard Soybean Meal on Intestinal Structure, Mucosal Enzyme Activities, and Pancreatic Response in Atlantic Salmon (Salmo salar L.). Aquac. Nutr. 2003, 9, 361–371. [Google Scholar] [CrossRef]
- Liu, Q.; Naganuma, T.; Ueno, A.; Tamamura, S.; Murakami, T. Effects of Byproduct Lactic Acid and Byproduct Betaine As Feed Additives on the Metabolomic Profiles of Blood, Meat, and Fat Tissue of Juvenile Bester Sturgeon (Acipenser ruthenus × Huso huso). J. Food Nutr. Res. 2025, 13, 146–155. [Google Scholar] [CrossRef]
- Battaglioni, S.; Benjamin, D.; Wälchli, M.; Maier, T.; Hall, M.N. mTOR Substrate Phosphorylation in Growth Control. Cell 2022, 185, 1814–1836. [Google Scholar] [CrossRef]
- Sabatini, D.M. Twenty-Five Years of mTOR: Uncovering the Link from Nutrients to Growth. Proc. Natl. Acad. Sci. USA 2017, 114, 11818–11825. [Google Scholar] [CrossRef]
- Peterson, T.R.; Laplante, M.; Thoreen, C.C.; Sancak, Y.; Kang, S.A.; Kuehl, W.M.; Gray, N.S.; Sabatini, D.M. DEPTOR Is an mTOR Inhibitor Frequently Overexpressed in Multiple Myeloma Cells and Required for Their Survival. Cell 2009, 137, 873–886. [Google Scholar] [CrossRef]
- Bayne, C.J.; Gerwick, L. The Acute Phase Response and Innate Immunity of Fish. Dev. Comp. Immunol. 2001, 25, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Kushner, I. The Phenomenon of the Acute Phase Response. Ann. N. Y. Acad. Sci. 1982, 389, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, A.; Müller, W.; Eens, M. Vitally Important—Does Early Innate Immunity Predict Recruitment and Adult Innate Immunity? Ecol. Evol. 2016, 6, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wu, X.; Gao, Y.; Gatlin, D.M.; Wu, M.; Yao, W.; Jin, Z.; Li, X.; Dong, Y. Effects of Dietary Carbohydrate Sources on Growth, Digestive Enzyme Activity, Gene Expression of Hepatic GLUTs and Key Enzymes Involved in Glycolysis-Gluconeogenesis of Giant Grouper Epinephelus lanceolatus Larvae. Aquaculture 2018, 484, 343–350. [Google Scholar] [CrossRef]
- Hartviksen, M.; Bakke, A.M.; Vecino, J.G.; Ringø, E.; Krogdahl, Å. Evaluation of the Effect of Commercially Available Plant and Animal Protein Sources in Diets for Atlantic Salmon (Salmo salar L.): Digestive and Metabolic Investigations. Fish Physiol. Biochem. 2014, 40, 1621–1637. [Google Scholar] [CrossRef]
- Romano, N.; Kumar, V.; Yang, G.; Kajbaf, K.; Rubio, M.B.; Overturf, K.; Brezas, A.; Hardy, R. Bile Acid Metabolism in Fish: Disturbances Caused by Fishmeal Alternatives and Some Mitigating Effects from Dietary Bile Inclusions. Rev. Aquac. 2020, 12, 1792–1817. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Hagey, L.R.; Krasowski, M.D. Bile Salts of Vertebrates: Structural Variation and Possible Evolutionary Significance. J. Lipid Res. 2010, 51, 226–246. [Google Scholar] [CrossRef]
