The Effects of Added Cellulases and Pectinases on Ruminal Fermentation Parameters and Bacterial Communities in Goats Supplemented with Macadamia Integrifolia Husks: An In Vitro Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Macadamia Integrifolia Husk Sample Collection
2.2. Experimental Design and Substrate Preparation
2.3. In Vitro Experimental and Sample Collected
2.4. Sample Analysis
2.5. Statistical Analysis
3. Results
3.1. Gas Production and In Vitro Nutrient Digestibility
3.2. In Vitro Fermentation Parameters in the Fermentation Fluid at 48 h
3.3. Bacterial Community Composition in the Fermentation Fluid at 48 h
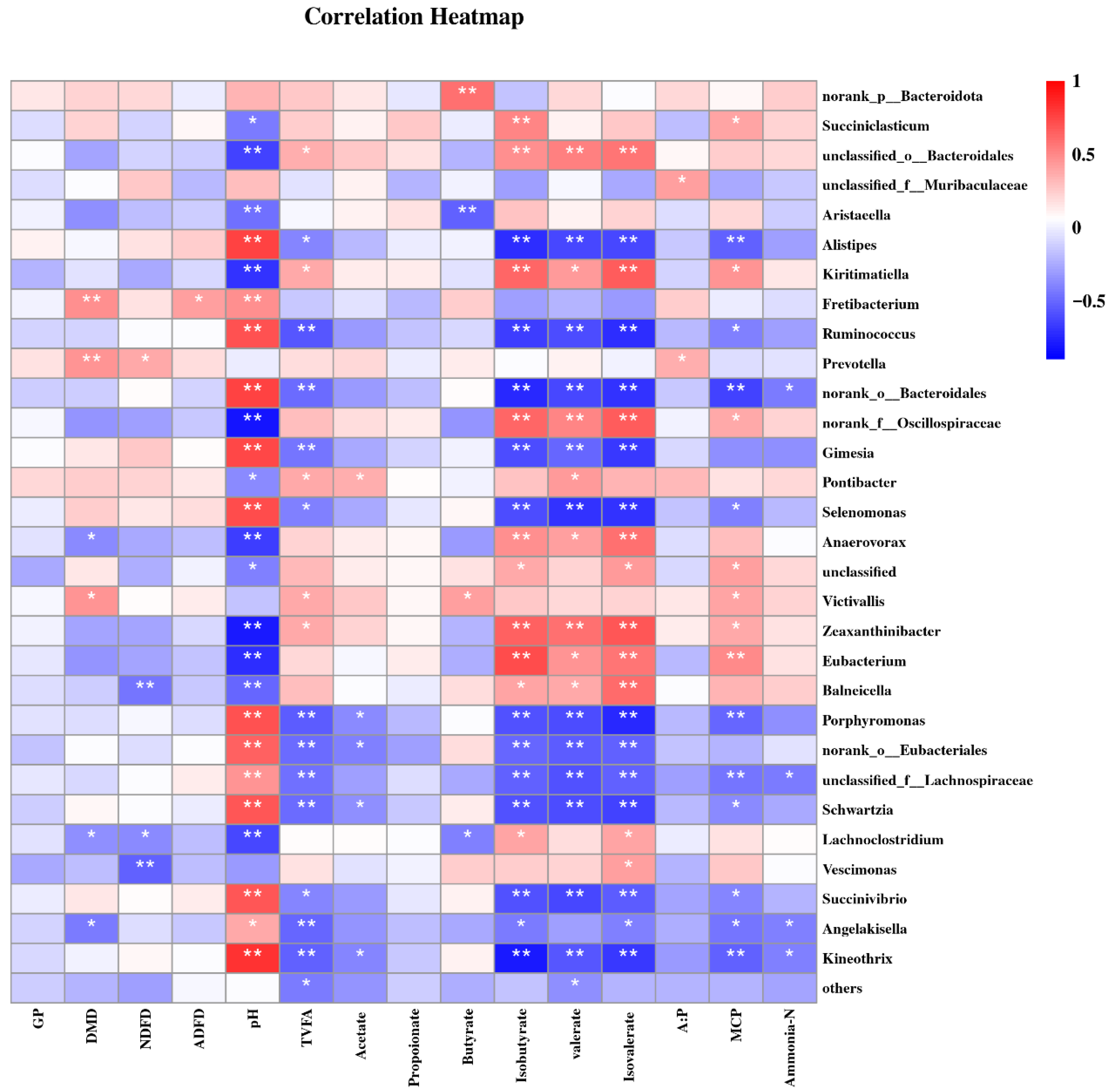
3.4. In Vitro Rumen Fermentation Parameters Correlated with Bacterial Community Composition
4. Discussion
4.1. Effect of Macadamia Integrifolia Husk with Supplementary Cellulases and Pectinases on Gas Production and Nutrients Digestibility in Goats
4.2. Effect of Macadamia Integrifolia Husk with Supplementary Cellulases and Pectinases on Rumen Fermentation Parameters in Goats
4.3. Effect of Macadamia Integrifolia Husk with Supplementary Cellulases and Pectinases on Bacterial Community Composition in Goats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tu, X.H.; Wu, B.F.; Xie, Y.; Xu, S.L.; Wu, Z.Y.; Lv, X.; Wei, F.; Du, L.Q.; Chen, H. A comprehensive study of raw and roasted macadamia nuts: Lipid profile, physicochemical, nutritional, and sensory properties. Food Sci. Nutr. 2021, 9, 1688–1697. [Google Scholar] [CrossRef]
- Guo, Q.; Barkla, B.J.; Barker, R.; Liu, L. Genotype-Driven proteomic diversity in macadamia nuts: Implications for allergenicity, nutritional quality, and breeding strategies. J. Agric. Food Chem. 2025, 73, 22272–22282. [Google Scholar] [CrossRef]
- Shuai, X.; Dai, T.; Chen, M.; Liang, R.; Du, L.; Chen, J.; Liu, C. Comparative Study of Chemical Compositions and Antioxidant Capacities of Oils Obtained from 15 Macadamia (Macadamia integrifolia) Cultivars in China. Foods 2021, 10, 1031. [Google Scholar] [CrossRef]
- Yang, F.; Tang, J.; Fu, X.M.; Tang, S.P.; Yang, H.X.; Dong, M.C.; Luo, X.P. Overview and prospect of production and marketing situation of macadamia in China. Fruit. Trees S. China 2025, 54, 1–5. (In Chinese) [Google Scholar] [CrossRef]
