IoT Monitoring of Indoor Air Quality in Dairy Goat Barns: The Role of Building Characteristics and Litter Management
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Monitored Barns
2.2. Monitoring Units
2.3. Data Processing and Statistical Analyss
3. Results
3.1. Air Quality and Ambient Conditions During the First Seven Days After Litter Renewal
3.2. Comparison Between Farms When the Litter Was of the Same Age
3.3. Effect of Season and Time of Day Within Farm
3.4. Effect of Litter Age Within Season
4. Discussion
4.1. THI
4.2. NH3 and CO2
4.3. PM2.5
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Sevi, A.; Casamassima, D.; Pulina, G.; Pazzona, A. Factors of welfare reduction in dairy sheep and goats. Ital. J. Anim. Sci. 2009, 8 (Suppl. 1), 81–101. [Google Scholar] [CrossRef]
- Ni, J.Q.; Erasmus, M.A.; Croney, C.C.; Li, C.; Li, Y. A critical review of advancement in scientific research on food animal welfare-related air pollution. J. Hazard. Mater. 2021, 408, 124468. [Google Scholar] [CrossRef] [PubMed]
- Von Borell, E.; Özpinar, A.; Eslinger, K.M.; Schnitz, A.L.; Zhao, Y.; Mitloehner, F.M. Acute and prolonged effects of ammonia on hematological variables, stress responses, performance, and behavior of nursery pigs. J. Swine Health Prod. 2007, 15, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.J.C.; Pines, M.K.; Latter, M.; Muller, T.; Petherick, J.C.; Norman, S.T.; Gaughan, J.B. Physiological and behavioral responses of sheep to gaseous ammonia. J. Anim. Sci. 2012, 90, 1562–1569. [Google Scholar] [CrossRef]
- Battini, M.; Barbieri, S.; Fioni, L.; Mattiello, S. Feasibility and validity of animal-based indicators for on-farm welfare assessment of thermal stress in dairy goats. Int. J. Biometeorol. 2016, 60, 289–296. [Google Scholar] [CrossRef]
- Sevi, A.; Albenzio, M.; Muscio, A.; Casamassima, D.; Centoducati, P. Effects of litter management on airborne particulates in sheep houses and on the yield and quality of ewe milk. Livest. Prod. Sci. 2003, 81, 1–9. [Google Scholar] [CrossRef]
- Zhuang, S.; Van Overbeke, P.; Vangeyte, J.; Sonck, B.; Demeyer, P. Evaluation of a cost-effective ammonia monitoring system for continuous real-time concentration measurements in a fattening pig barn. Sensors 2019, 19, 3669. [Google Scholar] [CrossRef]
- Philippe, F.X.; Laitat, M.; Nicks, B.; Cabaraux, J.F. Ammonia and greenhouse gas emissions during the fattening of pigs kept on two types of straw floor. Agric. Ecosyst. Environ. 2012, 150, 45–53. [Google Scholar] [CrossRef]
- Guarino, M.; Fabbri, C.; Navarotto, P.; Valli, L.; Moscatelli, G.; Rossetti, M.; Mazzotta, V. Ammonia, methane and nitrous oxide emissions and particulate matter concentrations in two different buildings for fattening pigs. In Proceedings of the International Symposium on Gaseous and Odor Emissions from Animal Production Facilities, Horsens, Denmark, 1–4 June 2003; pp. 140–149. [Google Scholar]
- Amon, B.; Kryvoruchko, V.; Fröhlich, M.; Amon, T.; Pöllinger, A.; Mösenbacher, I.; Hausleitner, A. Ammonia and greenhouse gas emissions from a straw flow system for fattening pigs: Housing and manure storage. Livest. Sci. 2007, 112, 199–207. [Google Scholar] [CrossRef]
- Mautone, A.; Finzi, A. State of the art and perspectives in ammonia and particulate matter measurement in pig houses. In Proceedings of the 2024 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Padova, Italy, 29–31 October 2024; pp. 128–133. [Google Scholar]
- Mautone, A.; Finzi, A. Air quality monitoring in piggeries through an IoT gas and environmental sensors device. In Proceedings of the 11th European Conference on Precision Livestock Farming, Bologna, Italy, 9–12 September 2024; pp. 1728–1736. [Google Scholar]
- Tugnolo, A.; Beghi, R.; Cocetta, G.; Finzi, A. An integrated device for rapid analysis of indoor air quality in farms: The cases of milking parlors and greenhouses for baby leaf cultivation. J. Clean. Prod. 2022, 369, 133401. [Google Scholar] [CrossRef]
- Ji, B.; Zheng, W.; Gates, R.S.; Green, A.R. Design and performance evaluation of the upgraded portable monitoring unit for air quality in animal housing. Comput. Electron. Agric. 2016, 124, 132–140. [Google Scholar] [CrossRef]
