Besnoitia besnoiti-Induced Neutrophil Extracellular Traps (NETs): Metabolic Signature, Signaling Pathways, Receptors and Implications on Pathogenesis
Simple Summary
Abstract
1. Introduction
1.1. The Apicomplexan Parasite Besnoitia Besnoiti: Taxonomy, Pathogeny and Clinical Signs
1.2. Innate Immune System and NET Formation
1.3. Literature Search Strategy
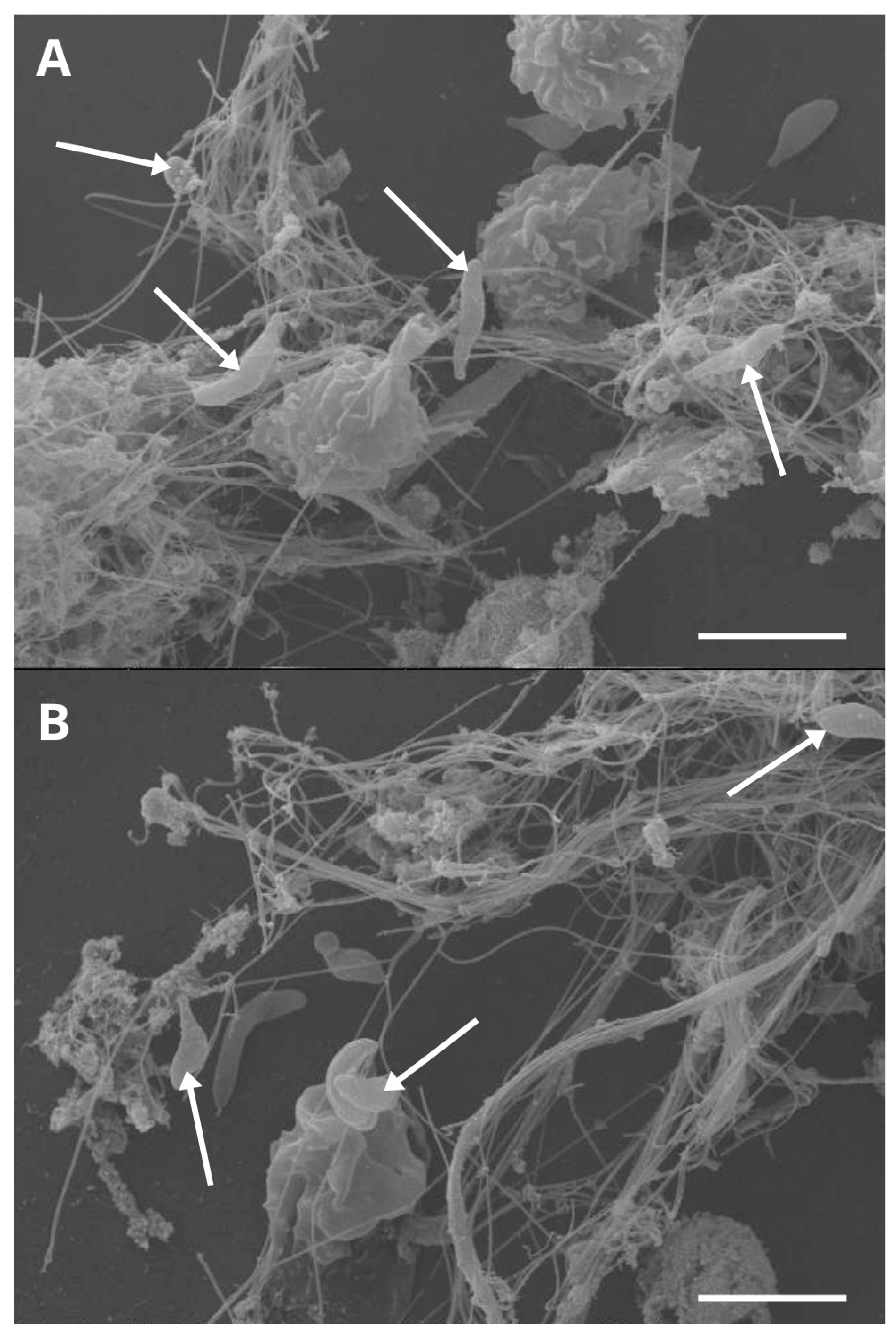
2. NETs-Derived Effects on Besnoitia besnoiti
3. Signaling Pathways Involved in Bovine NETosis Against B. besnoiti
Purinergic Signaling in Suicidal NETosis Against B. besnoiti
4. Metabolic Changes Linked to Bovine NETosis Against B. besnoiti
Pyruvate- and Lactate-Mediated Metabolic Pathways Are Implicated in B. besnoiti Tachyzoite-Induced NET Formation
5. Autophagy-Related Signaling and B. besnoiti-Induced Bovine NETosis
6. Interaction Between Bovine NET and Endothelium in the B. besnoiti-Generated Infection
7. Besnoitia bensoiti Bradyzoites Trigger Suicidal and Vital NETosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EFSA | European Food Safety Authority |
| ETs | Extracellular traps |
| MPO | Myeloperoxidase |
| NE | Neutrophil elastase |
| NETs | Neutrophil extracellular traps |
| NOX | Nicotinamide adenine dinucleotide phosphate oxidase |
| PMN | Polymorphonuclear neutrophils |
| ROS | Reactive oxygen species |
References
- Cortes, H.; Leitão, A.; Gottstein, B.; Hemphill, A. A Review on Bovine Besnoitiosis: A Disease with Economic Impact in Herd Health Management, Caused by Besnoitia besnoiti (Franco and Borges, 1916). Parasitology 2014, 141, 1406–1417. [Google Scholar] [CrossRef]
- Villa, L.; Gazzonis, A.L.; Perlotti, C.; Zanzani, S.A.; Sironi, G.; Manfredi, M.T. First Report of Demodex Bovis Infestation in Bovine Besnoitiosis Co-Infected Dairy Cattle in Italy. Parasitol. Int. 2020, 75, 102021. [Google Scholar] [CrossRef]
- Alobaidii, W.A.; Abdullah, D.A.; Alkatab, Y.N.M.; Ali, S.A.; Ola-Fadunsin, S.D.; Gimba, F.I. The First Molecular Investigation of Besnoitia besnoiti Infections among Cattle in Mosul, Iraq. Mol. Biol. Rep. 2024, 51, 585. [Google Scholar] [CrossRef]
- Álvarez-García, G.; García-Lunar, P.; Gutiérrez-Expósito, D.; Shkap, V.; Ortega-Mora, L.M. Dynamics of Besnoitia besnoiti Infection in Cattle. Parasitology 2014, 141, 1419–1435. [Google Scholar] [CrossRef]
- Olias, P.; Schade, B.; Mehlhorn, H. Molecular Pathology, Taxonomy and Epidemiology of Besnoitia Species (Protozoa: Sarcocystidae). Infect. Genet. Evol. 2011, 11, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Malatji, M.P.; Tembe, D.; Mukaratirwa, S. An Update on Epidemiology and Clinical Aspects of Besnoitiosis in Livestock and Wildlife in Sub-Saharan Africa: A Systematic Review. Parasite Epidemiol. Control 2023, 21, e00284. [Google Scholar] [CrossRef] [PubMed]
- Bentancourt Rossoli, J.V.; Moré, G.; Soto-Cabrera, A.; Moore, D.P.; Morrell, E.L.; Campero, L.M.; Basso, W.; Hecker, Y.P.; Scioscia, N.P. First Report of Natural Besnoitia akodoni Infection in Synanthropic (Muridae) and Wild (Cricetidae) Rodents from Argentina. Vet. Parasitol. Reg. Stud. Rep. 2025, 60, 101245. [Google Scholar] [CrossRef]
- Dubey, J.P.; Yabsley, M.J. Besnoitia neotomofelis n. sp. (Protozoa: Apicomplexa) from the Southern Plains Woodrat (Neotoma micropus). Parasitology 2010, 137, 1731–1747. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Silver, I.A.; Sadoughifar, R. Caprine Besnoitiosis: An Emerging Threat and Its Relationship to Some Other Infections of Ungulates by Besnoitia Species. Res. Vet. Sci. 2014, 97, 1–7. [Google Scholar] [CrossRef]