- Ahmed, M.F.; Popovich, D.G.; Whitby, C.P.; Rashidinejad, A. Phenolic compounds from macadamia husk: An updated focused review of extraction methodologies and antioxidant activities. Food Bioprod. Process 2024, 148, 165–175. [Google Scholar] [CrossRef]
- Yang, K.; Qing, Y.; Yu, Q.; Tang, X.; Chen, G.; Fang, R.; Liu, H. By-Product feeds: Current understanding and future perspectives. Agriculture 2021, 11, 207. [Google Scholar] [CrossRef]
- Haider, M.W.; Abbas, S.M.; Saeed, M.A.; Farooq, U.; Waseem, M.; Adil, M.; Tutu, C. Osei. Environmental and nutritional value of fruit and vegetable peels as animal feed: A comprehensive review. Anim. Res. One Health 2025, 3, 149–164. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, H.; Wang, Z.; Lan, X.; An, J.; Shen, W.; Wan, F. Recent advances in feed and nutrition of beef cattle in China—A review. Anim. Biosci. 2023, 36, 529–539. [Google Scholar] [CrossRef]
- Sakita, G.Z.; Bompadre, T.F.V.; Dineshkumar, D.; Lima, P.M.T.; Abdalla Filho, A.L.; Campioni, T.S.; de Oliva Neto, P.; Bremer Neto, H.; Louvandini, H.; Abdalla, A.L. Fibrolytic enzymes improving in vitro rumen degradability of tropical forages. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1267–1276. [Google Scholar] [CrossRef]
- Coblentz, W.K.; Akins, M.S. Silage review: Recent advances and future technologies for baled silages. J. Dairy Sci. 2018, 101, 4075–4092. [Google Scholar] [CrossRef]
- Musati, M.; Hervás, G.; Natalello, A.; Toral, P.G.; Luciano, G.; Priolo, A.; Frutos, P. Could we partially replace maize with nut skins for more sustainable sheep diets? In vitro ruminal fermentation and biohydrogenation. Anim. Feed. Sci. Technol. 2024, 318, 116113. [Google Scholar] [CrossRef]
- Musati, M.; Frutos, P.; Bertino, A.; Hervás, G.; Luciano, G.; Forte, C.; Priolo, A.; Lanza, M.; Bella, M.; Biondi, L.; et al. Dietary combination of linseed and hazelnut skin as a sustainable strategy to enrich lamb with health promoting fatty acids. Sci. Rep. 2024, 14, 10133. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Z.; Pei, C.; Degen, A.; Hao, L.; Cao, X.; Liu, H.; Zhou, J.; Long, R. A comparison between yaks and Qaidam cattle in in vitro rumen fermentation, methane emission, and bacterial community composition with poor quality substrate. Anim. Feed. Sci. Technol. 