- Pereira, W.F.; da Silva Fonseca, L.; Putti, F.F.; Góes, B.C.; de Paula Naves, L. Environmental monitoring in a poultry farm using an instrument developed with the internet of things concept. Comput. Electron. Agric. 2020, 170, 105257. [Google Scholar] [CrossRef]
- Feng, Z.; Zheng, L.; Ren, B.; Liu, D.; Huang, J.; Xue, N. Feasibility of low-cost particulate matter sensors for long-term environmental monitoring: Field evaluation and calibration. Sci. Total Environ. 2024, 945, 174089. [Google Scholar] [CrossRef]
- Tullo, E.; Finzi, A.; Guarino, M. Environmental impact of livestock farming and Precision Livestock Farming as a mitigation strategy. Sci. Total Environ. 2019, 650, 2751–2760. [Google Scholar] [CrossRef]
- Buoio, E.; Cialini, C.; Costa, A. Air quality assessment in pig farming: The Italian classyfarm. Animals 2023, 13, 2297. [Google Scholar] [CrossRef]
- Conti, C.; Borgonovo, F.; Guarino, M. Ammonia concentration and recommended threshold values in pig farming: A review. In Proceedings of the 2021 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Trento-Bolzano, Italy, 3–5 November 2021; pp. 162–166. [Google Scholar]
- Ullman, J.L.; Mukhtar, S.; Lacey, R.E.; Carey, J.B. A review of literature concerning odors, ammonia, and dust from broiler production facilities: 4. Remedial management practices. J. Appl. Poult. Res. 2004, 13, 521–531. [Google Scholar] [CrossRef]
- Seedorf, J.; Hartung, J. Survey of ammonia concentrations in livestock buildings. J. Agric. Sci. 1999, 133, 433–437. [Google Scholar] [CrossRef]
- Howard, A.; Botlaguduru, S.V.; Du, H.; Kommalapati, R.R.; Huque, Z. Measurements and comparative air quality analysis of a goat farm operation. Trans. ASABE 2019, 62, 1723–1733. [Google Scholar] [CrossRef]
- Cai, L.; Yu, J.; Zhang, J.; Qi, D. The effects of slatted floors and manure scraper systems on the concentrations and emission rates of ammonia, methane and carbon dioxide in goat buildings. Small Rumin. Res. 2015, 132, 103–110. [Google Scholar] [CrossRef]
- Hays, F.L.; Hahn, L.; Goret, E.; Johnson, H.D. Hydrogen sulfide (H2S) exposure in ruminants. J. Anim. Sci. 1972, 35, 189. [Google Scholar]
- Riedel, A.; Pieper, L.; Lautner, M.; Leiding, C.; Jung, M.; Schulze, M. Influence of deep-litter bedding materials on environmental and welfare-related factors in boar studs. Appl. Anim. Behav. Sci. 2024, 273, 106215. [Google Scholar] [CrossRef]
- Provolo, G.; Brandolese, C.; Grotto, M.; Marinucci, A.; Fossati, N.; Ferrari, O.; Beretta, E.; Riva, E. An Internet of Things framework for monitoring environmental conditions in livestock housing to improve animal welfare and assess environmental impact. Animals 2025, 15, 644. [Google Scholar] [CrossRef]
- Srivastava, A.; Yadav, P.; Mahajan, A.; Anand, M.; Yadav, S.; Madan, A.K.; Yadav, B. Appropriate THI model and its threshold for goats in semi-arid regions of India. J. Therm. Biol. 2021, 96, 102845. [Google Scholar] [CrossRef] [PubMed]
- Batschelet, E. Circular Statistics in Biology; Academic Press: London, UK, 1981. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models, R package version 3.1-164; R foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Salama, A.A.K.; Caja, G.; Hamzaoui, S.; Badaoui, B.; Castro-Costa, A.; Façanha, D.A.E.; Bozzi, R. Different levels of response to heat stress in dairy goats. Small Rumin. Res. 2014, 121, 73–79. [Google Scholar] [CrossRef]
- Rapetti, L.; Colombini, S.; Galassi, G.; Crovetto, G.M.; Malagutti, L. Relationship between milk urea level, protein feeding and urinary nitrogen excretion in high producing dairy goats. Small Rumin. Res. 2014, 121, 96–100. [Google Scholar] [CrossRef]
- Philippe, F.X.; Nicks, B. Review on greenhouse gas emissions from pig houses: Production of carbon dioxide, methane and nitrous oxide by animals and manure. Agric. Ecosyst. Environ. 2015, 199, 10–25. [Google Scholar] [CrossRef]
- Pedersen, S.; Sällvik, K. International Commission of Agricultural Engineering, Section II 4th Report of Working Group Climatization of Animal Houses Heat and Moisture Production at Animal and House Levels. 2002. Available online: https://www.cigr.org/sites/default/files/documets/CIGR_4TH_WORK_GR.pdf (accessed on 15 July 2025).