- Schares, G.; Jutras, C.; Bärwald, A.; Basso, W.; Maksimov, A.; Schares, S.; Tuschy, M.; Conraths, F.J.; Brodeur, V. Besnoitia tarandi in Canadian Woodland Caribou—Isolation, Characterization and Suitability for Serological Tests. Int. J. Parasitol. Parasites Wildl. 2019, 8, 1–9. [Google Scholar] [CrossRef]
- Berman, N.; Tirosh-Levy, S.; Steinman, A.; Minderigiu, A.; Blinder, E.; Leszkowicz Mazuz, M. First Detection of Anti-Besnoitia spp. Antibodies in Equids in Israel and the Palestinian Authority. Microorganisms 2023, 11, 929. [Google Scholar] [CrossRef] [PubMed]
- Tinkler, S.H.; Villa, L.; Manfredi, M.T.; Walshe, N.; Jahns, H. First Report of Besnoitia bennetti in Irish Donkeys: An Emerging Parasitic Disease in Europe. Ir. Vet. J. 2024, 77, 2. [Google Scholar] [CrossRef] [PubMed]
- Basso, W.; Lesser, M.; Grimm, F.; Hilbe, M.; Sydler, T.; Trösch, L.; Ochs, H.; Braun, U.; Deplazes, P. Bovine Besnoitiosis in Switzerland: Imported Cases and Local Transmission. Vet. Parasitol. 2013, 198, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-García, G.; Frey, C.F.; Mora, L.M.O.; Schares, G. A Century of Bovine Besnoitiosis: An Unknown Disease Re-Emerging in Europe. Trends Parasitol. 2013, 29, 407–415. [Google Scholar] [CrossRef]
- Habarugira, G.; Nkuranga, C.; Asiimwe, B.; Turikumwenayo, J.B.; Ojok, L. First Confirmed Case of Bovine Besnoitiosis in Rwanda. Vet. Parasitol. Reg. Stud. Rep. 2019, 17, 100294. [Google Scholar] [CrossRef]
- Jacinto, J.; Graziosi, G.; Galuppi, R.; Poluzzi, A.; Ogundipe, T.; Militerno, G.; Beltrame, A.; Gentile, A.; Dini, F.M. Bovine Besnoitiosis: Assessment of the Diagnostic Accuracy of Three Different Tests Using a Bayesian Latent Class Model Approach and Clinical Characterization of the Disease. Prev. Vet. Med. 2025, 235, 106415. [Google Scholar] [CrossRef]
- Vanhoudt, A.; Pardon, B.; De Schutter, P.; Bosseler, L.; Sarre, C.; Vercruysse, J.; Deprez, P. Eerste Bevestigd Geval van Boviene Besnoitiose in België Bij Een Ingevoerde Stier. Vlaams Diergeneeskd. Tijdschr. 2015, 84, 205–211. [Google Scholar] [CrossRef]
- Rostaher, A.; Mueller, R.S.; Majzoub, M.; Schares, G.; Gollnick, N.S. Bovine Besnoitiosis in Germany. Vet. Dermatol. 2010, 21, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Expósito, D.; Ferre, I.; Ortega-Mora, L.M.; Álvarez-García, G. Advances in the Diagnosis of Bovine Besnoitiosis: Current Options and Applications for Control. Int. J. Parasitol. 2017, 47, 737–751. [Google Scholar] [CrossRef]
- Ramakrishnan, C.; Krishnan, A.; Francisco, S.; Schmid, M.W.; Russo, G.; Leitão, A.; Hemphill, A.; Soldati-Favre, D.; Hehl, A.B. Dissection of Besnoitia besnoiti Intermediate Host Life Cycle Stages: From Morphology to Gene Expression. PLoS Pathog. 2022, 18, e1010955. [Google Scholar] [CrossRef]
- Muñoz-Caro, T.; Silva, L.M.R.; Ritter, C.; Taubert, A.; Hermosilla, C. Besnoitia besnoiti Tachyzoites Induce Monocyte Extracellular Trap Formation. Parasitol. Res. 2014, 113, 4189–4197. [Google Scholar] [CrossRef]
- Velásquez, Z.D.; Lopez-Osorio, S.; Pervizaj-Oruqaj, L.; Herold, S.; Hermosilla, C.; Taubert, A. Besnoitia besnoiti–Driven Endothelial Host Cell Cycle Alteration. Parasitol. Res. 2020, 119, 2563–2577. [Google Scholar] [CrossRef]
- Gollnick, N.S.; Scharr, J.C.; Schares, S.; Bärwald, A.; Schares, G.; Langenmayer, M.C. Naturally Acquired Bovine Besnoitiosis: Disease Frequency, Risk and Outcome in an Endemically Infected Beef Herd. Transbound. Emerg. Dis. 2018, 65, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Caro, T.; Hermosilla, C.; Silva, L.M.R.; Cortes, H.; Taubert, A. Neutrophil Extracellular Traps as Innate Immune Reaction against the Emerging Apicomplexan Parasite Besnoitia besnoiti. PLoS ONE 2014, 9, e91415. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, W.; Shen, F.; Du, W.; Xu, Z.; Liu, Z. The Emerging Role of Neutrophil Extracellular Traps in Arterial, Venous and Cancer-Associated Thrombosis. Front. Cardiovasc. Med. 2021, 8, 786387. [Google Scholar] [CrossRef]