2022, 291, 115395. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Washington, DC, USA, 2012. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy. Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Robertson, J.B.; Van Soest, P.J. The detergent system of analysis and its application to human foods. In The Analysis of Dietary Fibres in Food; James, W.P., Theander, O., Eds.; Marcel Dekker: New York, NY, USA, 1981; pp. 23–158. [Google Scholar]
- Hristov, A.N.; Ivan, M.; Rode, L.M.; McAllister, T.A. Fermentation characteristics and ruminal ciliate protozoal population in cattle fed medium- or high-concentrate barley-based diets. J. Anim. Sci. 2001, 79, 515–524. [Google Scholar] [CrossRef]
- Makkar, H.; Sharma, O.; Dawra, R.; Negi, S. Simple determination of microbial protein in rumen liquor. J. Dairy Sci. 1982, 65, 2170–2173. [Google Scholar] [CrossRef]
- Wang, B.; Mao, S.Y.; Yang, H.J.; Wu, Y.M.; Wang, J.K.; Li, S.L.; Shen, Z.M.; Liu, J.X. Effects of alfalfa and cereal straw as a forage source on nutrient digestibility and lactation performance in lactating dairy cows. J. Dairy. Sci. 2014, 97, 7706–7715. [Google Scholar] [CrossRef]
- Wei, M.; Cui, Z.; Li, J.; Yan, P. Estimation of metabolisable energy and net energy of rice straw and wheat straw for beef cattle by indirect calorimetry. Arch. Anim. Nutr. 2018, 72, 275–289. [Google Scholar] [CrossRef]
- Shen, R.R.; Sun, X.Y.; Liu, B.; Li, Y.Q.; Gao, Y.X.; Li, J.G.; Cao, Y.F.; Li, Q.F. Effects of different compound microorganism preparations on fermentation quality, nutritional components and rumen degradation rate of mixed silage of potato pulp and soybean straw. Chin. J. Anim. Nutr. 2019, 31, 3319–3329. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Z.J.; Guo, T.J.; Zhao, J.; Sang, D.J.; Shi, Y.; Cui, J.W. Effects of steam explosion and fermentation after steam explosion on nutrient value of cotton stalk. Chin. J. Anim. Nutr. 2018, 30, 3720–3725. (In Chinese) [Google Scholar]
- Yang, Z.; Zheng, Y.; Liu, S.; Xie, T.; Wang, Q.; Wang, Z.; Li, S.; Wang, W. Rumen metagenome reveals the mechanism of mitigation methane emissions by unsaturated fatty acid while maintaining the performance of dairy cows. Anim. Nutr. 2024, 18, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, S.; Razmazar, V.; Mirshekar, R. Gas Production: A Proposed in vitro Method to Estimate the Extent of Digestion of a Feedstuff in the Rumen. J. Biol. Sci. 2010, 10, 573–580. [Google Scholar] [CrossRef]
