Pilot Study: Exploring the Feasibility of Individual Voluntary Waiting Period Settings Using Postpartum Recovery Indicators in Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Group Definitions
2.2. Blood Samples and Analyses
2.3. Statistical Analyses
3. Results
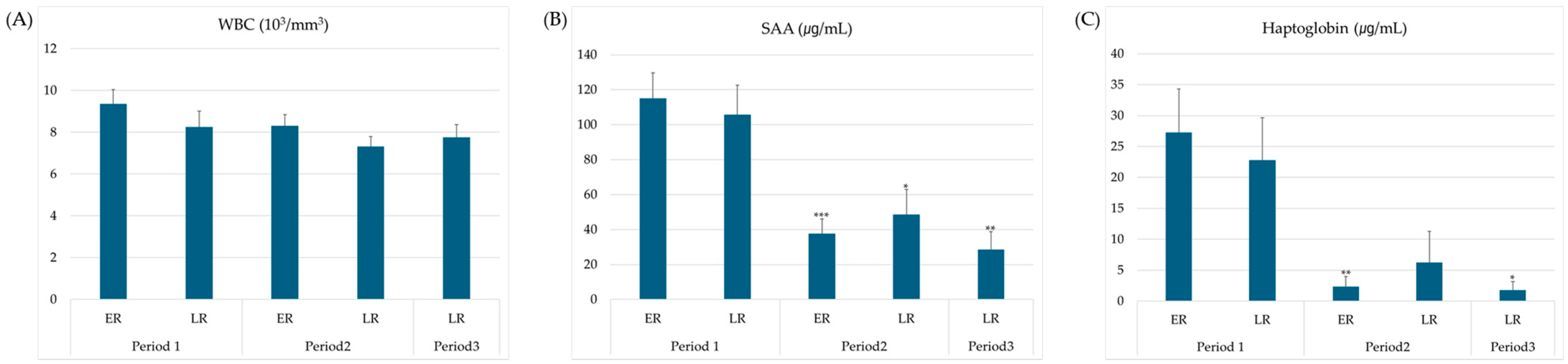
3.1. Inflammatory State
3.2. Acute-Phase Response
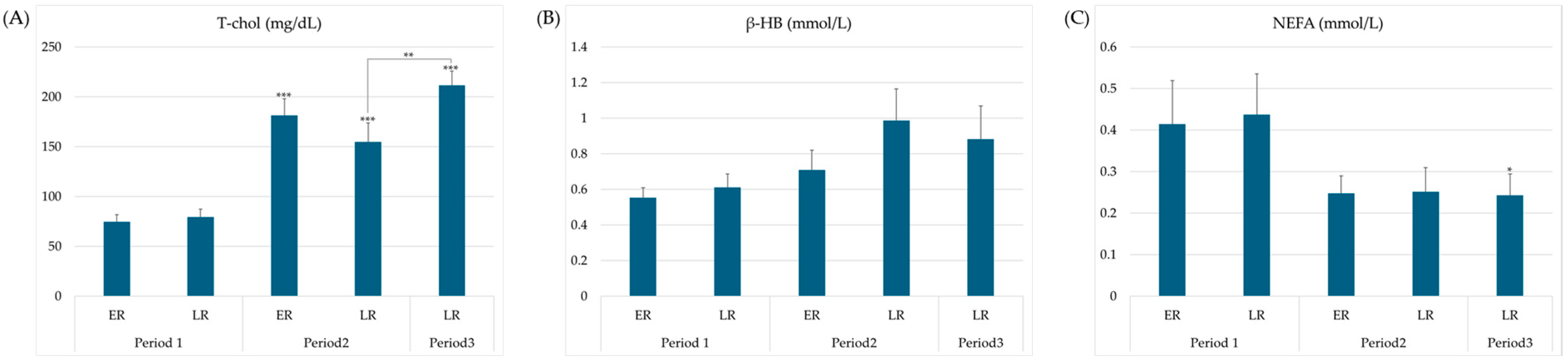
3.3. Feed Intake and Energy Balance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial insemination |
| APP | Acute phase protein |
| APR | Acute phase reaction |
| β-HB | Beta-hydroxybutyrate |
| COR | Cortisol |
| CRP | C-reactive protein |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| ER | Early recovery |
| HPT | Haptoglobin |
| IL | Interleukin |
| LR | Late recovery |
| NEB | Negative energy balance |
| NEFA | Non-esterified fatty acid |
| SAA | Serum amyloid A |
| SP | Substance P |
| T-Chol | Total cholesterol |
| TNF-α | Tumor necrosis factor-alpha |
| VWP | Voluntary waiting period |
| WBC | White blood cell (count) |
References
- Ricci, A.; Bonizi, G.; Sarasso, G.; Gallo, S.; Dondo, A.; Zoppi, S.; Vincenti, L. Subclinical endometritis in beef cattle in early and late postpartum: Cytology, bacteriology, haptoglobin and test strip sufficiency to evaluate the evolution of the disease. Theriogenology 2017, 94, 86–93. [Google Scholar] [CrossRef]
- Hussein, H.A.; El-Razik, K.A.E.A.; Gomaa, A.M.; Elbayoumy, M.K.; Abdelrahman, K.A.; Hosein, H.I. Milk amyloid A as a biomarker for diagnosis of subclinical mastitis in cattle. Vet. World 2018, 11, 34–41. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Nakada, K.; Hagiwara, K.; Kirisawa, R.; Iwai, H.; Moriyoshi, M.; Sawamukai, Y. Changes in interleukin-6 concentration in peripheral blood of pre- and post-partum dairy cattle and its relationship to postpartum reproductive diseases. J. Vet. Med. Sci. 2004, 66, 1403–1408. [Google Scholar] [CrossRef]
- Proudfoot, K.L.; Weary, D.M.; LeBlanc, S.J.; Mamedova, L.K.; von Keyserlingk, M.A.G. Exposure to an unpredictable and competitive social environment affects behavior and health of transition dairy cows. J. Dairy Sci. 2018, 101, 9309–9320. [Google Scholar] [CrossRef]
- Ferguson, J. Reproductive management in dairy herds. Clin. Theriogenology 2020, 12, 309–322. [Google Scholar]
- Kusaka, H.; Hasegawa, R.; Nishimoto, N.; Kawahata, M.; Miura, H.; Kikuchi, M.; Sakaguchi, M. Comparison of diagnostic methods for uterine health in dairy cattle on different days postpartum. Vet. Rec. 2020, 163, 91. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.M.; Waheeb, R.S.; Abd El-Rheem, S.M.; El-Amrawi, G.A. Evaluation of Medical and Economical Efficacy of Some Protocols for Treatment of Postpartum Clinical Endometritis in Holstein Dairy Cows. Alex. J. Vet. Sci. 2023, 79, 150–157. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, K.; Zhou, Y.; Zhao, J.; Wang, J.; Lu, W. Role of melatonin in bovine reproductive biotechnology. Molecules 2023, 28, 4940. [Google Scholar] [CrossRef] [PubMed]
