Effect of a Reduced-Protein Diet Supplemented with Essential Amino Acids on the Muscle Proteome of Female and Entire Male Finishing Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design and Muscle Sampling
2.2. Protein Extraction
2.3. Proteomics Data Acquisition
2.4. Data Analysis
3. Results
3.1. Growth Performance and Meat Traits
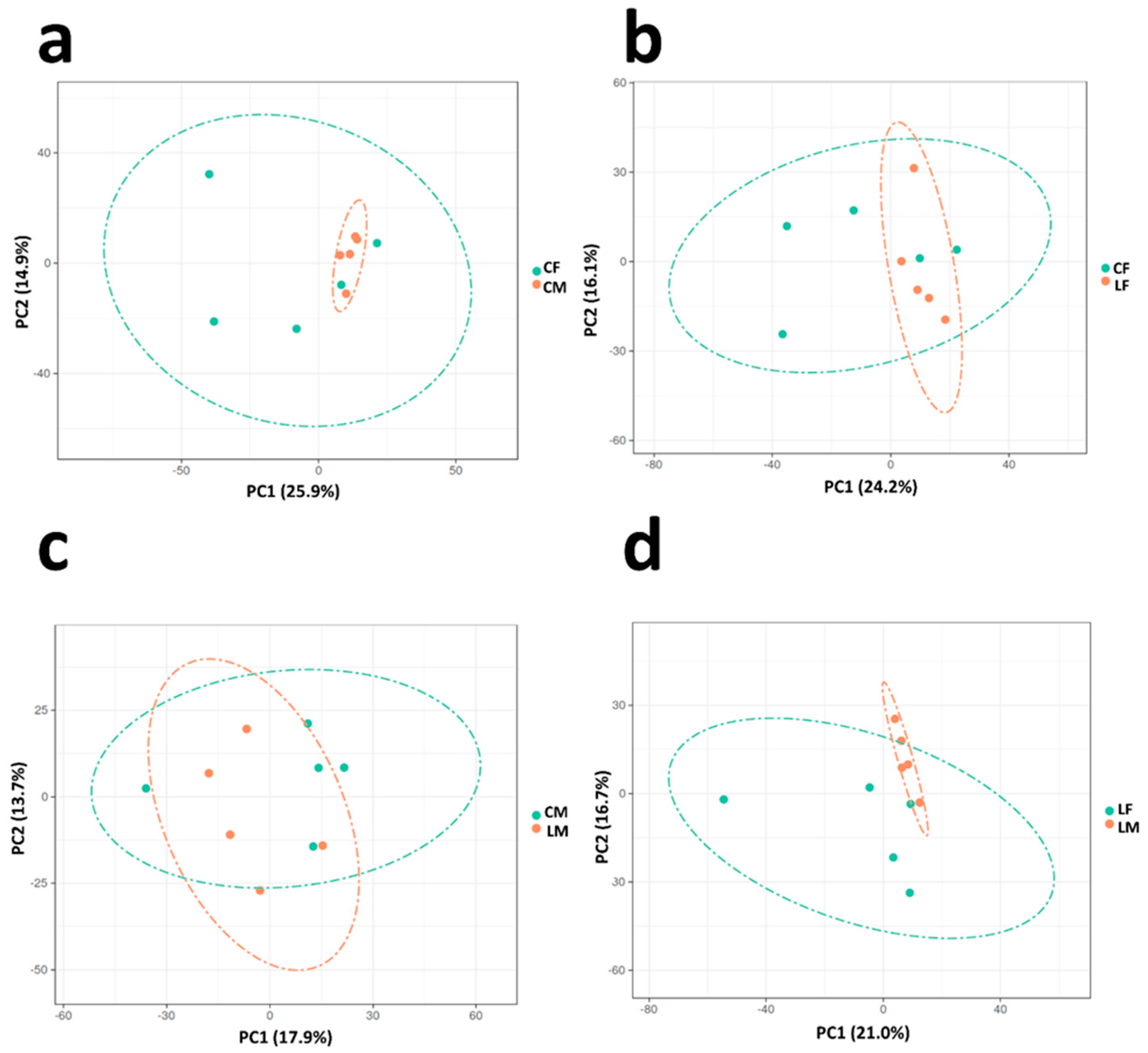
3.2. Differential Proteomics Analysis
3.2.1. Control Male vs. Control Female Comparison
| Accession Number | Protein Name | Unique Peptides | Abundance Ratio: CF/CM | Adjusted p Value: CF/CM | Gene | Main Biological Process |
|---|---|---|---|---|---|---|
| F1RVS9 | Peptidase inhibitor 16 | 2 | 0.347 | 0.001013751 | PI16 | Other biological processes |
| A0A5G2R6C5 | DNA-directed RNA polymerases I, II, and III subunit RPABC3 | 2 | 0.416 | 1.36756 × 106 | POLR2H | RNA metabolism |
| A0A5G2QW66 | Isochorismatase domain containing 2 | 2 | 0.433 | 0.012840564 | ISOC2 | Other biological processes |
| A0A287B510 | Ras homolog family member G | 2 | 2.011 | 0.003132865 | RHOG | Cell organization and biogenesis |
| A0A0H5ANC0 | Mimecan | 14 | 2.017 | 0.00139652 | OGN | Developmental processes |
| I3LCW1 | Fatty acid synthase | 62 | 2.069 | 0.001338133 | FASN | Other metabolic processes |
| A0A5G2R2Y0 | Microfibril-associated protein | 3 | 2.077 | 0.004970294 | MFAP5 | Cell organization and biogenesis |
| A4GR69 | Telethonin | 16 | 2.080 | 0.001519769 | TCAP | |
| A0A481D3P3 | Transmembrane protein 43 | 3 | 2.113 | 0.029580554 | TMEM43 | Cell organization and biogenesis |