- Christodoulou, C.; Kliem, K.E.; Auffret, M.D.; Humphries, D.J.; Newbold, J.R.; Davison, N.; Crompton, L.; Dhanoa, M.S.; Smith, L.G.; Stergiadis, S. In vitro rumen degradation, fermentation, and methane production of four agro-industrial protein-rich co-products, compared with soyabean meal. Anim. Feed. Sci. Technol. 2025, 319, 116151. [Google Scholar] [CrossRef]
- Bugoni, M.; Takiya, C.S.; Grigoletto, N.T.S.; Vittorazzi Júnior, P.C.; Nunes, A.T.; Chesini, R.G.; da Silva, G.G.; Durman, T.; Pettigrew, J.E.; Rennó, F.P. Feeding amylolytic and proteolytic exogenous enzymes: Effects on nutrient digestibility, ruminal fermentation, and performance in dairy cows. J. Dairy Sci. 2023, 106, 3192–3202. [Google Scholar] [CrossRef]
- Liu, Z.; Li, W.; Zhao, C.; Zhang, Y.; Li, Y.; Wang, L.; Li, X.; Yao, J.; Pellikaan, W.F.; Cao, Y. Effects of fibrolytic and amylolytic compound enzyme preparation on rumen fermentation, serum parameters and production performance in primiparous early-lactation dairy cows. J. Dairy Res. 2024, 91, 167–170. [Google Scholar] [CrossRef]
- Togtokhbayar, N.; Cerrillo, M.A.; Rodríguez, G.B.; Elghandour, M.M.; Salem, A.Z.; Urankhaich, C.; Jigjidpurev, S.; Odongo, N.E.; Kholif, A.E. Effect of exogenous xylanase on rumen in vitro gas production and degradability of wheat straw. Anim. Sci. J. 2015, 86, 765–771. [Google Scholar] [CrossRef]
- Zhang, M.; Qiu, Q.; Zhao, X.; Ouyang, K.; Liu, C. Characterization of novel multifunctional xylanase from rumen metagenome and its effects on in vitro microbial fermentation of wheat straw. Fermentation 2024, 10, 574. [Google Scholar] [CrossRef]
- Azzaz, H.H.; Murad, H.A.; Hassaan Noha, A.; Fahmy, M. Pectinase Production Optimization for Improving Dairy Animal’s Diets Degradation. Int. J. Dairy Sci. 2020, 15, 54–61. [Google Scholar] [CrossRef]