- Conejeros, I.; Velásquez, Z.D.; Grob, D.; Zhou, E.; Salecker, H.; Hermosilla, C.; Taubert, A. Histone H2A and Bovine Neutrophil Extracellular Traps Induce Damage of Besnoitia besnoiti-Infected Host Endothelial Cells but Fail to Affect Total Parasite Proliferation. Biology 2019, 8, 78. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Sollberger, G.; Tilley, D.O.; Zychlinsky, A. Neutrophil Extracellular Traps: The Biology of Chromatin Externalization. Dev. Cell 2018, 44, 542–553. [Google Scholar] [CrossRef]
- Kraus, R.F.; Gruber, M.A. Neutrophils—From Bone Marrow to First-Line Defense of the Innate Immune System. Front. Immunol. 2021, 12, 767175. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.R.; Muñoz-Caro, T.; Burgos, R.A.; Hidalgo, M.A.; Taubert, A.; Hermosilla, C. Far beyond Phagocytosis: Phagocyte-Derived Extracellular Traps Act Efficiently against Protozoan Parasites In Vitro and In Vivo. Mediat. Inflamm. 2016, 2016, 5898074. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, Z.A.; Khosrojerdi, A.; Aslani, S.; Hemmatzadeh, M.; Babaie, F.; Bairami, A.; Shomali, N.; Hosseinzadeh, R.; Safari, R.; Mohammadi, H. Multi-Facets of Neutrophil Extracellular Trap in Infectious Diseases: Moving beyond Immunity. Microb. Pathog. 2021, 158, 105066. [Google Scholar] [CrossRef]
- Tsioumpekou, M.; Krijgsman, D.; Leusen, J.H.W.; Olofsen, P.A. The Role of Cytokines in Neutrophil Development, Tissue Homing, Function and Plasticity in Health and Disease. Cells 2023, 12, 1981. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Neutrophils versus Protozoan Parasites: Plasmodium, Trichomonas, Leishmania, Trypanosoma, and Entameoba. Microorganisms 2024, 12, 827. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel Cell Death Program Leads to Neutrophil Extracellular Traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Wang, H.; Kim, S.J.; Lei, Y.; Wang, S.; Wang, H.; Huang, H.; Zhang, H.; Tsung, A. Neutrophil Extracellular Traps in Homeostasis and Disease. Signal Transduct. Target. Ther. 2024, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Ma, Y.; Opsomer, G.; Pascottini, O.B.; Guan, Y.; Dong, Q. Neutrophil Extracellular Traps in Cattle Health and Disease. Res. Vet. Sci. 2021, 139, 4–10. [Google Scholar] [CrossRef]
- Yipp, B.G.; Kubes, P. NETosis: How Vital Is It? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef]
- Yildiz, K.; Gokpinar, S.; Gazyagci, A.N.; Babur, C.; Sursal, N.; Azkur, A.K. Role of NETs in the Difference in Host Susceptibility to Toxoplasma Gondii between Sheep and Cattle. Vet. Immunol. Immunopathol. 2017, 189, 1–10. [Google Scholar] [CrossRef]
- Maksimov, P.; Hermosilla, C.; Kleinertz, S.; Hirzmann, J.; Taubert, A. Besnoitia besnoiti Infections Activate Primary Bovine Endothelial Cells and Promote PMN Adhesion and NET Formation under Physiological Flow Condition. Parasitol. Res. 2016, 115, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Conejeros, I.; Velásquez, Z.D.; Muñoz-Caro, T.; Gärtner, U.; Hermosilla, C.; Taubert, A. Simultaneous and Positively Correlated NET Formation and Autophagy in Besnoitia besnoiti Tachyzoite-Exposed Bovine Polymorphonuclear Neutrophils. Front. Immunol. 2019, 10, 1131. [Google Scholar] [CrossRef]