- Pedersen, S.; Blanes-Vidal, V.; Joergensen, H.; Chwalibog, A.; Haeussermann, A.; Heetkamp, M.J.W.; Aarnink, A.J.A. Carbon Dioxide Production in Animal Houses: A Literature Review. CIGR E-J. 2008, X. Manuscript BC 08 008. Available online: https://cigrjournal.org/index.php/Ejounral/article/view/1205 (accessed on 15 July 2025).
- Calvet, S.; van Dooren, H.J.C.; Ogink, N.W.; Mosquera, J. Measuring and assessing the role of deep litter to estimate the ventilation rate using the CO2 mass balance method. Biosyst. Eng. 2022, 224, 313–323. [Google Scholar] [CrossRef]
- WHO. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; WHO European Centre for Environment and Health: Bonn, Germany, 2021; Available online: https://iris.who.int/bitstream/handle/10665/345329/9789240034228-eng.pdf?sequence=1 (accessed on 10 September 2025).
- Bordignon, F.; Pravato, M.; Trocino, A.; Xiccato, G.; Marinello, F.; Pezzuolo, A. Environmental Gradients and Hen Spatial Distribution in a Cage-Free Aviary System: Internet of Things-Based Real-Time Monitoring for Proactive Management. Animals 2025, 15, 1225. [Google Scholar] [CrossRef]
- Janke, D.; Bornwin, M.; Coorevits, K.; Hempel, S.; van Overbeke, P.; Demeyer, P.; Amon, B. A low-cost wireless sensor network for barn climate and emission monitoring—Intermediate results. Atmosphere 2023, 14, 1643. [Google Scholar] [CrossRef]
- Rosa, E.; Rincón, L.; Merino, P. Real time electrochemical ammonia (NH3) sensors performance in a natural ventilation dairy cattle barn. In Proceedings of the 11th European Conference on Precision Livestock Farming, Bologna, Italy, 9–12 September 2024; pp. 1400–1405. [Google Scholar]
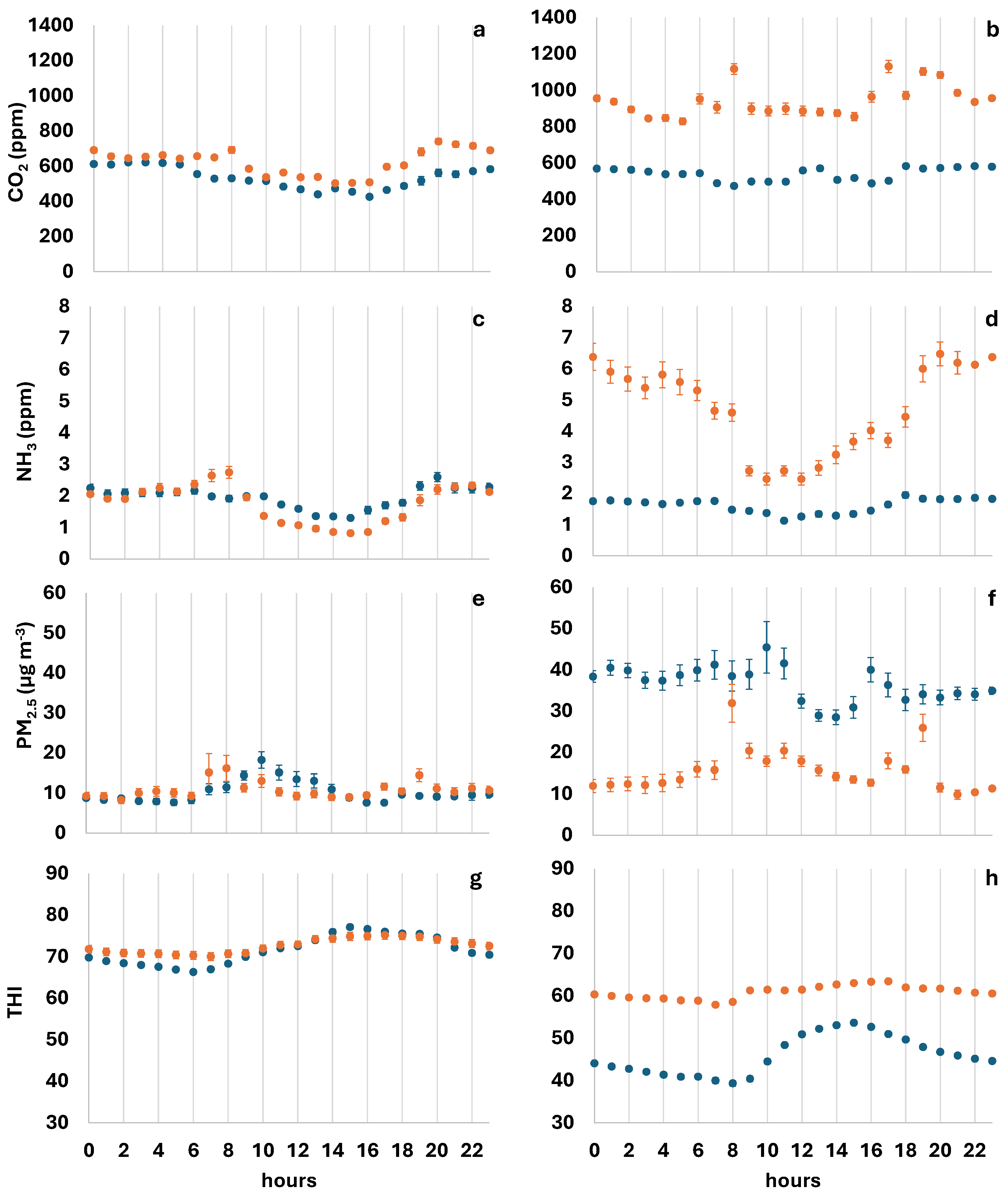
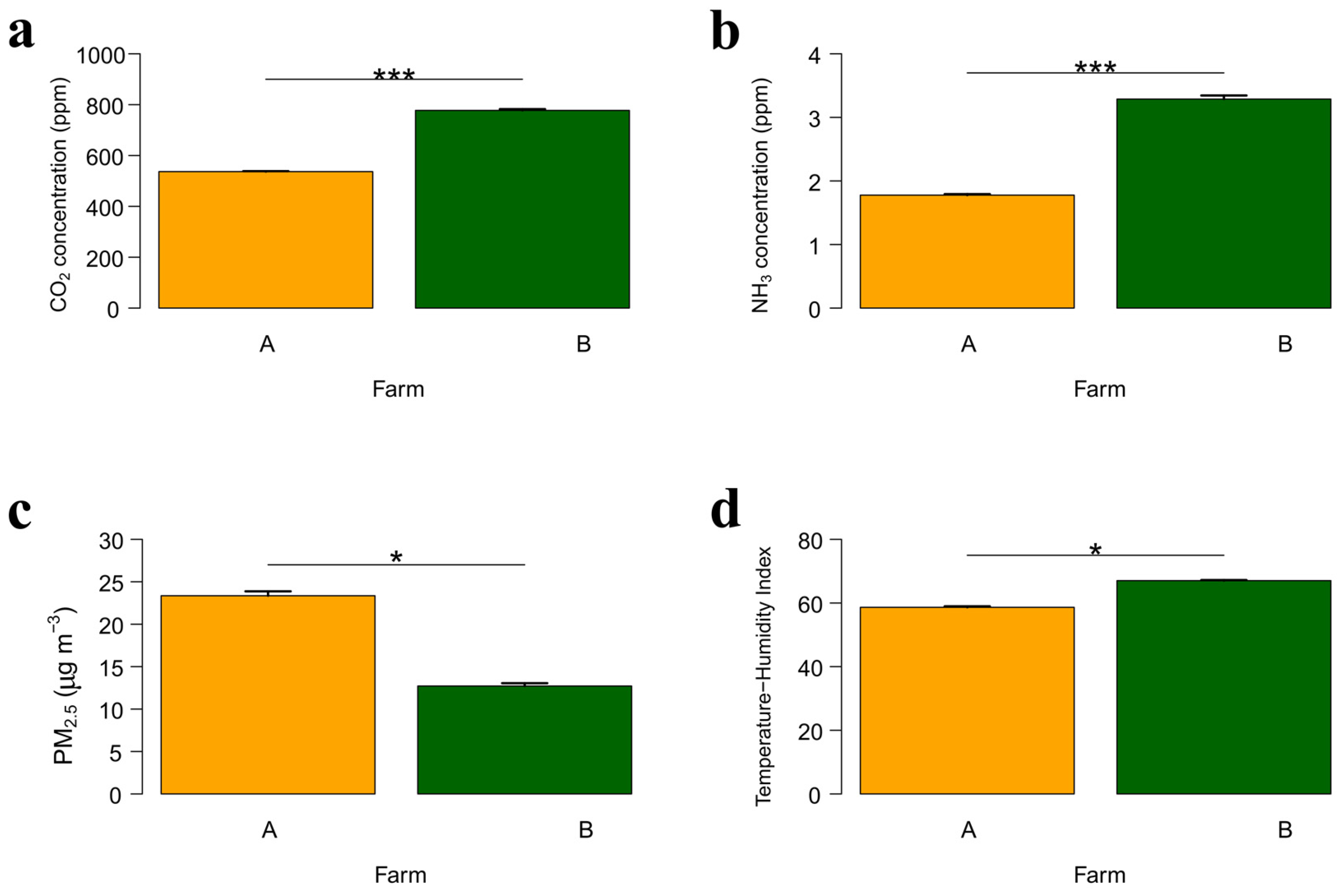
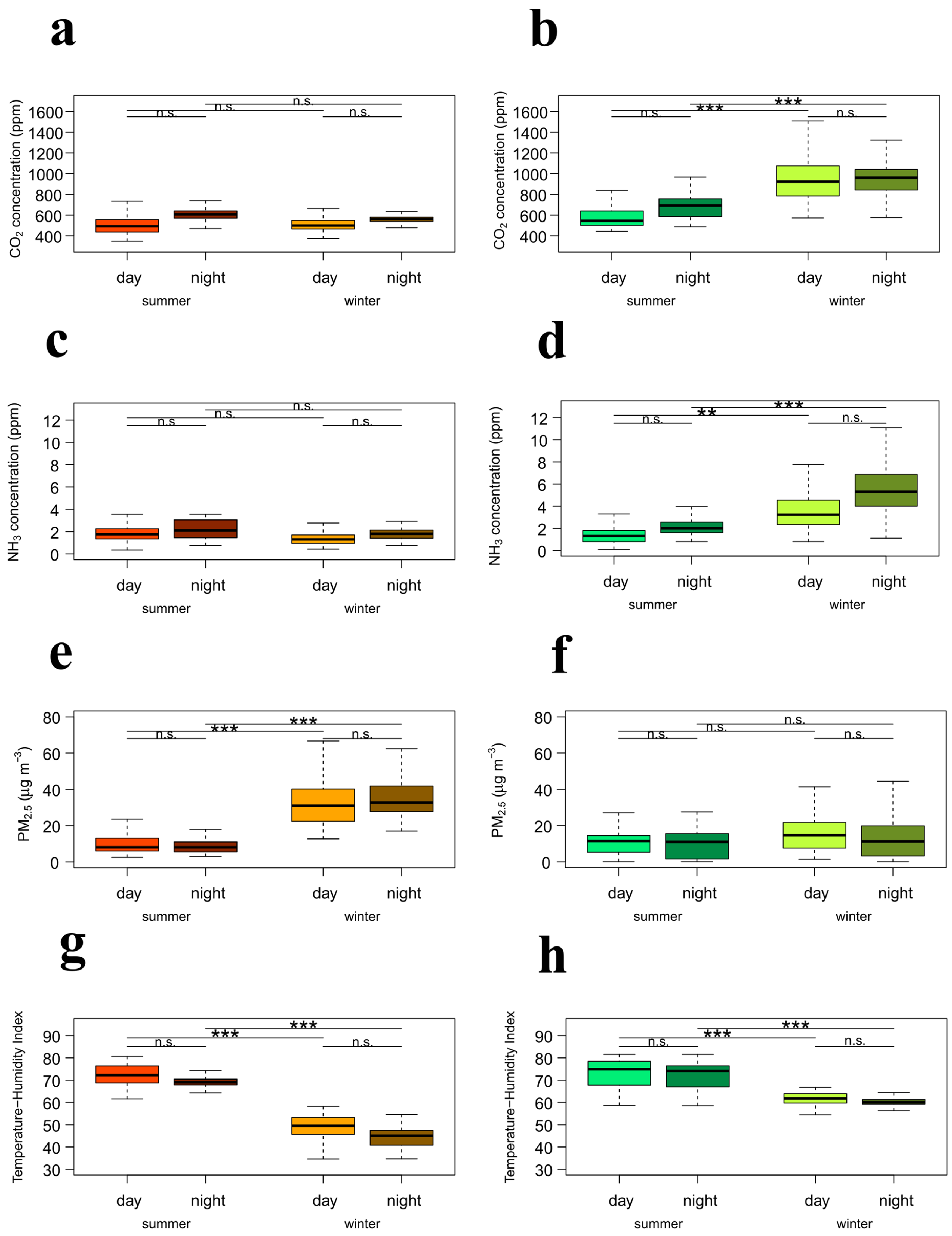

| Parameter | Farm A | Farm B | ||
|---|---|---|---|---|
| Winter | Summer | Winter | Summer | |