- Novriadi, R.; Spangler, E.; Rhodes, M.; Hanson, T.; Allen Davis, D. Effects of Various Levels of Squid Hydrolysate and Squid Meal Supplementation with Enzyme-Treated Soy on Growth Performance, Body Composition, Serum Biochemistry and Histology of Florida Pompano Trachinotus Carolinus. Aquaculture 2017, 481, 85–93. [Google Scholar] [CrossRef]
- Yamamoto, T.; Suzuki, N.; Furuita, H.; Sugita, T.; Tanaka, N.; Goto, T. Supplemental Effect of Bile Salts to Soybean Meal-Based Diet on Growth and Feed Utilization of Rainbow Trout Oncorhynchus Mykiss. Fish. Sci. 2007, 73, 123–131. [Google Scholar] [CrossRef]
- Yu, B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994, 74, 139–162. [Google Scholar] [CrossRef]
- Geronikaki, A.; Gavalas, A. Antioxidants and Inflammatory Disease: Synthetic and Natural Antioxidants with Anti-Inflammatory Activity. Comb. Chem. High Throughput Screen. 2006, 9, 425–442. [Google Scholar] [CrossRef]
- Sekhar, R.V.; Patel, S.G.; Guthikonda, A.P.; Reid, M.; Balasubramanyam, A.; Taffet, G.E.; Jahoor, F. Deficient Synthesis of Glutathione Underlies Oxidative Stress in Aging and Can Be Corrected by Dietary Cysteine and Glycine Supplementation. Am. J. Clin. Nutr. 2011, 94, 847–853. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Quinhones, E.B.; Jung, E.A.C.; Zeni, G.; Rocha, J.B.T. Anti-Inflammatory and Antinociceptive Activity of Diphenyl Diselenide. Inflamm. Res. 2003, 52, 56–63. [Google Scholar] [CrossRef]
- Saiga, A.I.; Tanabe, S.; Nishimura, T. Antioxidant Activity of Peptides Obtained from Porcine Myofibrillar Proteins by Protease Treatment. J. Agric. Food Chem. 2003, 51, 3661–3667. [Google Scholar] [CrossRef]
- Malbrouck, C.; Trausch, G.; Devos, P.; Kestemont, P. Hepatic Accumulation and Effects of Microcystin-LR on Juvenile Goldfish Carassius auratus L. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 135, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Liang, H.; Xu, G.; Zhu, J.; Wang, Y.; Li, S.; Ren, M.; Chen, X. Appropriate Dietary Phenylalanine Improved Growth, Protein Metabolism and Lipid Metabolism, and Glycolysis in Largemouth Bass (Micropterus salmoides). Fish Physiol. Biochem. 2024, 50, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Zhang, M.; Adhikari, B.; Xinfeng, C.; Xu, B. The Effect of Ultrasound-Assisted Immersion Freezing on Selected Physicochemical Properties of Mushrooms. Int. J. Refrig. 2014, 42, 121–133. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive Peptides from Muscle Sources: Meat and Fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef]
