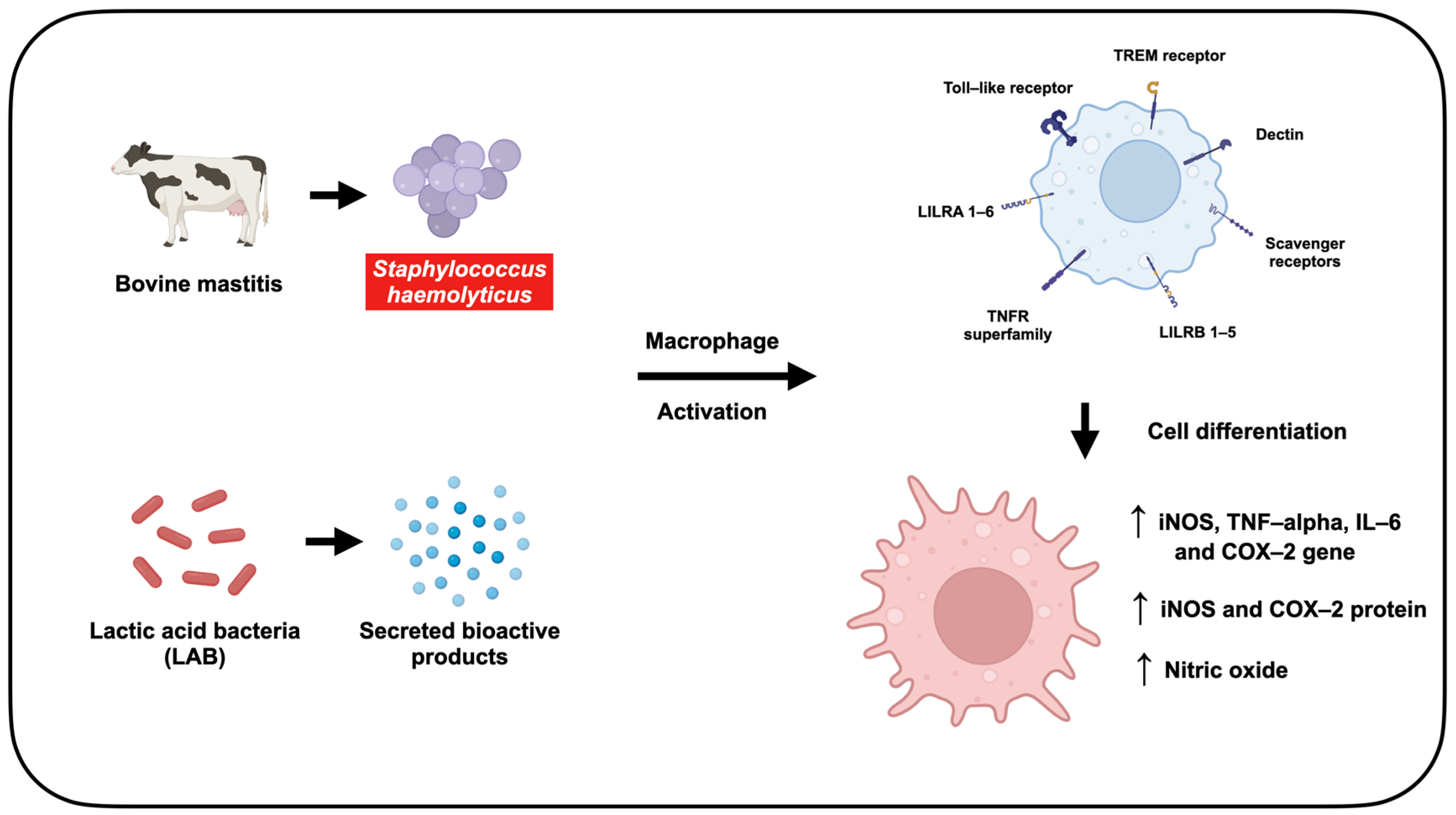
Lactic Acid Bacteria Metabolites Modulate Immune Response Against Staphylococcus haemolyticus-Infected RAW264.7 Murine Macrophage: A Novel Approach for Bovine Mastitis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Bacterial Strain
2.3. Preparation of LAB-Derived Metabolites
2.4. Preparation of Inactivated S. haemolyticus Bacterial Cells
2.5. Cell Culture
2.6. Cell Viability
2.7. Infection Studies with Inactivated S. haemolyticus
2.8. Measurement of Nitric Oxide Production
2.9. Morphological Analysis of Macrophage Activation
2.10. Analysis of Inflammatory Gene Expression by Quantitative Real-Time PCR
2.11. Immunoblotting Analysis of Protein Expression
2.12. DPPH Radical Scavenging Assay
2.13. ABTS Radical Scavenging Assay