| Wheater conditions | ||||
| Temperature (°C) | 5.9 ± 3.2 | 22.6 ± 3.8 | 11.5 ± 3.4 | 25.2 ± 7.3 |
| Humidity (%) | 73.1 ± 16.7 | 85.9 ± 15.0 | 61.0 ± 23.6 | 54.0 ± 25.3 |
| Precipitation (mm) | 0.0 ± 0.0 | 0.4 ± 2.3 | 0.0 ± 0.2 | 0.3 ± 1.3 |
| Altitude (m a.s.l.) | 300 | 600 | ||
| Barn orientation | E-W | E-W | ||
| Building material | Wood (roof); Concrete-wood (walls) | Wood (roof); Concrete (walls) | ||
| Goats (n.) | 120 | 110 | 66 | 59 |
| Stocking density (m2 head−1) | 1.54 | 1.68 | 1.73 | 1.93 |
| Airspace (m3 head−1) | 14.3 | 15.6 | 9.8 | 11 |
| Air inlet surface (m2 head−1) | 0.61 | 0.67 | 0.25 | 0.43 |
| Air outlet surface (m2 head−1) | 0.38 | 0.41 | 0.07 | 0.08 |
| Litter renewal frequency (d) | 90 | 60 | 7 | |
| Parameter | Sensor | Type | Range | Accuracy |
|---|---|---|---|---|
| NH3 | 4NH3-100 (*) | Electrochemical | 0–100 ppm | ±10% |
| CO2 | SCD30 (**) | Nondispersive infrared (NDIR) | 400–10,000 ppm | 30 ppm |
| H2S | 4H2S-100 (*) | Electrochemical | 0–100 ppm | ±2% |
| Temperature | SHT3x/SHT4x (**) | CMOSens | −40–+125 °C | ±0.1 °C |
| Humidity | SHT3x/SHT4x (**) | CMOSens | 0–100% | ±2% |
| PM | SPS30 (**) | Laser scattering | 0–1000 µg m−3 | ±10 µg m−3 (PM2.5) ±25 µg m−3 (PM10) |
| Variable | Effect | Estimate | SE | DF | t | p |
|---|---|---|---|---|---|---|
| CO2 | φ | 0.89 | - | - | - | - |
| Intercept | 536.50 | 27.32 | 3852 | 19.64 | <0.001 | |
| Farm B | 246.45 | 40.39 | 30 | 6.10 | <0.001 | |
| sin(hour) | 27.94 | 7.89 | 3852 | 3.54 | <0.001 | |
| cos(hour) | 55.47 | 7.07 | 3852 | 7.84 | <0.001 | |
| sin(hour) × cos(hour) | −42.38 | 12.86 | 3852 | −3.30 | 0.001 | |
| NH3 | Φ | 0.98 | - | - | - | - |
| Intercept | 1.63 | 0.17 | 3852 | 9.79 | <0.001 | |
| Farm B | 1.73 | 0.40 | 30 | 4.26 | <0.001 | |
| sin(hour) | 0.45 | 0.10 | 3852 | 4.29 | <0.001 | |
| cos(hour) | 0.59 | 0.10 | 3852 | 5.82 | <0.001 | |