- Günzel, D.; Fromm, M. Claudins and Other Tight Junction Proteins. In Comprehensive Physiology; Prakash, Y.S., Ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 1819–1852. ISBN 978-0-470-65071-4. [Google Scholar]
- Cai, W.; Li, X.; Cai, M.; Tang, Z.; Zhu, B.; Yang, M.; Hu, Y.; Dai, J. Effects of Replacing Fishmeal with Soybean Meal on Growth Performance, Liver Antioxidant Capacity and Intestinal Health in Juvenile Asian Red-Tailed Catfish (Hemibagrus wyckioides). Aquac. Rep. 2025, 40, 102646. [Google Scholar] [CrossRef]
- Chen, H.-Q.; Yang, J.; Zhang, M.; Zhou, Y.-K.; Shen, T.-Y.; Chu, Z.-X.; Zhang, M.; Hang, X.-M.; Jiang, Y.-Q.; Qin, H.-L. Lactobacillus plantarum Ameliorates Colonic Epithelial Barrier Dysfunction by Modulating the Apical Junctional Complex and PepT1 in IL-10 Knockout Mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 299, G1287–G1297. [Google Scholar] [CrossRef]
- Song, F.; Xu, D.; Zhou, H.; Xu, W.; Mai, K.; He, G. The Differences in Postprandial Free Amino Acid Concentrations and the Gene Expression of PepT1 and Amino Acid Transporters after Fishmeal Partial Replacement by Meat and Bone Meal in Juvenile Turbot (Scophthalmus maximus L.). Aquac. Res. 2017, 48, 3766–3781. [Google Scholar] [CrossRef]



| Ingredients (%) | EHPBM0 | EHPBM20 | EHPBM50 | EHPBM100 |
|---|---|---|---|---|
| Fish meal a | 30.00 | 24.00 | 15.00 | 0.00 |
| EHPBM b | 0.00 | 7.30 | 18.40 | 36.70 |
| Soy concentrated protein a | 15.60 | 15.60 | 15.60 | 15.60 |
| Wheat gluten | 5.00 | 5.00 | 5.00 | 5.00 |
| Swine blood meal | 5.00 | 5.00 | 5.00 | 5.00 |
| Chicken meal | 12.00 | 12.00 | 12.00 | 12.00 |
| Fish oil | 4.10 | 4.20 | 4.50 | 4.90 |
| Soybean oil | 3.00 | 3.00 | 3.00 | 3.00 |
| Wheat meal a | 5.50 | 5.50 | 5.50 | 5.50 |
| Tapioca starch meal | 6.00 | 6.00 | 6.00 | 6.00 |
| Microcrystalline cellulose | 4.70 | 4.40 | 2.90 | 0.00 |
| Zeolite | 3.90 | 2.60 | 1.30 | 0.00 |
| Calcium dihydrogen phosphate | 2.50 | 2.50 | 2.50 | 2.50 |
| Mineral and Vitamin premix c | 2.00 | 2.00 | 2.00 | 2.00 |
| Choline chloride | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin C | 0.10 | 0.10 | 0.10 | 0.10 |
| Mold inhibitor | 0.05 | 0.05 | 0.05 | 0.05 |
| Antioxidant | 0.05 | 0.05 | 0.05 | 0.05 |
| L-Lysine d | 0.00 | 0.13 | 0.34 | 0.68 |
| DL-Methionine d | 0.00 | 0.09 | 0.22 | 0.44 |
| Analyzed composition | ||||
| Crude protein (%) | 47.56 | 47.84 | 47.92 | 48.08 |
| Crude lipid (%) | 11.11 | 11.14 | 11.25 | 11.74 |
| Ash (%) | 14.41 | 12.90 | 11.91 | 10.02 |
| Energy (KJ/g) | 17.95 | 17.82 | 17.62 | 17.43 |
| Ingredients (%) | Fish Meal | EHPBM |