- Li, M.M.; White, R.R.; Guan, L.L.; Harthan, L.; Hanigan, M.D. Metatranscriptomic analyses reveal ruminal pH regulates fiber degradation and fermentation by shifting the microbial community and gene expression of carbohydrate-active enzymes. Anim. Microbiome 2021, 3, 32. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Newbold, J.R. Effect of ammonia concentration on rumen microbial protein production in vitro. Br. J. Nutr. 2022, 127, 847–849. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Mantovani, H.C.; Vedovatto, M.; Cardoso, A.S.; Rodrigues, A.A.; Homem, B.G.C.; de Abreu, M.J.I.; Rodrigues, A.N.; Cursino Batista, L.H.; de Oliveira, J.S.; et al. Impact of dietary exogenous feed enzymes on performance, nutrient digestibility, and ruminal fermentation parameters in beef cattle: A meta-analysis. Animal 2025, 19, 101481. [Google Scholar] [CrossRef] [PubMed]
- NASEM (The National Academies of Sciences, Engineering, and Medicine). Nutrient Requirements of Beef Cattle, 8th ed.; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Tan, Z.; Wang, L.; Wang, Z.; Xue, B.; Hu, R.; Peng, Q.H.; Xiao, J.X. Supplementing NSP enzymes in high concentrate diets can prevent foamy rumen bloat in goats. Sci. Rep. 2025, 15, 5127. [Google Scholar] [CrossRef] [PubMed]
- Ran, T.; Saleem, A.M.; Shen, Y.; Ribeiro, G.O.; Beauchemin, K.A.; Tsang, A.; Yang, W.; McAllister, T.A. Effects of a recombinant fibrolytic enzyme on fiber digestion, ruminal fermentation, nitrogen balance, and total tract digestibility of heifers fed a high forage diet1. J. Anim. Sci. 2019, 97, 3578–3587. [Google Scholar] [CrossRef] [PubMed]
- Baran, M.; Kmet, V. Effect of pectinase on rumen fermentation in sheep and lambs. Arch. Anim. Nutr. 1987, 37, 643–649. [Google Scholar] [CrossRef]
- Simon, A.L.; Copetti, P.M.; Lago, R.V.P.; Vitt, M.G.; Nascimento, A.L.; Silva, L.E.L.E.; Wagner, R.; Klein, B.; Martins, C.S.; Kozloski, G.V.; et al. Inclusion of exogenous enzymes in feedlot cattle diets: Impacts on physiology, rumen fermentation, digestibility and fatty acid profile in rumen and meat. Biotechnol. Rep 2023, 41, e00824. [Google Scholar] [CrossRef]
- Tricarico, J.M.; Johnston, J.D.; Dawson, K.A.; Hanson, K.C.; Mcleod, K.R.; Harmon, D.L. The effects of an Aspergillus oryzae extract containing alpha-amylase activity on ruminal fermentation and milk production in lactating Holstein cows. Anim. Sci. 2005, 81, 365–374. [Google Scholar] [CrossRef]