- López-Helguera, I.; López-Gatius, F.; Garcia-Ispierto, I. The influence of genital tract status in postpartum period on the subsequent reproductive performance in high producing dairy cows. Theriogenology 2012, 77, 1334–1342. [Google Scholar] [CrossRef]
- Burgers, E.E.A.; Goselink, R.M.A.; Bruckmaier, R.M.; Gross, J.J.; Jorritsma, R.; Kemp, B.; Kok, A.; van Knegsel, A.T.M. Effect of voluntary waiting period on metabolism of dairy cows during different phases of the lactation. J. Anim. Sci. 2023, 101, skad194. [Google Scholar] [CrossRef]
- Löf, E.; Gustafsson, H.; Emanuelson, U. Factors influencing the chance of cows being pregnant 30 days after the herd voluntary waiting period. J. Dairy Sci. 2014, 97, 2071–2080. [Google Scholar] [CrossRef]
- Ma, J.; Burgers, E.E.A.; Kok, A.; Goselink, R.M.A.; Lam, T.J.G.M.; Kemp, B.; van Knegsel, A.T.M. Consequences of extending the voluntary waiting period for insemination on reproductive performance in dairy cows. Anim. Reprod. Sci. 2022, 244, 107046. [Google Scholar] [CrossRef]
- Sitko, E.M.; Di Croce, F.A.; McNeel, A.K.; Weigel, D.J.; Giordano, J.O. Effect of reproductive management programs that prioritized artificial insemination at detected estrus or timed artificial insemination on the economic performance of primiparous Holstein cows of different genetic merit for fertility. J. Dairy Sci. 2023, 106, 6495–6514. [Google Scholar] [CrossRef]
- Inchaisri, C.; Jorritsma, R.; Vos, P.L.A.M.; van der Weijden, G.C.; Hogeveen, H. Economic consequences of reproductive performance in dairy cattle. Theriogenology 2010, 74, 835–846. [Google Scholar] [CrossRef] [PubMed]
- van Knegsel, A.T.M.; Burgers, E.E.A.; Ma, J.; Goselink, R.M.A.; Kok, A. Extending lactation length: Consequences for cow, calf, and farmer. J. Anim. Sci. 2022, 100, skac220. [Google Scholar] [CrossRef]
- Hansson, A.; Holtenius, K.; Båge, R.; Lindberg, M.; Kronqvist, C. Effect of voluntary waiting period length on milk yield, fertility, and culling in high-yielding, second-parity cows. J. Dairy Sci. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, R.; Pieper, L.; Gonzalez-Grajales, A.; Swinkels, J.; Gelfert, C.; Staufenbiel, R. Evaluation of different acute-phase proteins for herd health diagnostics in early postpartum Holstein Friesian dairy cows. J. Dairy Res. 2021, 88, 33–37. [Google Scholar] [CrossRef]
- Pascottini, O.B.; LeBlanc, S.J. Modulation of immune function in the bovine uterus peripartum. Theriogenology 2020, 150, 193–200. [Google Scholar] [CrossRef]
- Poole, R.K.; Ault-Seay, T.B.; Payton, R.R.; Myer, P.R.; Lear, A.S.; Pohler, K.G. Evaluation of reproductive tract cytokines in post-partum beef cows relating to reproductive microbiota and fertility outcomes. Front. Anim. Sci. 2021, 2, 704714. [Google Scholar] [CrossRef]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Associations of elevated nonesterified fatty acids and β-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J. Dairy Sci. 2010, 93, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, H.; Ding, Y.; Li, J.; Li, J. Changes in the blood routine, biochemical indexes and the pro-inflammatory cytokine expressions of peripheral leukocytes in postpartum dairy cows with metritis. BMC Vet. Res. 2019, 15, 157. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Duffield, T.F.; Foster, R.A.; Gartley, C.J.; Leslie, K.E.; Walton, J.S.; Johnson, W.H. A comparison of the cytobrush and uterine lavage techniques to evaluate endometrial cytology in clinically normal postpartum dairy cows. Can. Vet. J. 2005, 46, 255–259. [Google Scholar]
- Pohl, A.; Burfeind, O.; Heuwieser, W. The associations between postpartum serum haptoglobin concentration and metabolic status, calving difficulties, retained fetal membranes, and metritis. J. Dairy Sci. 2015, 98, 4544–4551. [Google Scholar] [CrossRef] [PubMed]
- Shinya, U.; Iwamura, Y.; Yamato, O.; Pambudi, D.; Widodo, O.S.; Taniguchi, M.; Takagi, M. Serum amyloid A concentrations of healthy and clinically diseased Japanese Black Breeding Cattle-preliminary measurements for determining the cut-off concentrations. Vet. Sci. 2022, 9, 198. [Google Scholar] [CrossRef]
- Trela, M.; Domańska, D.; Witkowska-Piłaszewicz, O. Diagnostic use of serum amyloid A in dairy cattle. Agriculture 2022, 12, 459. [Google Scholar] [CrossRef]
- Myers, M.J.; Smith, E.R.; Turfle, P.G. Biomarkers in Veterinary Medicine. Annu. Rev. Anim. Biosci. 2017, 5, 65–87. [Google Scholar] [CrossRef]
- Li, Z.; Hou, Y.; Zhao, M.; Li, T.; Liu, Y.; Chang, J.; Ren, L. Serum amyloid a, a potential biomarker both in serum and tissue, correlates with ovarian cancer progression. J. Ovarian Res. 2020, 13, 67. [Google Scholar] [CrossRef]
- Gädicke, P.; Letelier, R.; Chihuailaf, R.; Ruiz, A.; Junod, T. Variation in the multivariate relationships between serum amyloid, haptoglobin and biochemical profiles of aborted and non-aborted cows. SOJ Dairy Vet. Sci. 2021, 1, 1–6. [Google Scholar]
- Dong, J.; Li, J.; Li, J.; Cui, L.; Meng, X.; Qu, Y.; Wang, H. The proliferative effect of cortisol on bovine endometrial epithelial cells. Reprod. Biol. Endocrinol. 2019, 17, 97. [Google Scholar] [CrossRef]
- Fouz, R.; Rodríguez-Vermúdez, R.; Rodríguez-Godina, I.J.; Rodríquez-Domínguez, M.; Rico, M.; Diéguez, F.J. Evaluation of haptoglobin concentration in clinically healthy dairy cows: Correlation between serum and milk levels. J. Appl. Anim. Res. 2024, 52, 2300624. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.; Noh, H.; Hong, L.; Chun, E.; Kim, E.; Ro, Y.; Choi, W. Analysis of acute phase response using acute phase proteins following simultaneous vaccination of lumpy skin disease and foot-and-mouth disease. Vaccines 2024, 12, 556. [Google Scholar] [CrossRef]
- Kleinhenz, M.D.; Van Engen, N.K.; Smith, J.S.; Gorden, P.J.; Ji, J.; Wang, C.; Perkins, S.C.B.; Coetzee, J.F. The impact of transdermal flunixin meglumine on biomarkers of pain in calves when administered at the time of surgical castration without local anesthesia. Livest. Sci. 2018, 212, 1–6. [Google Scholar] [CrossRef]