2.14. Metabolomic Analysis Using LC-MS/MS
2.15. Statistical Analysis
3. Results
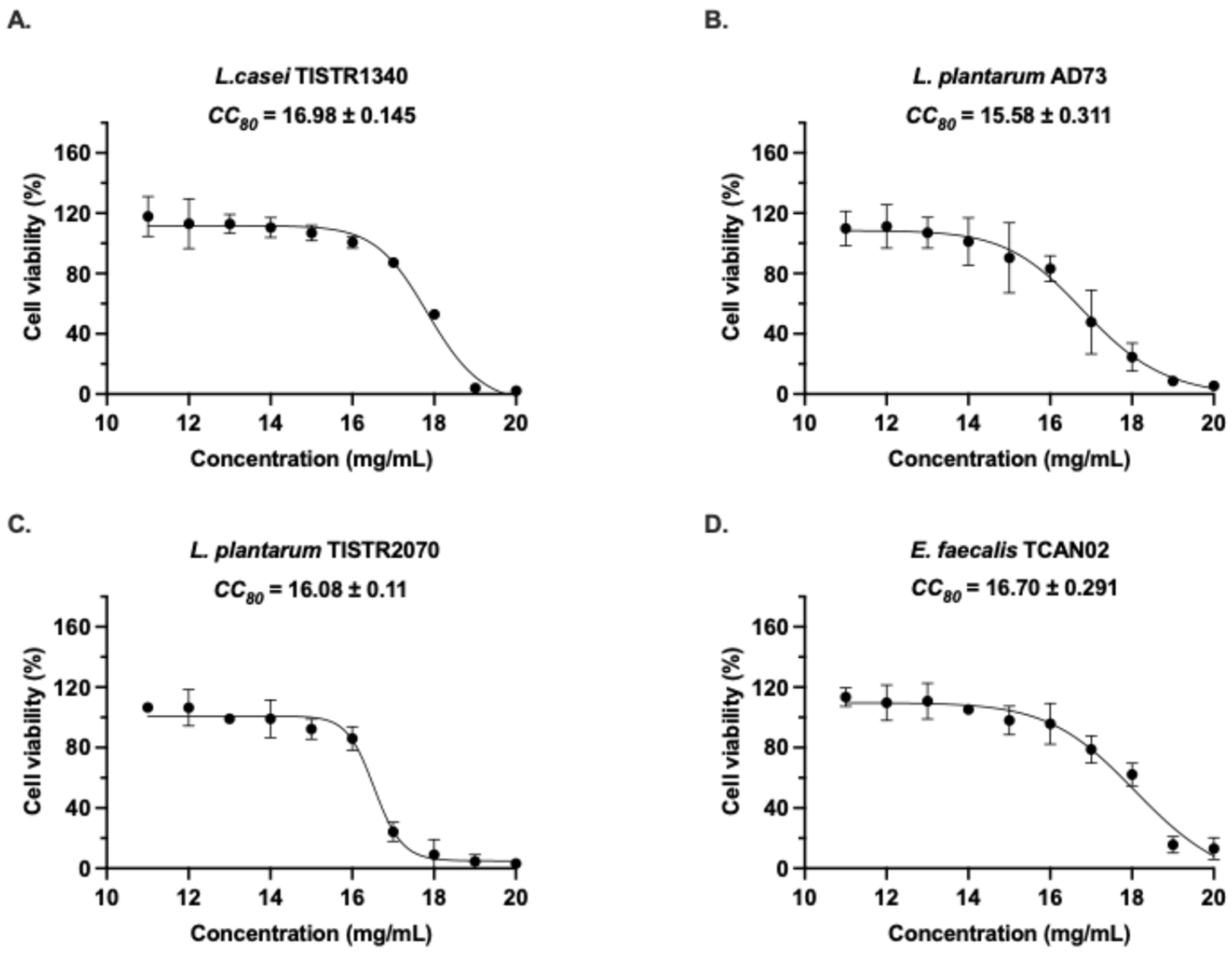
3.1. Cytotoxicity Evaluation of LAB-Derived Metabolites on RAW264.7 Murine Macrophages
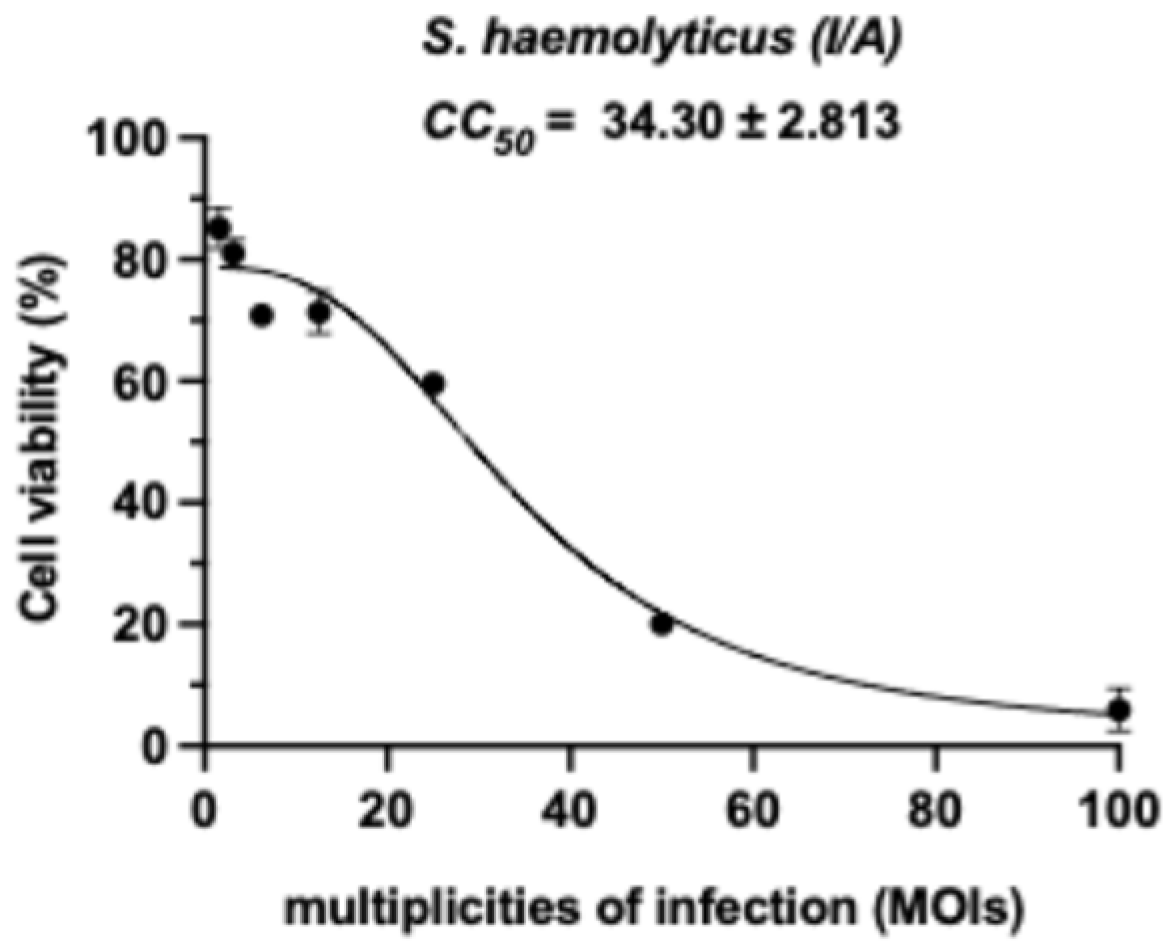
3.2. Cytotoxicity Evaluation of Inactivated S. haemolyticus (I/A) on RAW264.7 Murine Macrophages
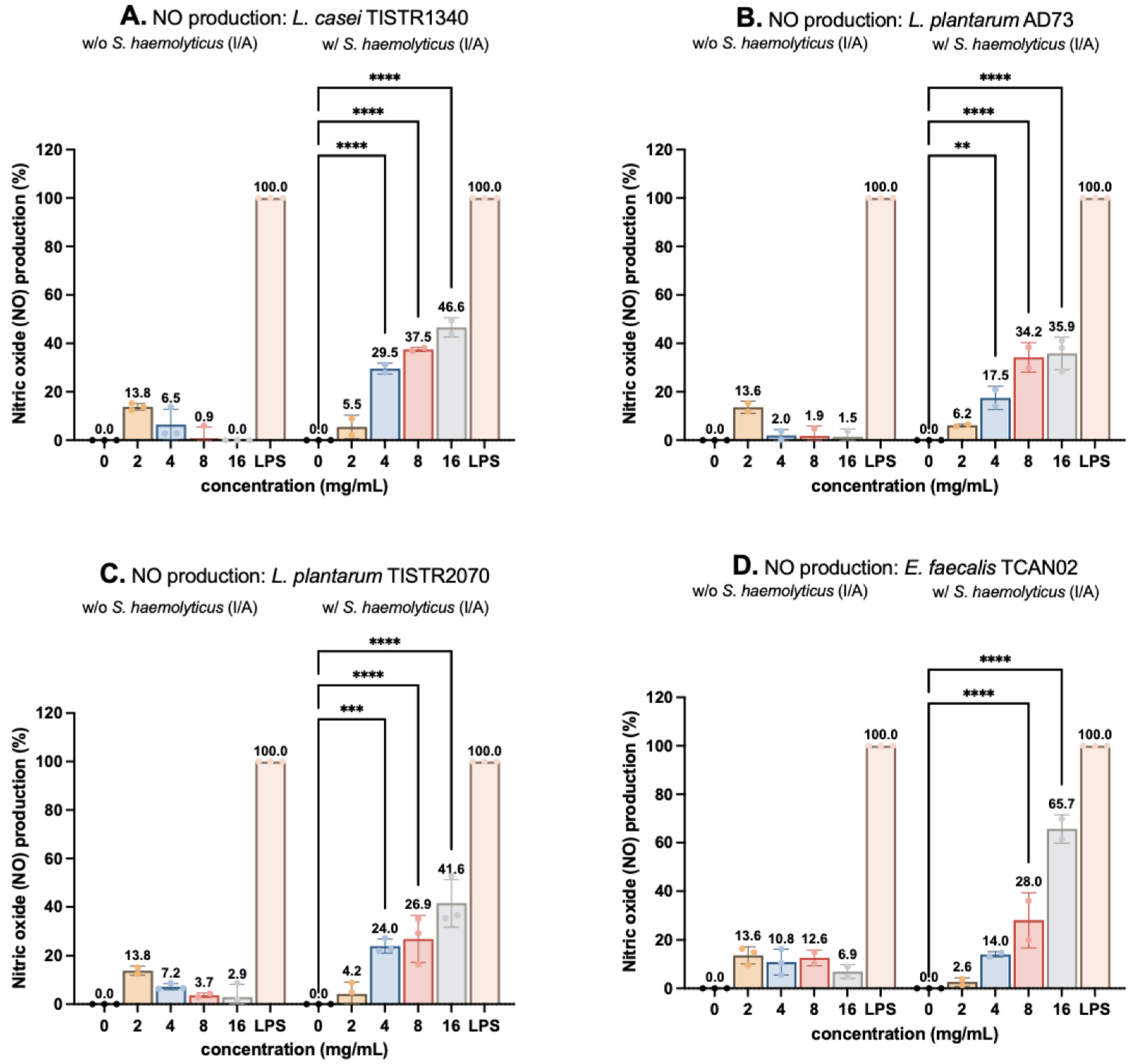
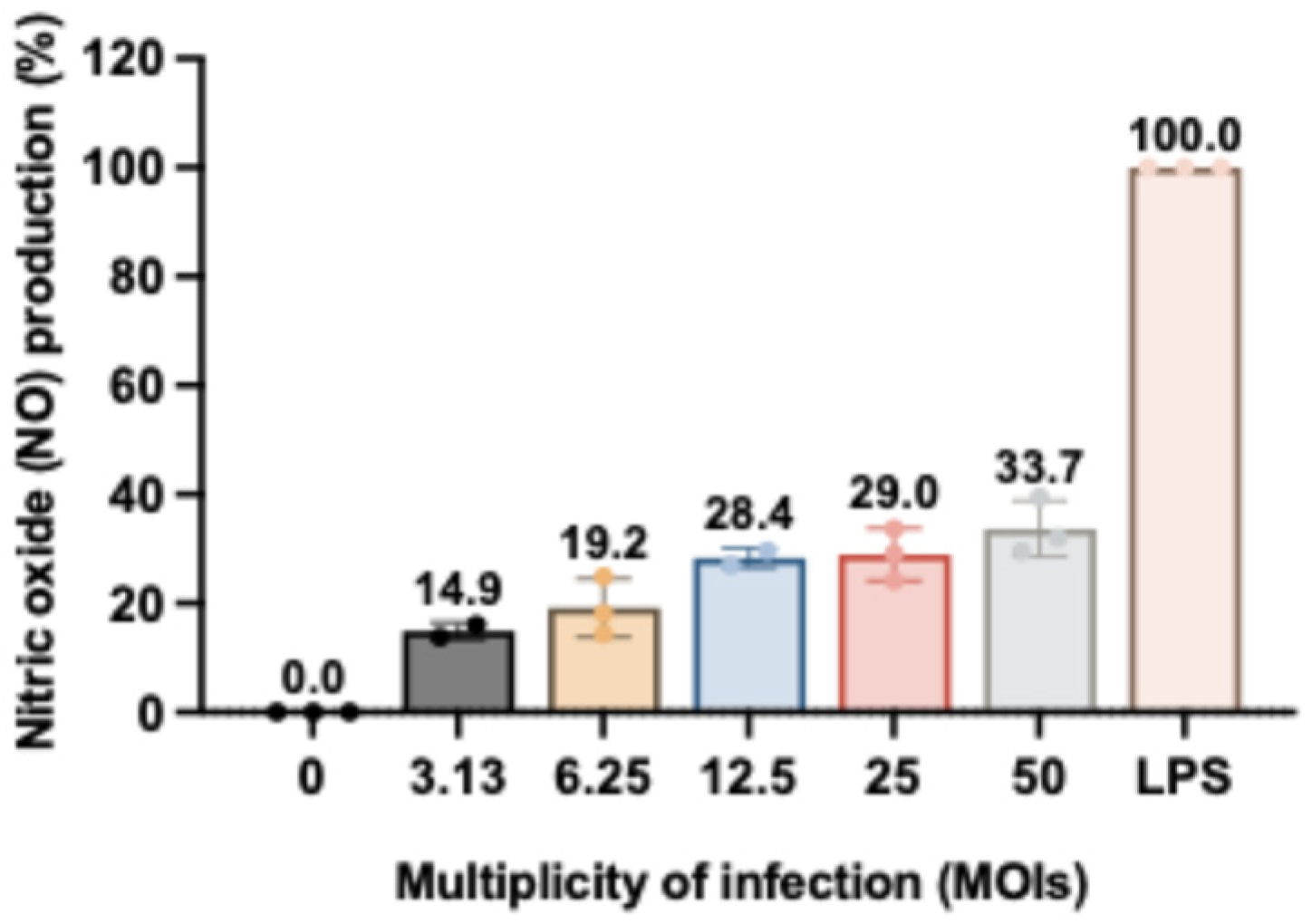
3.3. Nitric Oxide (NO) Production on RAW264.7 Murine Macrophages
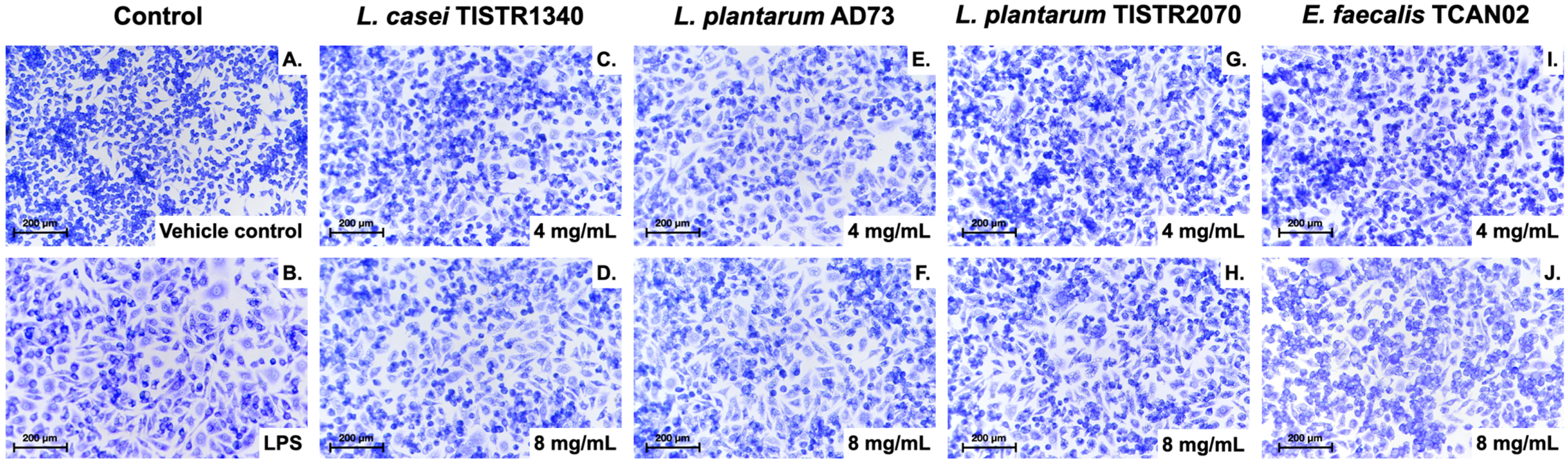
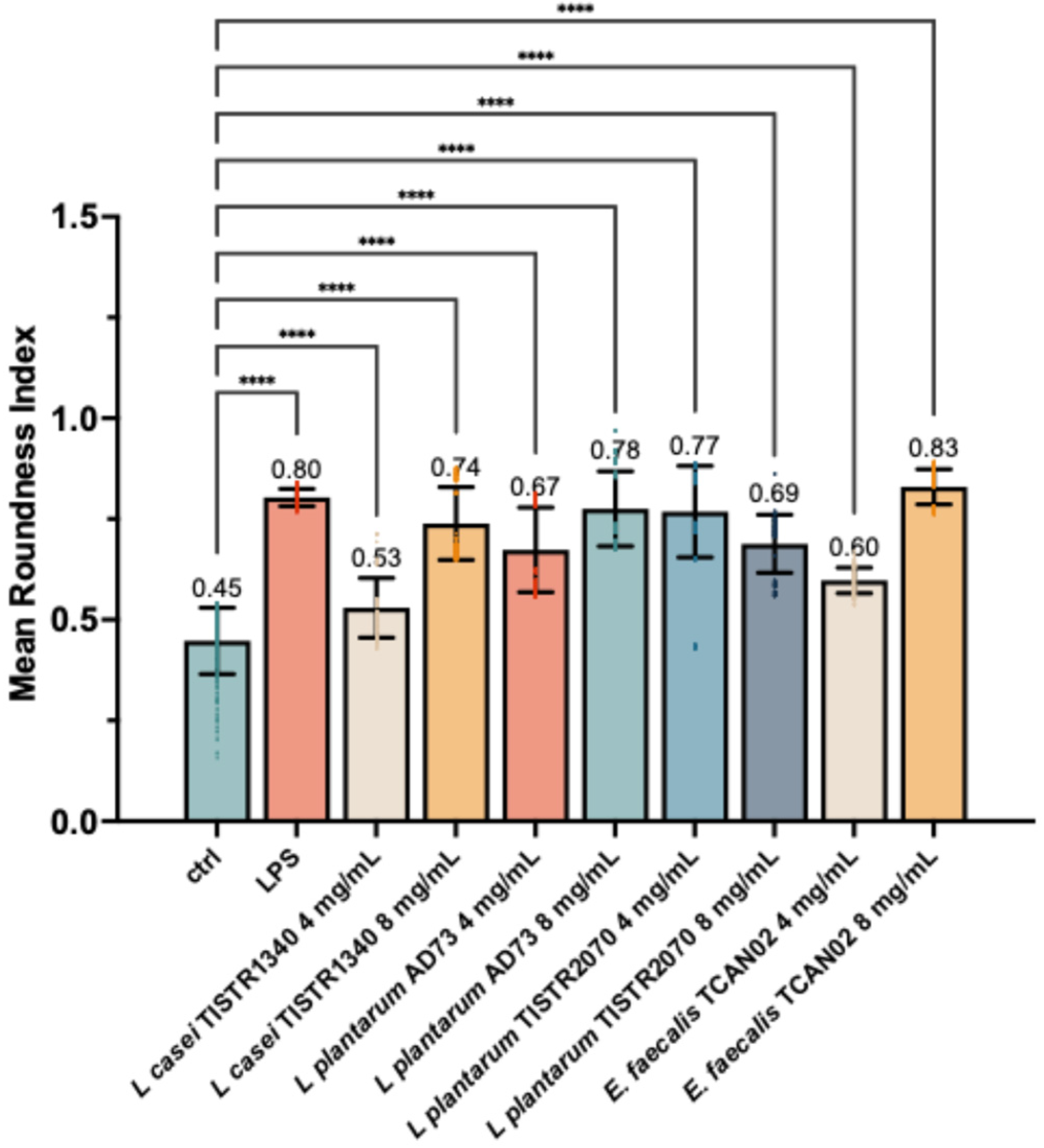
3.4. LAB-Derived Metabolites Induce Distinct Morphological Activation in S. haemolyticus (I/A)-Challenged RAW264.7 Murine Macrophages
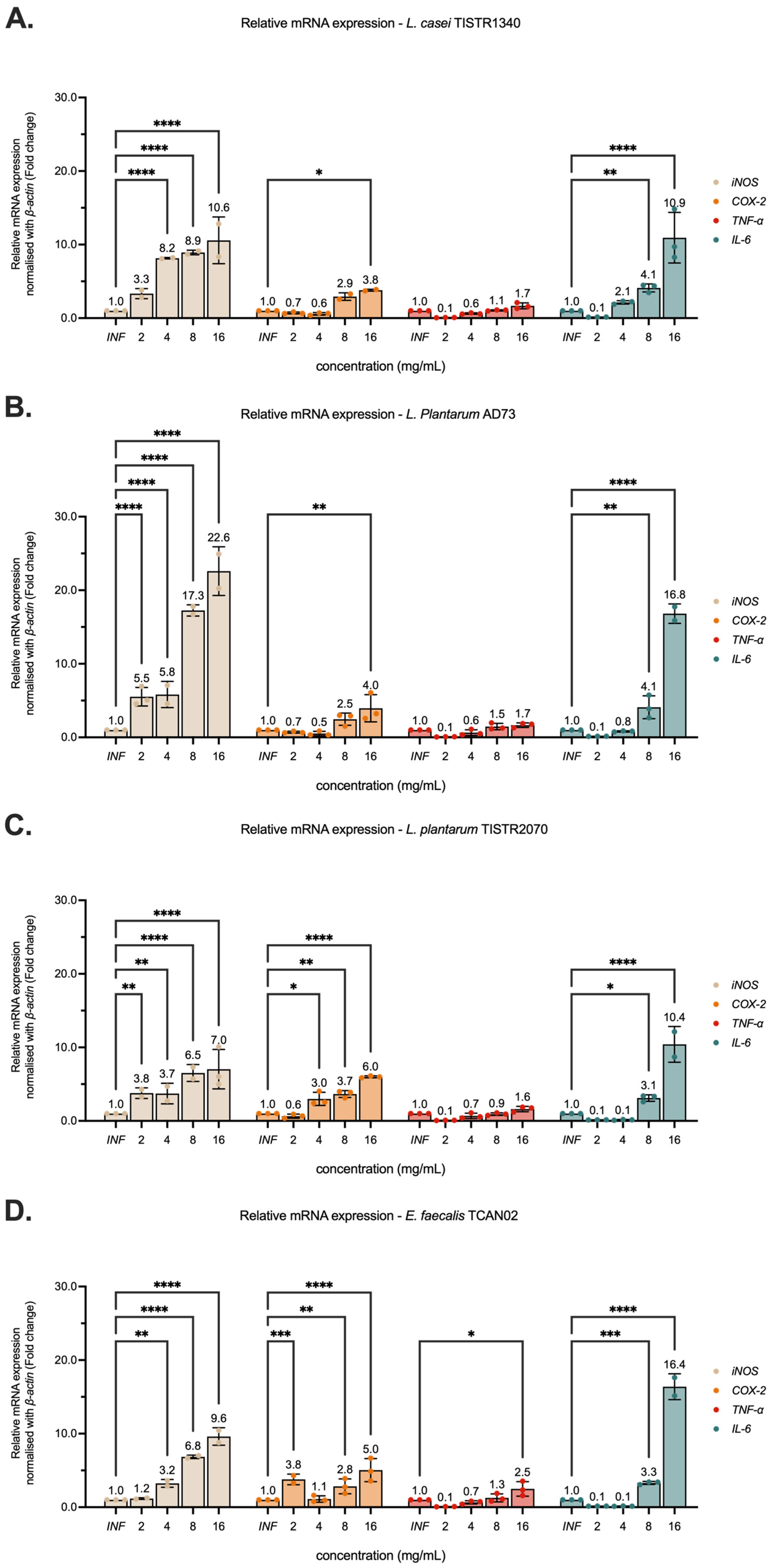