| sin(hour) × cos(hour) | −0.64 | 0.12 | 3852 | −5.47 | <0.001 | |
| PM2.5 | Φ | 0.74 | - | - | - | - |
| Intercept | 23.10 | 3.23 | 2552 | 7.16 | <0.001 | |
| Farm B | −10.45 | 4.64 | 30 | −2.25 | 0.032 | |
| sin(hour) | 2.92 | 0.69 | 2552 | 4.26 | <0.001 | |
| cos(hour) | −0.94 | 0.63 | 2552 | −1.50 | 0.135 | |
| sin(hour) × cos(hour) | −3.50 | 1.23 | 2552 | −2.85 | 0.004 | |
| THI | Φ | 0.99 | - | - | - | - |
| Intercept | 58.21 | 2.99 | 3852 | 19.50 | <0.001 | |
| Farm B | 8.31 | 3.65 | 30 | 2.28 | 0.03 | |
| sin(hour) | −2.36 | 0.12 | 3852 | −20.09 | <0.001 | |
| cos(hour) | −0.45 | 0.14 | 3852 | −3.21 | 0.001 | |
| sin(hour) × cos(hour) | 1.02 | 0.14 | 3852 | 7.43 | <0.001 |
| Variable | Farm | Effect | Estimate | SE | DF | t | p |
|---|---|---|---|---|---|---|---|
| CO2 | A | φ | 0.91 | - | - | - | - |
| Intercept | 529.91 | 16.63 | 1975 | 31.86 | <0.001 | ||
| Period (night) | 40.20 | 24.06 | 14 | 1.67 | 0.117 | ||
| Season (winter) | −9.48 | 22.71 | 14 | −0.41 | 0.683 | ||
| Period × Season | −20.56 | 26.91 | 14 | −0.76 | 0.458 | ||
| sin(hour) | 14.69 | 3.73 | 1975 | 3.94 | <0.001 | ||
| cos(hour) | 29.64 | 9.86 | 1975 | 3.01 | 0.002 | ||
| sin(hour) × cos(hour) | 55.05 | 7.64 | 1975 | 7.20 | <0.001 | ||
| CO2 | B | φ | 0.93 | - | - | - | - |
| Intercept | 627.76 | 18.94 | 1842 | 33.15 | <0.001 | ||
| Period (night) | −12.56 | 33.86 | 14 | −0.37 | 0.716 | ||
| Season (winter) | 382.52 | 70.94 | 14 | 5.39 | <0.001 | ||
| Period × Season | −112.63 | 80.20 | 14 | −1.40 | 0.182 | ||
| sin(hour) | −15.11 | 8.57 | 1842 | −1.76 | 0.078 | ||
| cos(hour) | 96.02 | 19.38 | 1842 | 4.96 | <0.001 | ||
| sin(hour) × cos(hour) | −125.92 | 80.20 | 14 | −5.67 | <0.001 | ||
| NH3 | A | φ | 0.92 | - | - | - | - |
| Intercept | 1.86 | 0.19 | 1975 | 9.77 | <0.001 | ||
| Period (night) | 0.05 | 0.17 | 14 | 0.27 | 0.790 | ||
| Season (winter) | −0.30 | 0.28 | 14 | −1.06 | 0.306 | ||