- Barragan, A.A.; Piñeiro, J.M.; Schuenemann, G.M.; Rajala-Schultz, P.J.; Sanders, D.E.; Lakritz, J.; Bas, S. Assessment of daily activity patterns and biomarkers of pain, inflammation, and stress in lactating dairy cows diagnosed with clinical metritis. J. Dairy Sci. 2018, 101, 8248–8258. [Google Scholar] [CrossRef]
- Nakajima, Y.; Momotani, E.; Murakami, T.; Ishikawa, Y.; Morimatsu, M.; Saito, M.; Suzuki, H.; Yasukawa, K. Induction of acute phase protein by recombinant human interleukin-6 (IL-6) in calves. Vet. Immunol. Immunopathol. 1993, 35, 385–391. [Google Scholar] [CrossRef]
- Bazzano, M.; Marchegiani, A.; Troisi, A.; McLean, A.; Laus, F. Serum amyloid A as a promising biomarker in domestic animals’ reproduction: Current knowledge and future perspective. Animals 2022, 12, 589. [Google Scholar] [CrossRef]
- Nakamura, M.; Miyamoto, T.; Camer, G.A.; Koyama, T.; Matsui, Y.; Sugiura, T.; Moriyoshi, M.; Nakada, K.; Sawamukai, Y. Postpartum clinicopathological and reproductive performance assessment and haptoglobin measurement of dairy cattle with retained fetal membrane. Philipp. Agric. Sci. 2020, 103, 235–244. [Google Scholar] [CrossRef]
- Shin, D.H.; Jeong, J.K.; Choi, I.S.; Moon, S.H.; Lee, S.C.; Kang, H.G.; Park, S.B.; Kim, I.H. Associations between serum haptoglobin concentration and peri- and postpartum disorders, milk yield, and reproductive performance in dairy cows. Livest. Sci. 2018, 213, 14–18. [Google Scholar] [CrossRef]
- Bustamante, H.A.; Rodríguez, A.R.; Herzberg, D.E.; Werner, M.P. Stress and pain response after oligofructose induced-lameness in dairy heifers. J. Vet. Sci. 2015, 16, 405–411. [Google Scholar] [CrossRef]
- Kasimanickam, G.; Kasimanickam, V. Impact of heat stress on embryonic development during first 16 days of gestation in dairy cows. Sci. Rep. 2021, 11, 14839. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Novo, A.; Pérez-Garnelo, S.S.; Villagrá, A.; Pérez-Villalobos, N.; Astiz, S. The effect of stress on reproduction and reproductive technologies in beef cattle-A review. Animals 2020, 10, 2096. [Google Scholar] [CrossRef] [PubMed]
- Mosher, R.A.; Coetzee, J.F.; Allen, P.S.; Havel, J.A.; Griffith, G.R.; Wang, C. Effects of sample handling methods on substance P concentrations and immunoreactivity in bovine blood samples. Am. J. Vet. Res. 2014, 75, 109–116. [Google Scholar] [CrossRef]
- Zieglgänsberger, W. Substance P and pain chronicity. Cell Tissue Res. 2019, 375, 227–241. [Google Scholar] [CrossRef]
- Ahmad, W.; Shabbir, M.A.B.; Ahmad, M.; Omer, M.O.; Mushtaq, R.M.Z.; Aroosa, S.; Iqbal, A.; Majeed, A. Insights into the prognostic role of serum interleukin-6 and hematobiochemical alterations in cattle during recent outbreaks of lumpy skin disease in Lodhran district, Pakistan. Vaccines 2023, 11, 113. [Google Scholar] [CrossRef]