3.5. Modulation of Pro-Inflammatory Cytokine Gene Expression on RAW264.7 Murine Macrophages
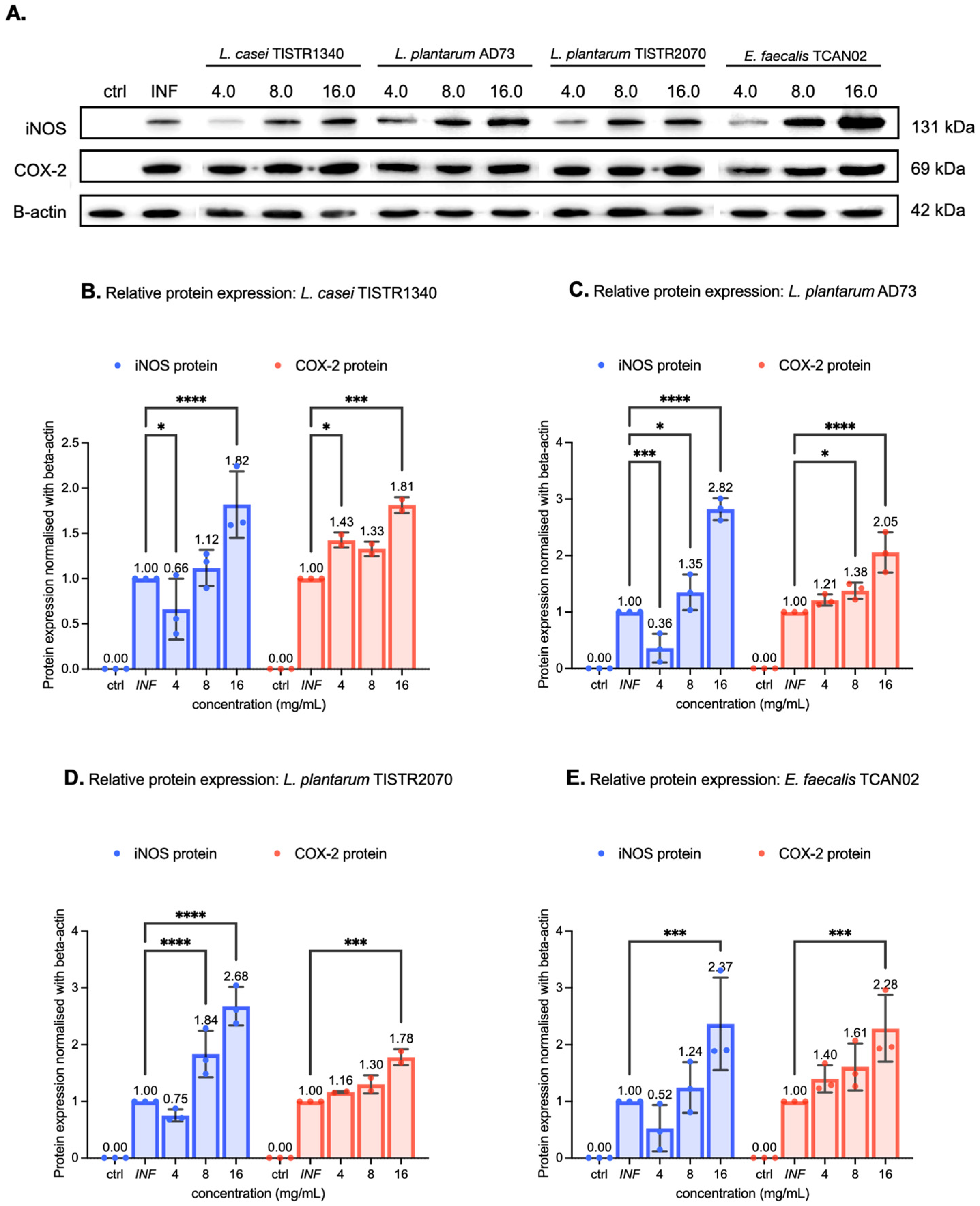
3.6. LAB-Derived Metabolites Potently Upregulate Inflammatory Mediators on RAW264.7 Murine Macrophages Exposed to S. haemolyticus
3.7. Antioxidant Activity of LAB-Derived Metabolites
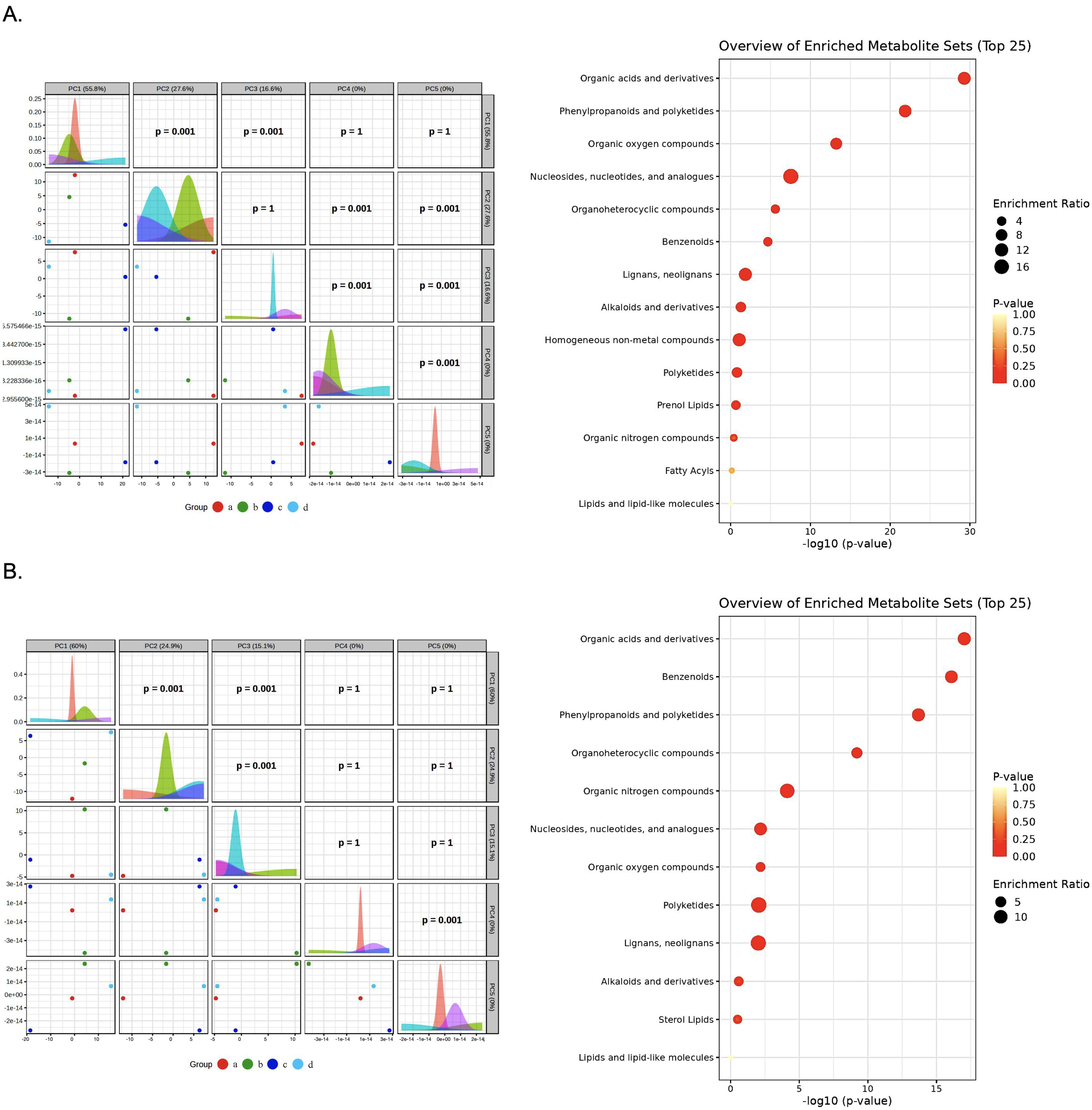
3.8. Multivariate Analysis Reveals Distinct Metabolic Profiles Between Bacterial Species
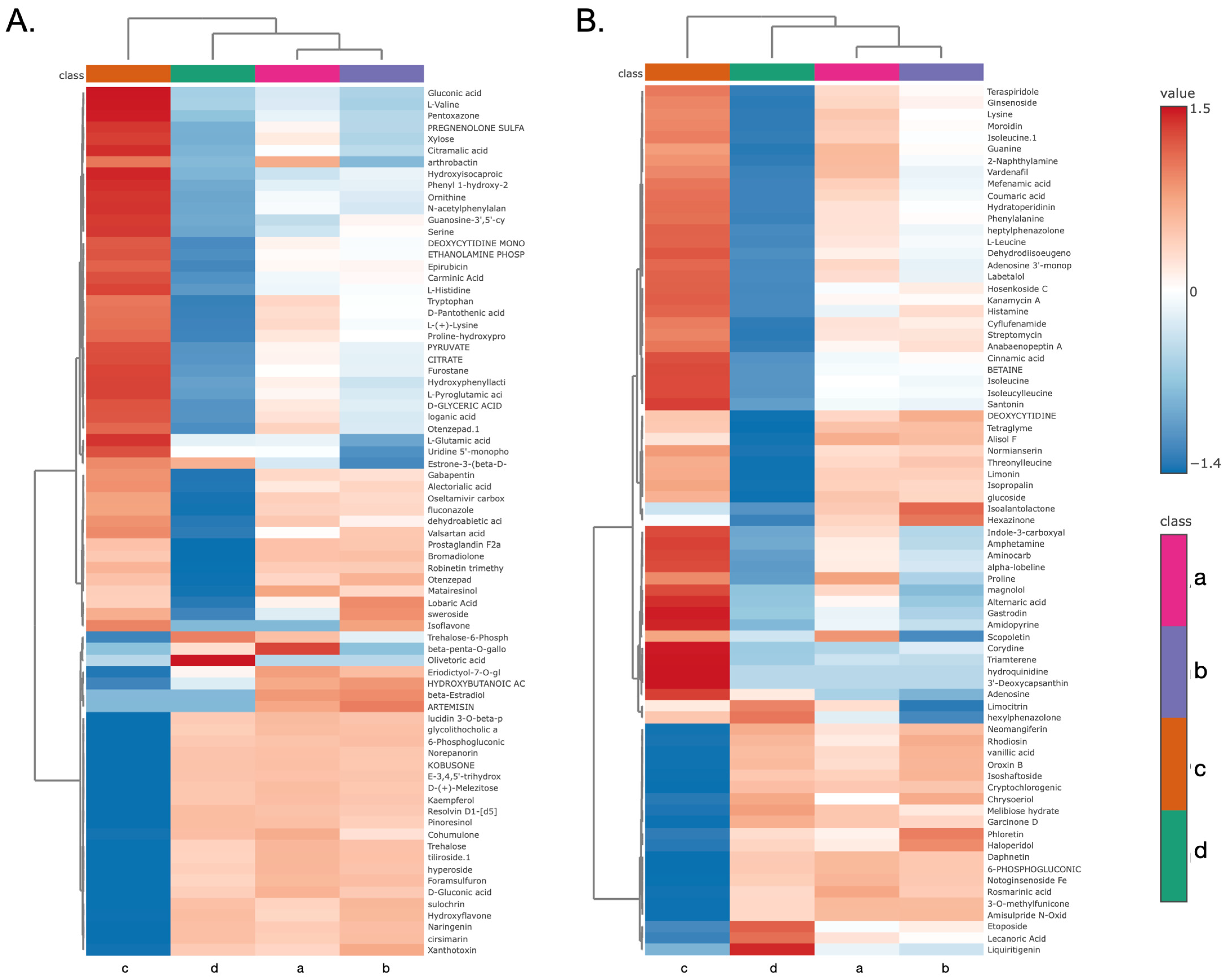
3.9. Hierarchical Clustering and Identification of Metabolites
3.10. Enrichment Analysis Define
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2, 2-azinobis (3-ethylbenzothiazoline-6 -sulfonic acid) |
| COX-2 | Cyclooxygenase-2 |
| DMEM | Dulbecco’s modified eagle’s medium |