- Liu, Z.K.; Li, Y.; Zhao, C.C.; Liu, Z.J.; Wang, L.M.; Li, X.Y.; Pellikaan, W.F.; Yao, J.H.; Cao, Y.C. Effects of a combination of fibrolytic and amylolytic enzymes on ruminal enzyme activities, bacterial diversity, blood profile and milk production in dairy cows. Animal 2022, 16, 100595. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, T.; Zhou, Y.; Li, F. Effects of enzyme + bacteria treatment on growth performance, rumen bacterial diversity, KEGG pathways, and the CAZy spectrum of Tan sheep. Bioengineered 2020, 11, 1221–1232. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Ren, J.; Xu, J.; Zhang, L.; Yan, F.; Liu, T.; Zhang, G.; Huws, S.A.; Yao, J.; et al. Multi-omics insights into microbiome-rumen epithelium interaction mechanisms underlying subacute rumen acidosis tolerance in dairy goats. Genome Biol. 2025, 26, 345. [Google Scholar] [CrossRef]
- Zhao, W.; Abdelsattar, M.M.; Wang, X.; Zhang, N.; Chai, J. In Vitro Modulation of Rumen Fermentation by Microbiota from the Recombination of Rumen Fluid and Solid Phases. Microbiol. Spectr. 2023, 11, e0338722. [Google Scholar] [CrossRef]
- McSweeney, C.S.; Halliday, M.; Mackie, R.I. Rumen Synergistota: New insights into their role in mimosine and fluoroacetate toxicity of ruminant livestock. Appl. Environ. Microbiol. 2025, 91, e0038025. [Google Scholar] [CrossRef]
- Li, J.; Yan, H.; Chen, J.; Duan, C.; Guo, Y.; Liu, Y.; Zhang, Y.; Ji, S. Correlation of ruminal fermentation parameters and rumen bacterial community by comparing those of the goat, sheep, and cow in vitro. Fermentation 2022, 8, 427. [Google Scholar] [CrossRef]
- Yu, J.; Cai, L.; Zhang, J.; Yang, A.; Wang, Y.; Zhang, L.; Guan, L.L.; Qi, D. Effects of thymol supplementation on goat rumen fermentation and rumen microbiota in vitro. Microorganisms 2020, 8, 1160. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; Plugge, C.M.; Akkermans, A.D.L.; De Vos, W.M. Victivallis vadensis gen. nov., sp. nov., a sugar-fermenting anaerobe from human faeces. Int. J. Syst. Evol. Microbiol. 2003, 53, 211e5. [Google Scholar] [CrossRef]
- Gomes Carvalho Alves, K.L.; Granja-Salcedo, Y.T.; Messana, J.D.; de Souza, V.C.; Generoso Ganga, M.J.; Detogni Colovate, P.H.; Kishi, L.T.; Berchielli, T.T. Rumen bacterial diversity in relation to nitrogen retention in beef cattle. Anaerobe 2021, 67, 102316. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Skliros, D.; Flemetakis, E.; Tsiplakou, E. Changes in the rumen bacteriome structure and enzymatic activities of goats in response to dietary supplementation with Schizochytrium spp. Microorganisms 2021, 9, 1528. [Google Scholar] [CrossRef]