- Giurgiu, O.V.; Berean, D.I.; Ionescu, A.; Ciupe, M.S.; Cimpean, C.R.; Radu, C.I.; Bitica, D.G.; Bogdan, S.; Bogdan, M.L. The effect of oral administration of zeolite on the energy metabolism and reproductive health of Romanian spotted breed in advanced gestation and post partum period. Vet. Anim. Sci. 2024, 23, 100333. [Google Scholar] [CrossRef]
- Koets, A.P.; de Schwartz, N.; Tooten, P.; Kankofer, M.; Broekhuijsen-Davies, J.M.; Rutten, V.P.M.G.; van Leengoed, L.A.M.G.; Taverne, M.A.M.; Gruys, E. Release of proinflammatory cytokines related to luteolysis and the periparturient acute phase response in prostaglandin-induced parturition in cows. Theriogenology 1998, 49, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Sack, G.H., Jr. Serum amyloid A—A review. Mol. Med. 2018, 24, 46. [Google Scholar] [CrossRef] [PubMed]
- Tschoner, T.; Feist, M. Substance P concentrations in the blood plasma and serum of adult cattle and calves during different painful procedures and conditions-a systematic review. BMC Vet. Res. 2022, 18, 232. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, J.F.; Lubbers, B.V.; Toerber, S.E.; Gehring, R.; Thomson, D.U.; Whithe, B.J.; Apley, M.D. Plasma concentrations of substance P and cortisol in beef calves after castration or stimulated castration. Appl. Anim. Behav. Sci. 2008, 69, 751–762. [Google Scholar]
- Berean, D.I.; Bogdan, L.M.; Cimpean, R. Comparative Evaluation of Ovsynch and Double Ovsynch Protocols with Single and Double Insemination in Holstein Dairy Cows: Reproductive Performance and Cost Analysis. Animals 2025, 15, 2380. [Google Scholar] [CrossRef]

| Group | ER | LR | ||
|---|---|---|---|---|
| Recovery day after calving | 30.3 ± 1.5 | 51.5 ± 2.7 | ||
| Parity | 1.5 ± 0.2 | 1.7 ± 0.2 | ||
| Analysis point (day after calving) | ER1 (period 1) | 4.9 ± 0.5 | LR1 (period 1) | 4.2 ± 0.8 |
| ER2 (period 2) | 30.3 ± 1.5 | LR2 (period 2) | 29.1 ± 1.1 | |
| LR3 (period 3) | 51.5 ± 2.7 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ro, Y.; Kim, J.; Chun, E.; Choe, E.; Lee, E.; Choi, W.; Kim, D. Pilot Study: Exploring the Feasibility of Individual Voluntary Waiting Period Settings Using Postpartum Recovery Indicators in Dairy Cows. Animals 2025, 15, 3331. https://doi.org/10.3390/ani15223331
Ro Y, Kim J, Chun E, Choe E, Lee E, Choi W, Kim D. Pilot Study: Exploring the Feasibility of Individual Voluntary Waiting Period Settings Using Postpartum Recovery Indicators in Dairy Cows. Animals. 2025; 15(22):3331. https://doi.org/10.3390/ani15223331
Chicago/Turabian StyleRo, Younghye, Jiyeon Kim, Eunwoo Chun, Eunhui Choe, Eunsong Lee, Woojae Choi, and Danil Kim. 2025. "Pilot Study: Exploring the Feasibility of Individual Voluntary Waiting Period Settings Using Postpartum Recovery Indicators in Dairy Cows" Animals 15, no. 22: 3331. https://doi.org/10.3390/ani15223331
APA StyleRo, Y., Kim, J., Chun, E., Choe, E., Lee, E., Choi, W., & Kim, D. (2025). Pilot Study: Exploring the Feasibility of Individual Voluntary Waiting Period Settings Using Postpartum Recovery Indicators in Dairy Cows. Animals, 15(22), 3331. https://doi.org/10.3390/ani15223331