| Period × Season | −0.02 | 0.20 | 14 | −0.09 | 0.929 | ||
| sin(hour) | −0.05 | 0.03 | 1975 | −1.61 | 0.108 | ||
| cos(hour) | 0.25 | 0.08 | 1975 | 3.23 | 0.001 | ||
| sin(hour) × cos(hour) | −0.20 | 0.06 | 1975 | −3.11 | 0.002 | ||
| NH3 | B | φ | 0.97 | - | - | - | - |
| Intercept | 1.99 | 0.21 | 1842 | 9.37 | <0.001 | ||
| Period (night) | −0.69 | 0.39 | 14 | −1.79 | 0.095 | ||
| Season (winter) | 2.16 | 0.63 | 14 | 3.43 | 0.004 | ||
| Period × Season | 1.70 | 0.95 | 14 | 1.79 | 0.095 | ||
| sin(hour) | 0.41 | 0.07 | 1842 | 5.97 | <0.001 | ||
| cos(hour) | 0.95 | 0.16 | 1842 | 6.09 | <0.001 | ||
| sin(hour) × cos(hour) | −0.97 | 0.18 | 1842 | −5.46 | <0.001 | ||
| PM2.5 | A | φ | 0.90 | - | - | - | - |
| Intercept | 9.21 | 2.50 | 1305 | 3.68 | <0.001 | ||
| Period (night) | 2.27 | 2.56 | 14 | 0.89 | <0.001 | ||
| Season (winter) | 25.37 | 5.81 | 14 | 4.37 | <0.001 | ||
| Period × Season | 1.12 | 5.52 | 14 | 0.20 | 0.842 | ||
| sin(hour) | 1.94 | 0.63 | 1305 | 3.08 | 0.002 | ||
| cos(hour) | −2.90 | 1.06 | 1305 | −2.75 | 0.006 | ||
| sin(hour) × cos(hour) | −4.44 | 1.15 | 1305 | −3.86 | <0.001 | ||
| PM2.5 | B | φ | 0.67 | - | - | - | - |
| Intercept | 11.28 | 3.03 | 1212 | 3.72 | <0.001 | ||
| Period (night) | −1.54 | 2.48 | 14 | −0.62 | 0.546 | ||
| Season (winter) | 5.75 | 4.43 | 14 | 1.30 | 0.215 | ||
| Period × Season | −2.36 | 3.27 | 14 | −0.72 | 0.482 | ||
| sin(hour) | 0.64 | 0.74 | 1212 | 0.86 | 0.390 | ||
| cos(hour) | 0.24 | 1.19 | 1212 | 0.21 | 0.838 | ||
| sin(hour) × cos(hour) | −1.14 | 1.14 | 1212 | −0.85 | 0.393 | ||
| THI | A | φ | 0.99 | - | - | - | - |
| Intercept | 71.8 | 0.81 | 1975 | 88.20 | <0.001 | ||
| Period (night) | 0.12 | 1.31 | 14 | 0.09 | 0.928 | ||
| Season (winter) | −26.07 | 1.23 | 14 | −21.14 | <0.001 | ||
| Period × Season | 0.41 | 2.13 | 14 | 0.19 | 0.850 | ||
| sin(hour) | −5.24 | 0.12 | 1975 | −43.15 | <0.001 | ||