| DPPH | 2, 2-diphenyl-1-picrylhydrazyl |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| MTT | 3-(4,5-dimethylthizaol-2-yl)-2,5-diphenyl tetrazolium bromide |
| RT-qPCR | Quantitative reverse transcription polymerase chain reaction |
| TNF- α | Tumor necrosis factor-alpha |
References
- Sinha, M.K.; Thombare, N.; Mondal, B. Subclinical mastitis in dairy animals: Incidence, economics, and predisposing factors. Sci. World J. 2014, 2014, 523984. [Google Scholar] [CrossRef]
- Sathiyabarathi, M.; Jeyakumar, S.; Manimaran, A.; Jayaprakash, G.; Pushpadass, H.A.; Sivaram, M.; Ramesha, K.; Das, D.; Kataktalware, M.A.; Prakash, M.A. Infrared thermography: A potential noninvasive tool to monitor udder health status in dairy cows. Vet. World 2016, 9, 1075. [Google Scholar] [CrossRef]
- Pyörälä, S.; Taponen, S. Coagulase-negative staphylococci—Emerging mastitis pathogens. Vet. Microbiol. 2009, 134, 3–8. [Google Scholar] [CrossRef]
- Persson Waller, K.; Aspán, A.; Nyman, A.; Persson, Y.; Grönlund Andersson, U. CNS species and antimicrobial resistance in clinical and subclinical bovine mastitis. Vet. Microbiol. 2011, 152, 112–116. [Google Scholar] [CrossRef]
- Shoaib, M.; Xu, J.; Meng, X.; Wu, Z.; Hou, X.; He, Z.; Shang, R.; Zhang, H.; Pu, W. Molecular epidemiology and characterization of antimicrobial-resistant Staphylococcus haemolyticus strains isolated from dairy cattle milk in Northwest, China. Front. Cell. Infect. Microbiol. 2023, 13, 1183390. [Google Scholar] [CrossRef] [PubMed]
- Ajose, D.J.; Abolarinwa, T.O.; Oluwarinde, B.O.; Montso, P.K.; Fayemi, O.E.; Aremu, A.O.; Ateba, C.N. Whole genome sequence analysis of multi-drug resistant and biofilm-forming Staphylococcus haemolyticus isolated from bovine milk. BMC Microbiol. 2024, 24, 426. [Google Scholar] [CrossRef] [PubMed]
- Tietze, K.; Dalpke, A.; Morath, S.; Mutters, R.; Heeg, K.; Nonnenmacher, C. Differences in innate immune responses upon stimulation with gram-positive and gram-negative bacteria. J. Periodontal Res. 2006, 41, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Health, National Immunisation Office (NIO). National Database of Antibiotic Resistant Organisms (NDARO). 2022. Available online: https://www.ncbi.nlm.nih.gov/pathogens/antimicrobial-resistance/ (accessed on 17 July 2025).