- Nagaraja, T.G. Microbiology of the rumen. In Rumenology; Millen, D.D., Arrigoni, M.D.B., Pacheco, R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 39–61. [Google Scholar]
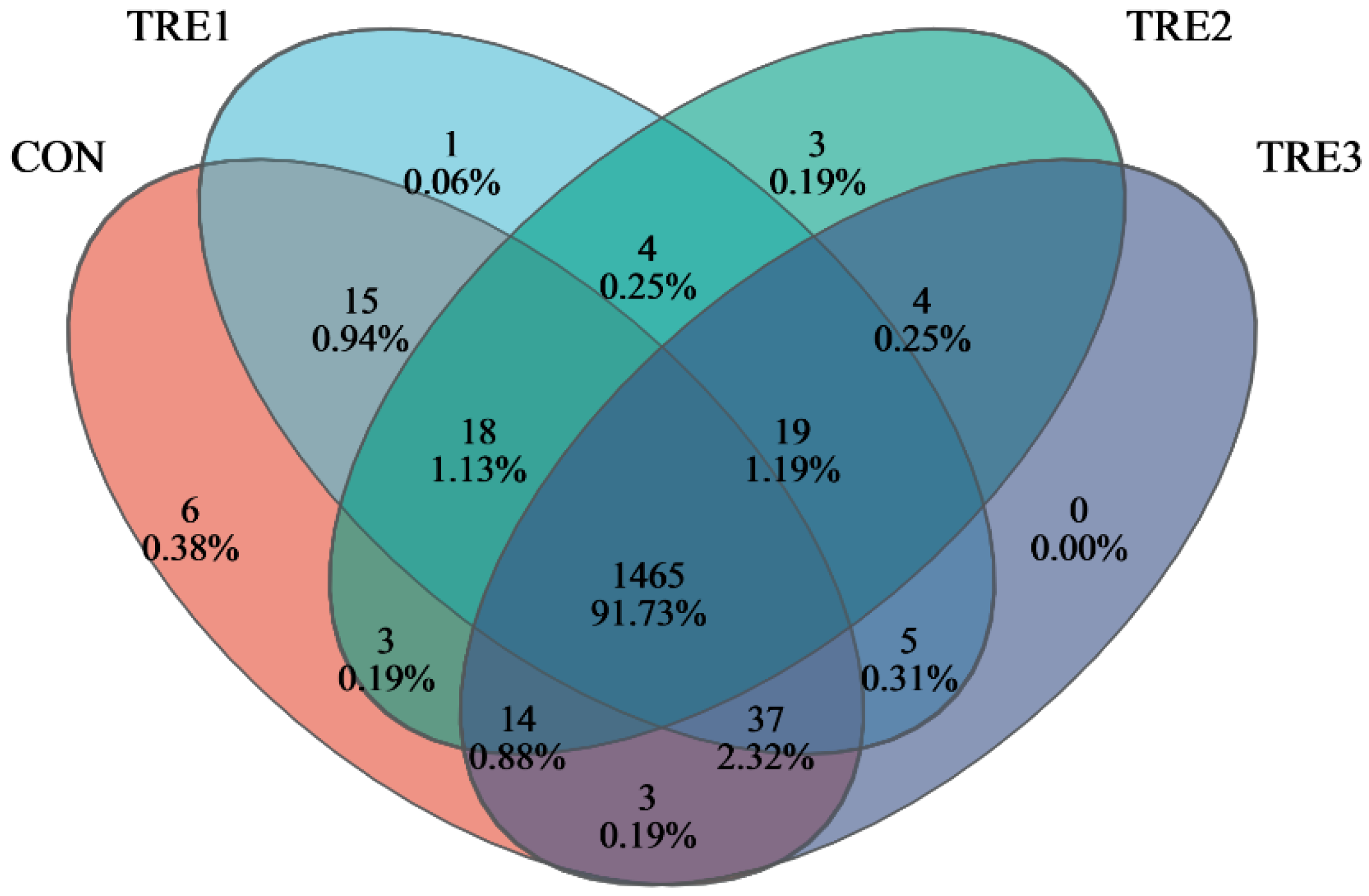
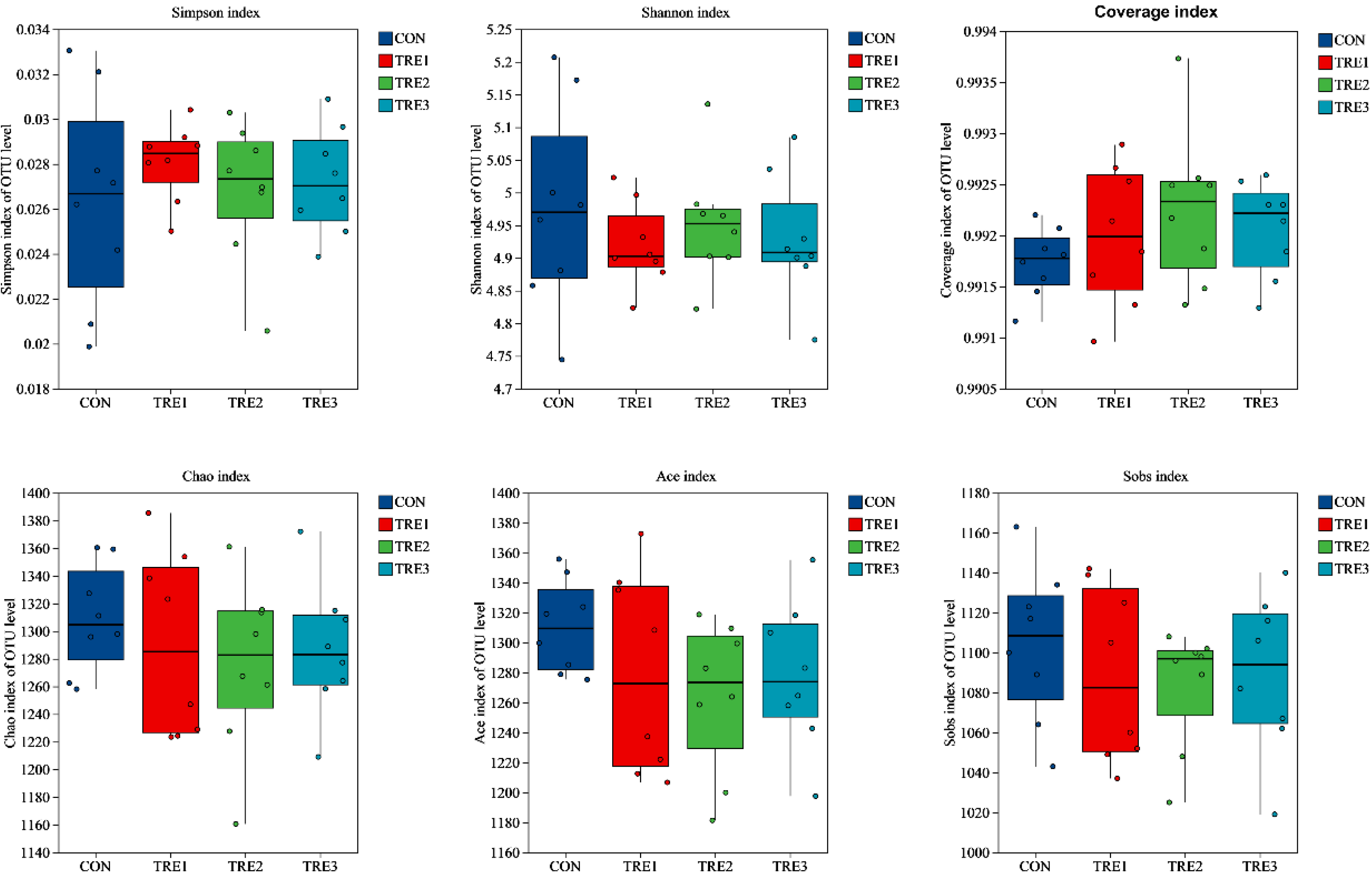
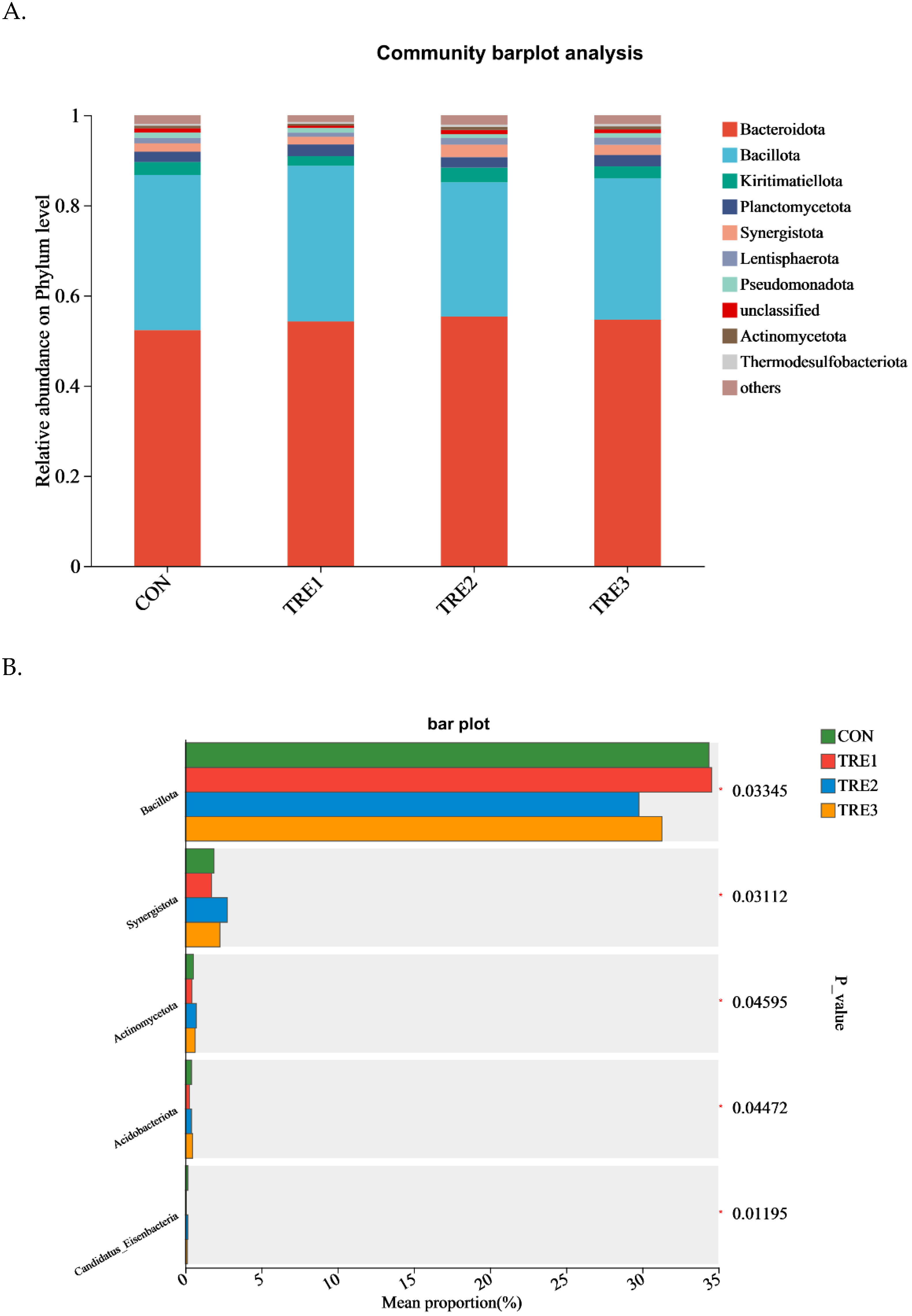

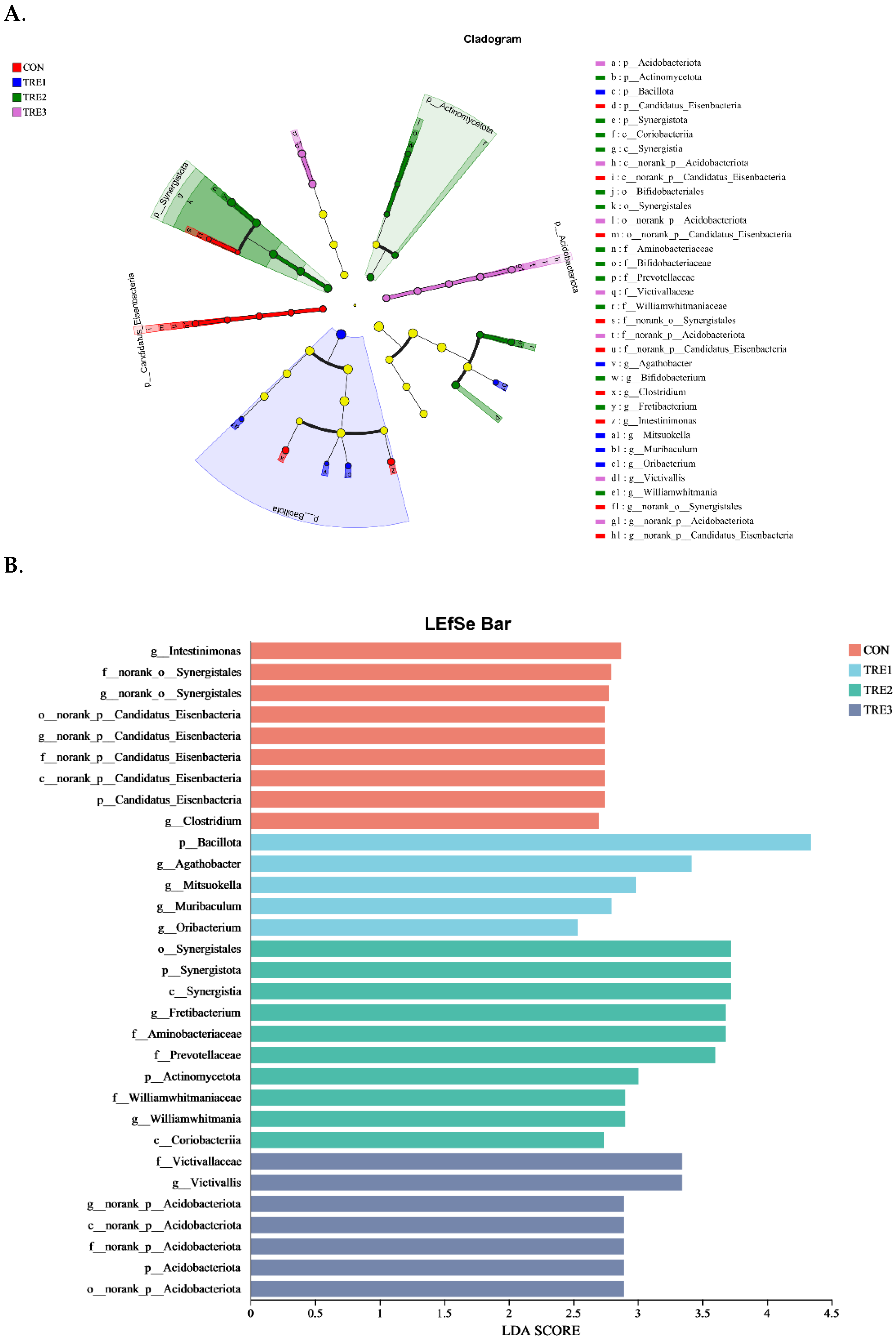
| Items | Chemical Composition, % |
|---|---|
| Dry matter | 93.7 |
| Crude protein | 8.14 |
| Organic matter | 89.9 |
| Ether extract | 8.53 |
| Neutral detergent fiber | 41.3 |
| Acid detergent fiber | 29.6 |
| Items | Times | CON | TRE1 | TRE2 | TRE3 | SEM | p |
| Gas production, mL/0.4 g of dry matter | 3 h | 33.33 | 35.03 | 33.70 | 34.88 | 0.354 | 0.237 |
| 6 h | 51.05 a | 55.65 b | 52.26 a | 55.83 b | 0.510 | <0.001 | |
| 9 h | 66.03 ab | 69.80 b | 65.21 a | 68.49 ab | 0.578 | 0.01 | |
| 12 h | 75.36 a | 80.64 b | 77.06 ab | 81.45 b | 0.805 | 0.014 | |
| 24 h | 97.28 a | 114.50 c | 105.53 b | 111.18 c | 1.292 | <0.001 | |
| 48 h | 126.53 a | 148.68 c | 134.83 b | 145.38 c | 1.683 | <0.001 | |
| Dry matter digestibility, % | 6 h | 29.20 a | 30.64 ab | 32.16 b | 33.14 b | 0.453 | <0.01 |
| 12 h | 33.17 a | 37.07 b | 36.58 b | 37.72 b | 0.428 | <0.001 | |
| 24 h | 40.64 a | 42.95 a | 42.23 a | 44.99 b | 1.137 | 0.048 | |
| 48 h | 45.60 a | 47.47 a | 51.41 b | 52.75 b | 0.579 | <0.001 | |
| Neutral detergent fiber digestibility, % | 6 h | 21.69 a | 23.38 b | 22.46 ab | 22.76 ab | 0.343 | 0.042 |
| 12 h | 28.94 a | 29.81 a | 30.02 ab | 31.44 b | 0.759 | 0.044 | |
| 24 h | 37.02 a | 39.85 b | 37.71 a | 41.26 b | 0.457 | <0.001 | |
| 48 h | 41.78 a | 45.12 b | 44.82 b | 45.92 b | 0.346 | <0.001 | |
| Acid detergent fiber digestibility, % | 6 h | 13.93 | 14.77 | 14.44 | 13.87 | 0.235 | 0.490 |
| 12 h | 21.09 | 20.18 | 21.65 | 20.70 | 0.365 | 0.570 | |
| 24 h | 30.83 a | 30.44 a | 32.55 b | 32.35 b | 0.374 | 0.040 | |
| 48 h | 33.71 a | 36.32 b | 36.40 b | 37.15 b | 0.290 | <0.001 |
| Items | CON | TRE1 | TRE2 | TRE3 | SEM | p |
|---|---|---|---|---|---|---|