- Zhou, E.; Conejeros, I.; Gärtner, U.; Mazurek, S.; Hermosilla, C.; Taubert, A. Metabolic Requirements of Besnoitia besnoiti Tachyzoite-Triggered NETosis. Parasitol. Res. 2020, 119, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Silva, L.M.R.; Conejeros, I.; Velásquez, Z.D.; Hirz, M.; Gärtner, U.; Jacquiet, P.; Taubert, A.; Hermosilla, C. Besnoitia besnoiti Bradyzoite Stages Induce Suicidal- and Rapid Vital-NETosis. Parasitology 2020, 147, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, G.; Conejeros, I.; Rojas-Barón, L.; Hermosilla, C.R.; Taubert, A. Besnoitia besnoiti-Induced Neutrophil Clustering and Neutrophil Extracellular Trap Formation Depend on P2X1 Purinergic Receptor Signaling. Front. Immunol. 2023, 14, 1244068. [Google Scholar] [CrossRef]
- Conejeros, I.; Velásquez, Z.D.; Rojas-Barón, L.; Espinosa, G.; Hermosilla, C.; Taubert, A. The CAMKK/AMPK Pathway Contributes to Besnoitia besnoiti-Induced NETosis in Bovine Polymorphonuclear Neutrophils. Int. J. Mol. Sci. 2024, 25, 8442. [Google Scholar] [CrossRef]
- Mendez, J.; Sun, D.; Tuo, W.; Xiao, Z. Bovine Neutrophils Form Extracellular Traps in Response to the Gastrointestinal Parasite Ostertagia ostertagi. Sci. Rep. 2018, 8, 17598. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Godínez, C.; Carrero, J.C. The State of Art of Neutrophil Extracellular Traps in Protozoan and Helminthic Infections. Biosci. Rep. 2019, 39, BSR20180916. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Turner, J.D.; Pionnier, N.; Midgley, A.; Guimaraes, A.F.; Johnston, K.L.; Edwards, S.W.; Taylor, M.J. Wolbachia Endosymbionts Induce Neutrophil Extracellular Trap Formation in Human Onchocerciasis. Sci. Rep. 2016, 6, 35559. [Google Scholar] [CrossRef]
- Muñoz-Caro, T.; Saraiva, E.M.; Mariante, R.M. Bibliometric Analysis of Neutrophil Extracellular Traps Induced by Protozoan and Helminth Parasites (2008–2024). Front. Immunol. 2025, 16, 1498453. [Google Scholar] [CrossRef]
- Burgos, R.A.; Werling, D.; Hermosilla, C.R. Editorial: The Emerging Role of Metabolism and Metabolic-Related Receptors on Neutrophil Extracellular Traps (NET) Formation. Front. Immunol. 2022, 13, 1028228. [Google Scholar] [CrossRef]
- Baz, A.A.; Hao, H.; Lan, S.; Li, Z.; Liu, S.; Chen, S.; Chu, Y. Neutrophil Extracellular Traps in Bacterial Infections and Evasion Strategies. Front. Immunol. 2024, 15, 1357967. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Berends, E.T.M.; Chan, R.; Schwab, E.; Roy, S.; Sen, C.K.; Torres, V.J.; Wozniak, D.J. Staphylococcus aureus Biofilms Release Leukocidins to Elicit Extracellular Trap Formation and Evade Neutrophil-Mediated Killing. Proc. Natl. Acad. Sci. USA 2018, 115, 7416–7421. [Google Scholar] [CrossRef] [PubMed]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.H.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.Y.; Surette, M.G.; Sugai, M.; et al. A Novel Mechanism of Rapid Nuclear Neutrophil Extracellular Trap Formation in Response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef] [PubMed]
- Döhrmann, S.; LaRock, C.N.; Anderson, E.L.; Cole, J.N.; Ryali, B.; Stewart, C.; Nonejuie, P.; Pogliano, J.; Corriden, R.; Ghosh, P.; et al. Group A Streptococcal M1 Protein Provides Resistance against the Antimicrobial Activity of Histones. Sci. Rep. 2017, 7, 43039. [Google Scholar] [CrossRef]
- Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; Bernuth, H.V.; Zychlinsky, A. Diverse Stimuli Engage Different Neutrophil Extracellular Trap Pathways. eLife 2017, 6, e24437. [Google Scholar] [CrossRef]
- Dai, J.; Lai, L.; Tang, H.; Wang, W.; Wang, S.; Lu, C.; Yao, H.; Fan, H.; Wu, Z. Streptococcus suis Synthesizes Deoxyadenosine and Adenosine by 5′-Nucleotidase to Dampen Host Immune Responses. Virulence 2018, 9, 1509–1520. [Google Scholar] [CrossRef]