|---|---|---|
| Crude protein | 65.60 | 53.00 |
| Crude lipid | 9.50 | 4.00 |
| Methionine | 1.85 | 0.34 |
| Lysine | 5.05 | 2.35 |
| Threonine | 2.74 | 1.56 |
| Genes a | (5′-3′) Forward Sequence | (5′-3′) Reverse Sequence | GenBank |
|---|---|---|---|
| gapdh | ACTGTCACTCCTCCATCTT | CACGGTTGCTGTATCCAA | AZA04761.1 |
| Hepatic protein metabolism | |||
| mtor | TTTGGAACCAAACCCCGTCA | ATCAGCTCACGGCAGTATCG | XM_038723321.1 |
| rps6k | TCCAGAGACTCGTGACACCT | AGCTTGGCATACTCTGAGGC | XM_038713349.1 |
| 4ebp1 | CCAGGATCATCTATGACGAAAG | TGCAGCGATATTGTTGTTGTTC | XM_038703879.1 |
| igf-1 | CCTCTGCCTGTGTATAATCA | TGTCCGTCTTAGCCATCT | [45] |
| Hepatic glucose metabolism | |||
| glut2 | GTGTTTGCTGTGCTGCTCCT | GCTCCGTATCGTCTTTGGG | [46] |
| g6pase | ACACAGCAGCCCTGTTCTAC | CCGTTCACACAGTACGGGAT | XM_038735542.1 |
| fbp1 | GCGATTGGCGAATTTATC | ACTCTGTGACGGCGGGTT | [47] |
| pepck | GGCAAAACCTGGAAGCAAGG | ATAATGGCGTCGATGGGGAC | MT431525.1 |
| pk | CACGCAACACTGGCATCATC | TCGAAGCTCTCACATGCCTC | MT431526.1 |
| pfkfb2 | GGTGGCACTGGAAGATGTCA | TGGTGGCATCAAAAACAGCG | XP_018556727.1 |
| g6pdh | ATGTGTCGCAGGAATGAG | TGATGAAGAAGAGGTGTGAA | XP_010777945. |
| Hepatic lipid metabolism | |||
| fabp1 | CTGGAGACTATTACTGGAGAG | ACACAATGCCACCAAGAG | Cluster-21914.4188 |
| acc | TTACATCGCAGCCAACAG | CTCTCCACCTTCCTCTACA | XP_022609673.1 |
| elovl2-l | GGACACAACAATACAAGATGG | GAACAGGTAGCACAGCAAT | Cluster-21914.20999 |
| lpl-1 | CTCCGCAGCCTACACTAA | CCAGCAGATGAATCCTCTC | FJ436090.1 |
| cpt1 | TTACCGTATGGCTATGACTG | GGCTCCGATAACACCTCT | XP_027141042.1 |
| ppar-α | AGGCTTCATCACCAGAGA | TCCGCAGCAGATAATAGTAG | MK614719.1 |
| ppar-γ | GAGTTCTCAGTCAAGTTCAAC | AATGTAGCACCGTCTCCT | MK614721.1 |
| Intestinal barrier and transport | |||
| zo-1 | ATCTCAGCAGGGATTCGACG | CTTTTGCGGTGGCGTTGG | XM_038701018.1 |
| clau | CCAGGGAAGGGGAGCAATG | GCTCTTTGAACCAGTGCGAC | XM_038713307.1 |
| pept1 | CCTATTTGCCTCGCTTTTGGTTGC | CATTAACCTTCGCCGTGAATGGG | MZ773078.1 |
| lat1 | CGCTGCCGAACCCATTTTTG | TTGAGCGTGAGCGTCTTTGT | XM_038706332.1 |
| nf-κb | AGAAGACGACTCGGGGATGA | GCTTCTGCAGGTTCTGGTCT | XM_038699793.1 |
| tnf-α | CTTCGTCTACAGCCAGGCATCG | TTTGGCACACCGACCTCACC | XM_038710731.1 |
| Indexes | EHPBM0 | EHPBM20 | EHPBM50 | EHPBM100 | p1 | p2 |
|---|---|---|---|---|---|---|
| IBW (g) | 6.61 ± 0.03 | 6.60 ± 0.03 | 6.59 ± 0.03 | 6.62 ± 0.03 | 0.348 | 0.791 |
| FBW (g) | 40.40 ± 1.11 b | 37.35 ± 0.46 b | 37.13 ± 0.73 b | 29.66 ± 1.15 a | 0.488 | 0.369 |
| FCR | 0.81 ± 0.02 a | 0.83 ± 0.02 a | 0.85 ± 0.01 a | 1.01 ± 0.03 b | 0.489 | 0.117 |
| WGR (%) | 511.24 ± 17.35 b | 465.68 ± 5.30 b | 463.18 ± 9.46 b | 348.38 ± 19.28 a | 0.429 | 0.276 |