| pH | 6.38 | 6.37 | 6.38 | 6.39 | 0.04 | 0.999 |
| Ammonia-N, mg/100 mL | 30.57 | 32.61 | 31.78 | 32.41 | 0.35 | 0.150 |
| MCP, mg/100 mL | 19.32 a | 20.21 ab | 20.20 ab | 21.17 b | 0.222 | 0.024 |
| TVFAs, mmol/L | 45.21 a | 47.14 ab | 46.31 ab | 48.33 c | 0.290 | <0.001 |
| Acetate, mmol/L | 31.50 a | 32.73 bc | 32.33 ab | 33.66 c | 0.183 | <0.001 |
| Propionate, mmol/L | 8.01 a | 8.31 bc | 8.12 ab | 8.44 c | 0.040 | <0.001 |
| Butyrate, mmol/L | 0.40 | 0.42 | 0.41 | 0.43 | 0.014 | 0.826 |
| Iso-butyrate, mmol/L | 2.40 | 2.54 | 2.46 | 2.61 | 0.061 | 0.645 |
| Valerate, mmol/L | 1.94 | 2.09 | 1.99 | 2.13 | 0.082 | 0.855 |
| Iso-valerate, mmol/L | 0.86 | 1.04 | 1.00 | 1.07 | 0.031 | 0.706 |
| Acetate–Propionate | 3.94 | 3.94 | 3.98 | 3.99 | 0.017 | 0.541 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, F.; Han, J.; Zhu, D.; Song, X.; Zeng, H.; Zhang, X.; Chen, A.; Li, Z.; Huang, S.; Liu, J.; et al. The Effects of Added Cellulases and Pectinases on Ruminal Fermentation Parameters and Bacterial Communities in Goats Supplemented with Macadamia Integrifolia Husks: An In Vitro Study. Animals 2025, 15, 3337. https://doi.org/10.3390/ani15223337
Cai F, Han J, Zhu D, Song X, Zeng H, Zhang X, Chen A, Li Z, Huang S, Liu J, et al. The Effects of Added Cellulases and Pectinases on Ruminal Fermentation Parameters and Bacterial Communities in Goats Supplemented with Macadamia Integrifolia Husks: An In Vitro Study. Animals. 2025; 15(22):3337. https://doi.org/10.3390/ani15223337
Chicago/Turabian StyleCai, Faguo, Jiancheng Han, Donghong Zhu, Ximei Song, Hui Zeng, Xiaosong Zhang, Anmiao Chen, Zehua Li, Shiyang Huang, Jingbo Liu, and et al. 2025. "The Effects of Added Cellulases and Pectinases on Ruminal Fermentation Parameters and Bacterial Communities in Goats Supplemented with Macadamia Integrifolia Husks: An In Vitro Study" Animals 15, no. 22: 3337. https://doi.org/10.3390/ani15223337
APA StyleCai, F., Han, J., Zhu, D., Song, X., Zeng, H., Zhang, X., Chen, A., Li, Z., Huang, S., Liu, J., Li, M., Liu, H., & Zhou, H. (2025). The Effects of Added Cellulases and Pectinases on Ruminal Fermentation Parameters and Bacterial Communities in Goats Supplemented with Macadamia Integrifolia Husks: An In Vitro Study. Animals, 15(22), 3337. https://doi.org/10.3390/ani15223337