- Krivošíková, K.; Šupčíková, N.; Gaál Kovalčíková, A.; Janko, J.; Pastorek, M.; Celec, P.; Podracká, Ľ.; Tóthová, Ľ. Neutrophil Extracellular Traps in Urinary Tract Infection. Front. Pediatr. 2023, 11, 1154139. [Google Scholar] [CrossRef]
- Floyd, M.; Winn, M.; Cullen, C.; Sil, P.; Chassaing, B.; Yoo, D.; Gewirtz, A.T.; Goldberg, J.B.; McCarter, L.L.; Rada, B. Swimming Motility Mediates the Formation of Neutrophil Extracellular Traps Induced by Flagellated Pseudomonas aeruginosa. PLoS Pathog. 2016, 12, e1005987. [Google Scholar] [CrossRef]
- Ladero-Auñon, I.; Molina, E.; Holder, A.; Kolakowski, J.; Harris, H.; Urkitza, A.; Anguita, J.; Werling, D.; Elguezabal, N. Bovine Neutrophils Release Extracellular Traps and Cooperate With Macrophages in Mycobacterium avium Subsp. Paratuberculosis Clearance In Vitro. Front. Immunol. 2021, 12, 645304. [Google Scholar] [CrossRef]
- Gondaira, S.; Nishi, K.; Fujiki, J.; Iwano, H.; Watanabe, R.; Eguchi, A.; Hirano, Y.; Higuchi, H.; Nagahata, H. Innate Immune Response in Bovine Neutrophils Stimulated with Mycoplasma bovis. Vet. Res. 2021, 52, 58. [Google Scholar] [CrossRef]
- Guimarães-Costa, A.B.; Nascimento, M.T.C.; Froment, G.S.; Soares, R.P.P.; Morgado, F.N.; Conceição-Silva, F.; Saraiva, E.M. Leishmania amazonensis Promastigotes Induce and Are Killed by Neutrophil Extracellular Traps. Proc. Natl. Acad. Sci. USA 2009, 106, 6748–6753. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK Pathway Is Required for Neutrophil Extracellular Trap Formation. Nat. Chem. Biol. 2011, 7, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 Channel and Mitochondrial ROS Mediate NADPH Oxidase-Independent NETosis Induced by Calcium Influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef]
- Leshner, M.; Wang, S.; Lewis, C.; Zheng, H.; Chen, X.A.; Santy, L.; Wang, Y. PAD4 Mediated Histone Hypercitrullination Induces Heterochromatin Decondensation and Chromatin Unfolding to Form Neutrophil Extracellular Trap-like Structures. Front. Immun. 2012, 3, 307. [Google Scholar] [CrossRef]
- Osca-Verdegal, R.; Beltrán-García, J.; Paes, A.B.; Nacher-Sendra, E.; Novella, S.; Hermenegildo, C.; Carbonell, N.; García-Giménez, J.L.; Pallardó, F.V. Histone Citrullination Mediates a Protective Role in Endothelium and Modulates Inflammation. Cells 2022, 11, 4070. [Google Scholar] [CrossRef]
- Cekic, C.; Linden, J. Purinergic Regulation of the Immune System. Nat. Rev. Immunol. 2016, 16, 177–192. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, Y.; Sumi, Y.; Li, A.; To, U.K.; Elkhal, A.; Inoue, Y.; Woehrle, T.; Zhang, Q.; Hauser, C.; et al. Purinergic Signaling: A Fundamental Mechanism in Neutrophil Activation. Sci. Signal. 2010, 3, ra45. [Google Scholar] [CrossRef]
- Wang, X.; Chen, D. Purinergic Regulation of Neutrophil Function. Front. Immunol. 2018, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Mousawi, F.; Li, D.; Roger, S.; Li, J.; Yang, X.; Jiang, L.-H. Adenosine Triphosphate Release and P2 Receptor Signaling in Piezo1 Channel-Dependent Mechanoregulation. Front. Pharmacol. 2019, 10, 1304. [Google Scholar] [CrossRef] [PubMed]
- Rubenich, D.S.; Omizzollo, N.; Szczepański, M.J.; Reichert, T.E.; Whiteside, T.L.; Ludwig, N.; Braganhol, E. Small Extracellular Vesicle-Mediated Bidirectional Crosstalk between Neutrophils and Tumor Cells. Cytokine Growth Factor Rev. 2021, 61, 16–26. [Google Scholar] [CrossRef]
- Conejeros, I.; López-Osorio, S.; Zhou, E.; Velásquez, Z.D.; Del Río, M.C.; Burgos, R.A.; Alarcón, P.; Chaparro-Gutiérrez, J.J.; Hermosilla, C.; Taubert, A. Glycolysis, Monocarboxylate Transport, and Purinergic Signaling Are Key Events in Eimeria Bovis-Induced NETosis. Front. Immunol. 2022, 13, 842482. [Google Scholar] [CrossRef]