- Galdeano, C.M.; Perdigón, G. Role of viability of probiotic strains in their persistence in the gut and in mucosal immune stimulation. J. Appl. Microbiol. 2004, 97, 673–681. [Google Scholar] [CrossRef]
- Perdigón, G.; Fuller, R.; Raya, R. Lactic acid bacteria and their effect on the immune system. Curr. Issues Intest. Microbiol. 2001, 2, 27–42. [Google Scholar]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory effects of probiotics on cytokine profiles. BioMed Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef]
- Wallace, T.D.; Bradley, S.; Buckley, N.D.; Green-Johnson, J.M. Interactions of Lactic Acid Bacteria with Human Intestinal Epithelial Cells: Effects on Cytokine Production. J. Food Prot. 2003, 66, 466–472. [Google Scholar] [CrossRef]
- Weid, T.v.d.; Bulliard, C.; Schiffrin, E.J. Induction by a Lactic Acid Bacterium of a Population of CD4+ T Cells with Low Proliferative Capacity That Produce Transforming Growth Factor β and Interleukin-10. Clin. Diagn. Lab. Immunol. 2001, 8, 695–701. [Google Scholar] [CrossRef]
- Arrioja-Bretón, D.; Mani-López, E.; Palou, E.; López-Malo, A. Antimicrobial activity and storage stability of cell-free supernatants from lactic acid bacteria and their applications with fresh beef. Food Control 2020, 115, 107286. [Google Scholar] [CrossRef]
- Dehlink, E.; Domig, K.J.; Loibichler, C.; Kampl, E.; Eiwegger, T.; Georgopoulos, A.; Kneifel, W.; Urbanek, R.; Szepfalusi, Z. Heat-and Formalin-Inactivated Probiotic Bacteria Induce Comparable Cytokine Patterns in Intestinal Epithelial Cell–Leucocyte Cocultures. J. Food Prot. 2007, 70, 2417–2421. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, L.; Liu, K. In vitro anti-inflammatory effects of arctigenin, a lignan from Arctium lappa L., through inhibition on iNOS pathway. J. Ethnopharmacol. 2009, 122, 457–462. [Google Scholar] [CrossRef]
- Suriyaprom, S.; Srisai, P.; Intachaisri, V.; Kaewkod, T.; Pekkoh, J.; Desvaux, M.; Tragoolpua, Y. Antioxidant and Anti-Inflammatory Activity on LPS-Stimulated RAW 264.7 Macrophage Cells of White Mulberry (Morus alba L.) Leaf Extracts. Molecules 2023, 28, 4395. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, D.; Clinkenbeard, K.D. Yersinia pestis Intracellular Parasitism of Macrophages from Hosts Exhibiting High and Low Severity of Plague. PLoS ONE 2012, 7, e42211. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-S.; Lee, S.-H.; Kim, S.-K.; Ahn, C.-B.; Je, J.-Y. Aminoethyl-chitosan inhibits LPS-induced inflammatory mediators, iNOS and COX-2 expression in RAW264. 7 mouse macrophages. Process Biochem. 2011, 46, 465–470. [Google Scholar] [CrossRef]
- Deng, Y.; Li, L.; Zhu, J.H.; Li, P.P.; Deng, Y.X.; Luo, H.H.; Yang, Y.Y.; He, B.C.; Su, Y. COX-2 promotes the osteogenic potential of BMP9 through TGF-β1/p38 signaling in mesenchymal stem cells. Aging 2021, 13, 11336–11351. [Google Scholar] [CrossRef]
- Taddesse, Y.; Im, E.J.; Kwak, D.; Lee, Y.C.; Hyun, E.; Hong, M.; Jiao, P.; Jia, Q.; Goo, Y.-K.; Hong, S.-B. Stellera chamaejasme methanolic extract attenuates nitric oxide production and enhance heme oxygenase 1 expression in murine macrophages. Chiang Mai J. Sci. 2017, 44, 858–868. [Google Scholar]
- He, J.; Li, J.; Liu, H.; Yang, Z.; Zhou, F.; Wei, T.; Dong, Y.; Xue, H.; Tang, L.; Liu, M. Scandoside exerts anti-inflammatory effect via suppressing NF-κB and MAPK signaling pathways in LPS-induced RAW 264.7 macrophages. Int. J. Mol. Sci. 2018, 19, 457. [Google Scholar] [CrossRef] [PubMed]
- Sittisart, P.; Chitsomboon, B. Intracellular ROS Scavenging Activity and Downregulation of Inflammatory Mediators in RAW264.7 Macrophage by Fresh Leaf Extracts of Pseuderanthemum palatiferum. J. Evid. Based Complement. Altern. Med. 2014, 2014, 309095. [Google Scholar] [CrossRef]
- Yao, F.; Xue, Q.; Li, K.; Cao, X.; Sun, L.; Liu, Y. Phenolic Compounds and Ginsenosides in Ginseng Shoots and Their Antioxidant and Anti-Inflammatory Capacities in LPS-Induced RAW264.7 Mouse Macrophages. Int. J. Mol. Sci. 2019, 20, 2951. [Google Scholar] [CrossRef]
- Reshi, A.A.; Husain, I.; Bhat, S.; Rehman, M.U.; Razak, R.; Bilal, S.; Mir, M.R. Bovine mastitis as an evolving disease and its impact on the dairy industry. Int. J. Curr. Res. Rev. 2015, 7, 48. [Google Scholar]
- Gomes, F.; Henriques, M. Control of bovine mastitis: Old and recent therapeutic approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef]
- Menzies, F.D.; Gordon, A.W.; McBride, S.H. An epidemiological study of bovine toxic mastitis. In Proceedings of the British Mastitis Conference 2003, Lancashire, UK, 15–17 September 2003; pp. 1–13. [Google Scholar]
- Leroy, F.; Van Coillie, E.; Braem, G.; Piessens, V.; Verbist, B.; De Vuyst, L.; De Vliegher, S. Subtyping of Staphylococcus haemolyticus isolates from milk and corresponding teat apices to verify the potential teat-skin origin of intramammary infections in dairy cows. J. Dairy Sci. 2015, 98, 7893–7898. [Google Scholar] [CrossRef]
- Awandkar, S.; Bhikane, A.; Kulkarni, M. Antibiotic resistance trends in clinical bovine mastitis. Biolife 2013, 1, 139–143. [Google Scholar]
- Kim, D.-M.; Kim, Y.; Seo, J.-W.; Lee, J.; Park, U.; Ha, N.-Y.; Koh, J.; Park, H.; Lee, J.-W.; Ro, H.-J. Enhanced eosinophil-mediated inflammation associated with antibody and complement-dependent pneumonic insults in critical COVID-19. Cell Rep. 2021, 37, 109798. [Google Scholar] [CrossRef]
- Geng, L.; Hu, W.; Liu, Y.; Wang, J.; Zhang, Q. A heteropolysaccharide from Saccharina japonica with immunomodulatory effect on RAW 264.7 cells. Carbohydr. Polym. 2018, 201, 557–565. [Google Scholar] [CrossRef]
- Liu, W.-B.; Lin, Z.-W.; Zhou, Y.; Ye, B.-C. Overexpression of capsular polysaccharide biosynthesis protein in Lactobacillus plantarum P1 to enhance capsular polysaccharide production for Di-n-butyl phthalate adsorption. J. Microbiol. Biotechnol. 2021, 31, 1545. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Jagelaviciute, J. Investigation of antibacterial activity and probiotic properties of strains belonging to Lactobacillus and Bifidobacterium genera for their potential application in functional food and feed products. Probiotics Antimicro. Prot. 2021, 13, 1387–1403. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095505. [Google Scholar] [CrossRef]