| cos(hour) | −2.23 | 0.32 | 1975 | −7.05 | <0.001 | ||
| sin(hour) × cos(hour) | 3.62 | 0.21 | 1975 | 17.34 | <0.001 | ||
| THI | B | φ | 0.99 | - | - | - | - |
| Intercept | 72.58 | 1.54 | 1842 | 47.09 | <0.001 | ||
| Period (night) | 0.03 | 1.24 | 14 | 0.03 | 0.979 | ||
| Season (winter) | −12.26 | 2.18 | 14 | −5.63 | <0.001 | ||
| Period × Season | 0.10 | 1.43 | 14 | 0.07 | 0.943 | ||
| sin(hour) | −2.06 | 0.06 | 1842 | −33.29 | 0.0000 | ||
| cos(hour) | −0.32 | 0.18 | 1842 | −1.84 | 0.662 | ||
| sin(hour) × cos(hour) | 0.72 | 0.19 | 1842 | 3.71 | <0.001 |
| Season | Variable | Effect | Estimate | SE | DF | t-Value | p |
|---|---|---|---|---|---|---|---|
| Summer | CO2 | φ | 0.85 | - | - | - | - |
| (Intercept) | 606.97 | 17.21 | 1415 | 35.28 | <0.001 | ||
| New vs. old litter | −62.81 | 21.56 | 8 | −2.91 | 0.0195 | ||
| sin(hour) | 49.87 | 9.49 | 1415 | 5.26 | <0.001 | ||
| cos(hour) | 73.65 | 9.05 | 1415 | 8.14 | <0.001 | ||
| sin(hour) × cos(hour) | −15.92 | 17.27 | 1415 | −0.92 | 0.357 | ||
| NH3 | φ | 0.96 | - | - | - | - | |
| (Intercept) | 2.87 | 0.29 | 1415 | 9.76 | <0.001 | ||
| New vs. old litter | −0.82 | 0.36 | 8 | −2.30 | 0.050 | ||
| sin(hour) | 0.18 | 0.13 | 1415 | 1.45 | 0.147 | ||
| cos(hour) | 0.48 | 0.12 | 1415 | 4.13 | <0.001 | ||
| sin(hour) × cos(hour) | −0.41 | 0.16 | 1415 | −2.52 | 0.012 | ||
| PM2.5 | φ | 0.92 | - | - | - | - | |
| (Intercept) | 11.72 | 1.84 | 880 | 6.37 | <0.001 | ||
| New vs. old litter | −0.13 | 2.58 | 8 | −0.05 | 0.962 | ||
| sin(hour) | 1.55 | 0.85 | 880 | 1.82 | 0.069 | ||
| cos(hour) | −0.43 | 0.80 | 880 | −0.54 | 0.589 | ||
| sin(hour) × cos(hour) | −1.82 | 1.37 | 880 | −1.33 | 0.183 | ||
| Winter | CO2 | φ | 0.89 | - | - | - | - |
| (Intercept) | 571.11 | 11.53 | 1600 | 49.53 | <0.001 | ||
| New vs. old litter | −38.46 | 14.25 | 10 | −2.70 | 0.022 | ||
| sin(hour) | −12.09 | 7.46 | 1600 | −1.62 | 0.105 | ||