- Awasthi, D.; Nagarkoti, S.; Sadaf, S.; Chandra, T.; Kumar, S.; Dikshit, M. Glycolysis Dependent Lactate Formation in Neutrophils: A Metabolic Link between NOX-Dependent and Independent NETosis. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2019, 1865, 165542. [Google Scholar] [CrossRef]
- Morrison, T.; Watts, E.R.; Sadiku, P.; Walmsley, S.R. The Emerging Role for Metabolism in Fueling Neutrophilic Inflammation. Immunol. Rev. 2023, 314, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Espinosa, O.; Rojas-Espinosa, O.; Moreno-Altamirano, M.M.B.; López-Villegas, E.O.; Sánchez-García, F.J. Metabolic Requirements for Neutrophil Extracellular Traps Formation. Immunology 2015, 145, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Ledderose, C.; Seier, T.; Graf, A.F.; Brix, B.; Chong, E.; Junger, W.G. Mitochondria Regulate Neutrophil Activation by Generating ATP for Autocrine Purinergic Signaling. J. Biol. Chem. 2014, 289, 26794–26803. [Google Scholar] [CrossRef]
- Maianski, N.A.; Geissler, J.; Srinivasula, S.M.; Alnemri, E.S.; Roos, D.; Kuijpers, T.W. Functional Characterization of Mitochondria in Neutrophils: A Role Restricted to Apoptosis. Cell Death Differ. 2004, 11, 143–153. [Google Scholar] [CrossRef]
- Gray, L.R.; Tompkins, S.C.; Taylor, E.B. Regulation of Pyruvate Metabolism and Human Disease. Cell. Mol. Life Sci. 2014, 71, 2577–2604. [Google Scholar] [CrossRef]
- Yiew, N.K.H.; Vazquez, J.H.; Martino, M.R.; Kennon-McGill, S.; Price, J.R.; Allard, F.D.; Yee, E.U.; Layman, A.J.; James, L.P.; McCommis, K.S.; et al. Hepatic Pyruvate and Alanine Metabolism Are Critical and Complementary for Maintenance of Antioxidant Capacity and Resistance to Oxidative Insult. Mol. Metab. 2023, 77, 101808. [Google Scholar] [CrossRef] [PubMed]
- Skendros, P.; Mitroulis, I.; Ritis, K. Autophagy in Neutrophils: From Granulopoiesis to Neutrophil Extracellular Traps. Front. Cell Dev. Biol. 2018, 6, 109. [Google Scholar] [CrossRef]
- Itakura, A.; McCarty, O.J.T. Pivotal Role for the mTOR Pathway in the Formation of Neutrophil Extracellular Traps via Regulation of Autophagy. Am. J. Physiol. Cell Physiol. 2013, 305, C348–C354. [Google Scholar] [CrossRef]
- Cao, J.-F.; Chen, J. Pseudomonas plecoglossicida Infection Induces Neutrophil Autophagy-Driven NETosis in Large Yellow Croaker Larimichthys crocea. Front. Immunol. 2024, 15, 1521080. [Google Scholar] [CrossRef]
- Park, S.Y.; Shrestha, S.; Youn, Y.-J.; Kim, J.-K.; Kim, S.-Y.; Kim, H.J.; Park, S.-H.; Ahn, W.-G.; Kim, S.; Lee, M.G.; et al. Autophagy Primes Neutrophils for Neutrophil Extracellular Trap Formation during Sepsis. Am. J. Respir. Crit. Care Med. 2017, 196, 577–589. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Hong, C.-W.; Kim, E.Y.; Lee, J.M. Current Understanding on the Metabolism of Neutrophils. Immune Netw. 2020, 20, e46. [Google Scholar] [CrossRef]
- Alba, G.; El Bekay, R.; Álvarez-Maqueda, M.; Chacón, P.; Vega, A.; Monteseirín, J.; Santa María, C.; Pintado, E.; Bedoya, F.J.; Bartrons, R.; et al. Stimulators of AMP-activated Protein Kinase Inhibit the Respiratory Burst in Human Neutrophils. FEBS Lett. 2004, 573, 219–225. [Google Scholar] [CrossRef]
- Silwal, P.; Kim, J.K.; Yuk, J.-M.; Jo, E.-K. AMP-Activated Protein Kinase and Host Defense against Infection. Int. J. Mol. Sci. 2018, 19, 3495. [Google Scholar] [CrossRef]