- Mayer, B.; Hemmens, B. Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem. Sci. 1997, 22, 477–481. [Google Scholar] [CrossRef]
- Granger, D.L.; Hibbs, J.; Perfect, J.R.; Durack, D.T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J. Clin. Investig. 1988, 81, 1129–1136. [Google Scholar] [CrossRef]
- Nathan, C.; Shiloh, M.U. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 2000, 97, 8841–8848. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of nitric oxide production in biological systems by using Griess reaction assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]
- Denis, M. Interleukin-6 in mouse hypersensitivity pneumonitis: Changes in lung free cells following depletion of endogenous IL-6 or direct administration of IL-6. J. Leukoc. Biol. 1992, 52, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Ulich, T.R.; Yin, S.; Guo, K.; Yi, E.S.; Remick, D.; del Castillo, J. Intratracheal injection of endotoxin and cytokines. II. Interleukin-6 and transforming growth factor beta inhibit acute inflammation. Am. J. Pathol. 1991, 138, 1097–1101. [Google Scholar] [PubMed]
- Tsan, M.F.; White, J.E.; Del Vecchio, P.J.; Shaffer, J.B. IL-6 enhances TNF-alpha- and IL-1-induced increase of Mn superoxide dismutase mRNA and O2 tolerance. Am. J. Physiol. 1992, 263, L22–L26. [Google Scholar] [CrossRef]
- Lee, A.G.; Kang, S.; Im, S.; Pak, Y.K. Cinnamic acid attenuates peripheral and hypothalamic inflammation in high-fat diet-induced obese mice. Pharmaceutics 2022, 14, 1675. [Google Scholar] [CrossRef]
- Ryyti, R.; Hämäläinen, M.; Leppänen, T.; Peltola, R.; Moilanen, E. Phenolic compounds known to be present in lingonberry (Vaccinium vitis-idaea L.) enhance macrophage polarization towards the anti-inflammatory M2 phenotype. Biomedicines 2022, 10, 3045. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Patil, P.; Raina, P.; Kaul-Ghanekar, R. Matairesinol repolarizes M2 macrophages to M1 phenotype to induce apoptosis in triple-negative breast cancer cells. Immunopharm. Immunotoxicol. 2025, 47, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jia, B.; Shang, J.; Wang, X.; Xu, L.; Liu, X.; Fang, M.; Zeng, F.; Zeng, H.-L.; Gong, Z. Effects of rosmarinic acid on immune response and intestinal microbiota in ovalbumin-induced intestinal allergy mice. J. Sci. Food Agric. 2024, 104, 3002–3012. [Google Scholar] [CrossRef] [PubMed]
| Genes | Sense Primer Sequence 5′-3′ | Antisense Primer Sequence 5′-3′ | References |
|---|---|---|---|
| iNOS | TTCCAGAATCCCTGGACAAGC | TGGTCAAACTCTTGGGGTTCG | [20] |
| COX-2 | AGAAGGAAATGGCTGCAGAA | GCTCGGCTTCCAGTATTGAG | [21] |
| TNF-α | AGCCCCCAGTCTGTATCCTTC | CATTCGAGGCTCCAGTGAATTCG | [20] |
| IL-6 | GCTGGAGTCACAGAAGGAGTG | GCATAACGCACTAGGTTTGCC | [22] |
| β-actin | TGCTGTCCCTGTATGCCTCTG | GCTGTAGCCACGCTCGGTCA | [23] |
| Bacterial Strain | Antioxidant Activity | |
|---|---|---|
| DPPH Assay (mg GAE/g Sample) | ABTS Assay (mg TE/g Sample) | |
| L. casei TISTR 1340 | 1.84 ± 0.21 * | 12.11 ± 0.99 |
| L. plantarum TISTR 2070 | 1.65 ± 0.15 | 9.64 ± 0.13 |
| L. plantarum AD73 | 1.59 ± 0.01 | 14.97 ± 2.52 ** |
| E. faecalis TCAN02 | 1.25 ± 0.36 | 7.44 ± 0.10 |
| Mode of Detection | Metabolites | Ontology | Predominant Strain(s) |
|---|---|---|---|
| Negative mode | gluconic acid | carbohydrate-derived acids | L. plantarum AD73 |
| glutamic acid | Alpha amino acids and derivatives | L. plantarum AD73 | |
| Kaempferol | Flavonoid-O-glycosides | E. faecalis TCAN02 | |
| lobaric acid | Depsides and depsidones | L. casei TISTR1340 | |
| matairesinol | Phenylpropanoid | E. faecalis TCAN02 | |
| Olivetoric acid | Phenolic Acid | L. plantarum TISTR2070 | |
| sweroside | secoiridoid glycosides | L. casei TISTR1340 | |
| Positive mode | Adenosine | Purine nucleosides | L. plantarum AD73 |
| Chrysoeriol | 3′-O-methylated flavonoids | E. faecalis TCAN02 | |
| Cinnamic acid | Hydroxycinnamic acids | L. plantarum AD73 | |
| gastrodin | Phenolic glucoside | L. plantarum AD73 | |
| Genistein | Isoflavones | E. faecalis TCAN02 | |
| ginsenoside | Triterpenoids | L. plantarum TISTR2070 | |
| Isoscopoletin | 8-prenylated flavones | L. plantarum AD73 | |
| limocitrin | Flavonols | L. plantarum TISTR2070 | |
| Luteolin | Flavonoid 8-C-glycosides | E. faecalis TCAN02 | |
| phloretin | 2′-Hydroxy-dihydrochalcones | L. casei TISTR1340 | |
| Rosmarinic acid | Coumaric acids and derivatives | E. faecalis TCAN02 | |
| scopoletin | 7-hydroxycoumarins | E. faecalis TCAN02 | |
| vanillic acid | M-methoxybenzoic acids and derivatives | L. plantarum AD73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheepchirasuk, N.; Suriyaprom, S.; Kaewkod, T.; Intachaisri, V.; Chitov, T.; Panya, A.; Suriyasathaporn, W.; Tragoolpua, Y. Lactic Acid Bacteria Metabolites Modulate Immune Response Against Staphylococcus haemolyticus-Infected RAW264.7 Murine Macrophage: A Novel Approach for Bovine Mastitis. Animals 2025, 15, 3338. https://doi.org/10.3390/ani15223338
Cheepchirasuk N, Suriyaprom S, Kaewkod T, Intachaisri V, Chitov T, Panya A, Suriyasathaporn W, Tragoolpua Y. Lactic Acid Bacteria Metabolites Modulate Immune Response Against Staphylococcus haemolyticus-Infected RAW264.7 Murine Macrophage: A Novel Approach for Bovine Mastitis. Animals. 2025; 15(22):3338. https://doi.org/10.3390/ani15223338
Chicago/Turabian StyleCheepchirasuk, Nitsanat, Sureeporn Suriyaprom, Thida Kaewkod, Varachaya Intachaisri, Thararat Chitov, Aussara Panya, Witaya Suriyasathaporn, and Yingmanee Tragoolpua. 2025. "Lactic Acid Bacteria Metabolites Modulate Immune Response Against Staphylococcus haemolyticus-Infected RAW264.7 Murine Macrophage: A Novel Approach for Bovine Mastitis" Animals 15, no. 22: 3338. https://doi.org/10.3390/ani15223338
APA StyleCheepchirasuk, N., Suriyaprom, S., Kaewkod, T., Intachaisri, V., Chitov, T., Panya, A., Suriyasathaporn, W., & Tragoolpua, Y. (2025). Lactic Acid Bacteria Metabolites Modulate Immune Response Against Staphylococcus haemolyticus-Infected RAW264.7 Murine Macrophage: A Novel Approach for Bovine Mastitis. Animals, 15(22), 3338. https://doi.org/10.3390/ani15223338