| SGR (%/days) | 3.35 ± 0.05 b | 3.21 ± 0.02 b | 3.20 ± 0.03 b | 2.77 ± 0.08 a | 0.443 | 0.175 |
| SR (%) | 92.50 ± 2.50 | 98.75 ± 1.25 | 91.25 ± 4.27 | 93.75 ± 3.15 | 0.315 | 0.345 |
| FI | 2.15 ± 0.03 a | 2.17 ± 0.02 a | 2.20 ± 0.01 a | 2.36 ± 0.02 b | 0.505 | 0.487 |
| Indices | EHPBM0 | EHPBM20 | EHPBM50 | EHPBM100 | p1 | p2 |
|---|---|---|---|---|---|---|
| Moisture (%) | 71.88 ± 0.18 a | 71.96 ± 0.04 a | 72.80 ± 0.34 ab | 73.46 ± 0.27 b | 0.653 | 0.248 |
| Lipid (%) | 7.37 ± 0.06 b | 7.38 ± 0.07 b | 6.57 ± 0.35 ab | 5.89 ± 0.31 a | 0.592 | 0.090 |
| Protein (%) | 17.07 ± 0.07 | 16.82 ± 0.09 | 16.91 ± 0.20 | 17.09 ± 0.24 | 0.510 | 0.150 |
| Ash (%) | 2.85 ± 0.06 b | 2.78 ± 0.20 ab | 2.23 ± 0.15 a | 2.24 ± 0.08 a | 0.587 | 0.142 |
| Indexes | EHPBM0 | EHPBM20 | EHPBM50 | EHPBM100 | p1 | p2 |
|---|---|---|---|---|---|---|
| SOD (U/mgprot) | 20.55±0.98 a | 80.63±2.11 b | 74.40±2.40 b | 89.11±1.62 c | 0.588 | 0.311 |
| CAT (U/mgprot) | 322.04±6.11 a | 425.35±15.57 b | 464.71±14.09 b | 1622.60±11.68 c | 0.502 | 0.544 |
| MDA (nmol/mgprot) | 1.48±0.08 b | 0.94±0.06 a | 1.11±0.06 a | 1.07±0.12 a | 0.565 | 0.083 |
| GSH (μmol/gprot) | 117.31±4.53 a | 129.58±7.76 a | 121.23±3.17 a | 173.65±8.71 b | 0.670 | 0.404 |
| GSH-Px (U/mgprot) | 26.70±1.80 a | 39.25±1.54 a | 129.11±3.58 b | 133.98±6.60 b | 0.178 | 0.099 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Mi, H.; Huang, D.; Liang, H.; Shan, W.; Ren, M.; Zhang, L.; Teng, T. Effects of Replacing Fishmeal with Enzymatically Hydrolyzed Pork Bone Meal (EHPBM) on Growth, Antioxidant Capacity, and Nutritional Metabolism in Micropterus salmoides. Animals 2025, 15, 3359. https://doi.org/10.3390/ani15233359
Bai X, Mi H, Huang D, Liang H, Shan W, Ren M, Zhang L, Teng T. Effects of Replacing Fishmeal with Enzymatically Hydrolyzed Pork Bone Meal (EHPBM) on Growth, Antioxidant Capacity, and Nutritional Metabolism in Micropterus salmoides. Animals. 2025; 15(23):3359. https://doi.org/10.3390/ani15233359
Chicago/Turabian StyleBai, Xinlan, Haifeng Mi, Dongyu Huang, Hualiang Liang, Wu Shan, Mingchun Ren, Lu Zhang, and Tao Teng. 2025. "Effects of Replacing Fishmeal with Enzymatically Hydrolyzed Pork Bone Meal (EHPBM) on Growth, Antioxidant Capacity, and Nutritional Metabolism in Micropterus salmoides" Animals 15, no. 23: 3359. https://doi.org/10.3390/ani15233359
APA StyleBai, X., Mi, H., Huang, D., Liang, H., Shan, W., Ren, M., Zhang, L., & Teng, T. (2025). Effects of Replacing Fishmeal with Enzymatically Hydrolyzed Pork Bone Meal (EHPBM) on Growth, Antioxidant Capacity, and Nutritional Metabolism in Micropterus salmoides. Animals, 15(23), 3359. https://doi.org/10.3390/ani15233359