- Bae, H.; Zmijewski, J.W.; Deshane, J.S.; Tadie, J.; Chaplin, D.D.; Takashima, S.; Abraham, E. AMP-activated Protein Kinase Enhances the Phagocytic Ability of Macrophages and Neutrophils. FASEB J. 2011, 25, 4358–4368. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, J.K.; Sharma, A.; Sukumaran, P.; Sun, Y.; Mishra, B.B.; Singh, B.B.; Sharma, J. Oxidant Sensor Cation Channel TRPM2 Regulates Neutrophil Extracellular Trap Formation and Protects against Pneumoseptic Bacterial Infection. FASEB J. 2018, 32, 6848–6859. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, S.; Ye, S.; Tang, S.; Hu, D.; Wei, L.; Xiao, F. Hexavalent Chromium Inhibits the Formation of Neutrophil Extracellular Traps and Promotes the Apoptosis of Neutrophils via AMPK Signaling Pathway. Ecotoxicol. Environ. Saf. 2021, 223, 112614. [Google Scholar] [CrossRef] [PubMed]
- Conejeros, I.; Velásquez, Z.D.; Espinosa, G.; Rojas-Baron, L.; Grabbe, M.; Hermosilla, C.; Taubert, A. AMPK and CAMKK Activation Participate in Early Events of Toxoplasma Gondii-Triggered NET Formation in Bovine Polymorphonuclear Neutrophils. Front. Vet. Sci. 2025, 12, 1557509. [Google Scholar] [CrossRef]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK Activators: Mechanisms of Action and Physiological Activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK1 (hATG1) by AMP-Activated Protein Kinase Connects Energy Sensing to Mitophagy. Science 2011, 331, 456–461. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Yuan, M.; Fan, H.; Cai, Z. Role of AMPK in Autophagy. Front. Physiol. 2022, 13, 1015500. [Google Scholar] [CrossRef]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.-Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.-L. ULK1 Induces Autophagy by Phosphorylating Beclin-1 and Activating VPS34 Lipid Kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.R.; Lütjohann, D.; Hamid, P.; Velasquez, Z.D.; Kerner, K.; Larrazabal, C.; Failing, K.; Hermosilla, C.; Taubert, A. Besnoitia besnoiti Infection Alters Both Endogenous Cholesterol de Novo Synthesis and Exogenous LDL Uptake in Host Endothelial Cells. Sci. Rep. 2019, 9, 6650. [Google Scholar] [CrossRef] [PubMed]
- Larrazabal, C.; Hermosilla, C.; Taubert, A.; Silva, L.M.R. Besnoitia besnoiti Tachyzoite Replication in Bovine Primary Endothelial Cells Relies on Host Niemann–Pick Type C Protein 1 for Cholesterol Acquisition. Front. Vet. Sci. 2024, 11, 1454855. [Google Scholar] [CrossRef]
- Mai, J.; Virtue, A.; Shen, J.; Wang, H.; Yang, X.-F. An Evolving New Paradigm: Endothelial Cells—Conditional Innate Immune Cells. J. Hematol. Oncol. 2013, 6, 61. [Google Scholar] [CrossRef]
- Jiménez-Meléndez, A.; Ramakrishnan, C.; Hehl, A.B.; Russo, G.; Álvarez-García, G. RNA-Seq Analyses Reveal That Endothelial Activation and Fibrosis Are Induced Early and Progressively by Besnoitia besnoiti Host Cell Invasion and Proliferation. Front. Cell. Infect. Microbiol. 2020, 10, 218. [Google Scholar] [CrossRef]
- Erpenbeck, L.; Gruhn, A.L.; Kudryasheva, G.; Günay, G.; Meyer, D.; Busse, J.; Neubert, E.; Schön, M.P.; Rehfeldt, F.; Kruss, S. Effect of Adhesion and Substrate Elasticity on Neutrophil Extracellular Trap Formation. Front. Immunol. 2019, 10, 2320. [Google Scholar] [CrossRef]
- Lögters, T.; Margraf, S.; Altrichter, J.; Cinatl, J.; Mitzner, S.; Windolf, J.; Scholz, M. The Clinical Value of Neutrophil Extracellular Traps. Med. Microbiol. Immunol. 2009, 198, 211–219. [Google Scholar] [CrossRef]
- Díaz-Godínez, C.; Fonseca, Z.; Néquiz, M.; Laclette, J.P.; Rosales, C.; Carrero, J.C. Entamoeba Histolytica Trophozoites Induce a Rapid Non-Classical NETosis Mechanism Independent of NOX2-Derived Reactive Oxygen Species and PAD4 Activity. Front. Cell. Infect. Microbiol. 2018, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones. PLoS ONE 2012, 7, e32366. [Google Scholar] [CrossRef]