| cos(hour) | 25.50 | 6.96 | 1600 | 3.66 | 0.0003 | ||
| sin(hour) × cos(hour) | −0.19 | 12.71 | 1600 | −0.02 | 0.988 | ||
| NH3 | φ | 0.85 | - | - | - | - | |
| (Intercept) | 2.36 | 0.16 | 1600 | 15.14 | <0.001 | ||
| New vs. old litter | −0.80 | 0.22 | 10 | −3.72 | 0.004 | ||
| sin(hour) | −0.02 | 0.04 | 1600 | −0.45 | 0.654 | ||
| cos(hour) | 0.34 | 0.04 | 1600 | 8.20 | <0.001 | ||
| sin(hour) × cos(hour) | −0.19 | 0.08 | 1600 | −2.37 | 0.018 | ||
| PM2.5 | φ | 0.92 | - | - | - | - | |
| (Intercept) | 18.17 | 4.39 | 1061 | 4.14 | <0.001 | ||
| New vs. old litter | 18.39 | 6.55 | 10 | 2.81 | 0.019 | ||
| sin(hour) | −0.36 | 2.42 | 1061 | −0.15 | 0.883 | ||
| cos(hour) | 2.01 | 2.22 | 1061 | 0.90 | 0.366 | ||
| sin(hour) × cos(hour) | 2.20 | 3.19 | 1061 | 0.69 | 0.490 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celozzi, S.; Ambrosini, R.; Rapetti, L.; Mattiello, S.; Finzi, A. IoT Monitoring of Indoor Air Quality in Dairy Goat Barns: The Role of Building Characteristics and Litter Management. Animals 2025, 15, 3332. https://doi.org/10.3390/ani15223332
Celozzi S, Ambrosini R, Rapetti L, Mattiello S, Finzi A. IoT Monitoring of Indoor Air Quality in Dairy Goat Barns: The Role of Building Characteristics and Litter Management. Animals. 2025; 15(22):3332. https://doi.org/10.3390/ani15223332
Chicago/Turabian StyleCelozzi, Stefania, Roberto Ambrosini, Luca Rapetti, Silvana Mattiello, and Alberto Finzi. 2025. "IoT Monitoring of Indoor Air Quality in Dairy Goat Barns: The Role of Building Characteristics and Litter Management" Animals 15, no. 22: 3332. https://doi.org/10.3390/ani15223332
APA StyleCelozzi, S., Ambrosini, R., Rapetti, L., Mattiello, S., & Finzi, A. (2025). IoT Monitoring of Indoor Air Quality in Dairy Goat Barns: The Role of Building Characteristics and Litter Management. Animals, 15(22), 3332. https://doi.org/10.3390/ani15223332