- Corsiero, E.; Pratesi, F.; Prediletto, E.; Bombardieri, M.; Migliorini, P. NETosis as Source of Autoantigens in Rheumatoid Arthritis. Front. Immunol. 2016, 7, 485. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense against Candida albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef] [PubMed]
- Zlatina, K.; Lütteke, T.; Galuska, S. Individual Impact of Distinct Polysialic Acid Chain Lengths on the Cytotoxicity of Histone H1, H2A, H2B, H3 and H4. Polymers 2017, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.V.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-Induced NETosis Is a Dynamic Process Involving Neutrophil Multitasking In Vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef]
| Authors (Year) | Host Species/ Cell Type | Parasite Stage | Experimental Approach | Main Findings |
|---|---|---|---|---|
| Muñoz-Caro et al. (2014) [24] | Bovine neutrophils | Tachyzoites | Staining and antibodies for detection of NETs compounds. Spectrofluorometric analysis for quantification of NETs. Treatments with DNase I and inhibitors of NOX, NE and MPO. |
|
| Maksimov et al. (2016) [40] | Bovine neutrophils, BUVEC | Tachyzoites | PMN adhesion assay under physiological flow conditions. |
|
| Conejeros et al. (2019) [26] | Bovine neutrophils, BUVEC | Tachyzoites | Confocal and fluorescence microscopy for detection of extracellular DNA and protein markers of NETs. Fluorescence analysis for estimation of NET-, DNA- and histone 2A (H2A)-induced endothelial cell death. Protein isolectin GS-IB4. |
|
| Zhou et al. (2019) [41] | Bovine neutrophils | Tachyzoites | “Cell-free”-NETs and “anchored”-NETs estimation. Immunofluorescence analysis. Immunoblotting-based analysis. |
|
| Zhou et al. (2020) [42] | Bovine neutrophils | Tachyzoites | Estimation of metabolic conversion rates through pharmacological inhibition and supernatant analysis. |
|
| Zhou et al. (2020) [43] | Bovine neutrophils | Bradyzoites | Histopathological examination. Immunofluorescence microscopy analysis. Live cell 3d holotomographic microscopy. |
|
| Espinosa et al. (2023) [44] | Bovine neutrophils | Tachyzoites | Immunofluorescence microscopy. Picogreen-derived fluorescence intensities. Luminometry. Seahorse XF analyzer. Flow cytometry. |
|
| Conejeros et al. (2024) [45] | Bovine neutrophils | Tachyzoites | Protein Extraction and Western blot. Seahorse XF analyzer. Flow cytometry. Immunofluorescence. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turra, N.; Conejeros, I.; Hermosilla, C.; Burgos, R.A.; Taubert, A. Besnoitia besnoiti-Induced Neutrophil Extracellular Traps (NETs): Metabolic Signature, Signaling Pathways, Receptors and Implications on Pathogenesis. Animals 2025, 15, 3326. https://doi.org/10.3390/ani15223326
Turra N, Conejeros I, Hermosilla C, Burgos RA, Taubert A. Besnoitia besnoiti-Induced Neutrophil Extracellular Traps (NETs): Metabolic Signature, Signaling Pathways, Receptors and Implications on Pathogenesis. Animals. 2025; 15(22):3326. https://doi.org/10.3390/ani15223326
Chicago/Turabian StyleTurra, Nicolás, Iván Conejeros, Carlos Hermosilla, Rafael Agustín Burgos, and Anja Taubert. 2025. "Besnoitia besnoiti-Induced Neutrophil Extracellular Traps (NETs): Metabolic Signature, Signaling Pathways, Receptors and Implications on Pathogenesis" Animals 15, no. 22: 3326. https://doi.org/10.3390/ani15223326
APA StyleTurra, N., Conejeros, I., Hermosilla, C., Burgos, R. A., & Taubert, A. (2025). Besnoitia besnoiti-Induced Neutrophil Extracellular Traps (NETs): Metabolic Signature, Signaling Pathways, Receptors and Implications on Pathogenesis. Animals, 15(22), 3326. https://doi.org/10.3390/ani15223326