| F1S6B5 | Fibromodulin | 10 | 2.220 | 0.000363034 | FMOD | Cell organization and biogenesis |
| I3LNY6 | Nestin | 33 | 2.230 | 0.000224964 | NES | Cell cycle |
| A0A287ADH9 | Chloride intracellular channel protein | 5 | 2.263 | 0.028770902 | CLIC4 | Transport |
| A0A286ZXM0 | PDZ domain-containing protein | 2 | 2.394 | 0.015442094 | AHNAK2 | Other biological processes |
| F1S6B4 | Prolargin | 16 | 2.403 | 7.71728 × 10−6 | PRELP | Other metabolic processes |
| A0A4 × 1SL89 | Haptoglobin | 17 | 2.434 | 4.9501 × 10−6 | HP | Protein metabolism |
| F1SFI6 | Fetuin B | 11 | 2.448 | 0.010355923 | FETUB | Other biological processes |
| A0A4X1W2B1 | Tropomyosin 3 | 5 | 2.503 | 0.000321367 | TPM3 | |
| Q06AA4 | U1 small nuclear ribonucleoprotein A | 3 | 2.505 | 0.030643582 | SNRPA | RNA metabolism |
| A0A287BC27 | Leucine-rich repeat flightless-interacting protein 2 | 4 | 2.554 | 0.002376756 | LRRFIP2 | Other biological processes |
| A0A4X1VZ89 | Heterochromatin protein 1-binding protein 3 | 6 | 2.791 | 0.020612845 | HP1BP3 | Cell organization and biogenesis |
| A0A8D0S0Y6 | Non-histone chromosomal protein HMG-17 | 4 | 2.861 | 0.000110911 | HMGN2 | No biological process assigned |
| A0A286ZLR2 | Non-specific serine/threonine protein kinase | 2 | 2.930 | 0.018827026 | DCLK1 | Cell organization and biogenesis |
| F1RQI0 | Collagen type XII alpha 1 chain | 60 | 3.034 | 1.58739 × 10−8 | COL12A1 | Developmental processes |
| I3L7P7 | Copper transport protein ATOX1 | 4 | 3.280 | 6.98032 × 10−5 | ATOX1 | Transport |
| A0A287BQ93 | Mitogen-activated protein kinase | 3 | 3.600 | 0.001823497 | MAPK12 | Signal transduction |
| F1S3E0 | Transmembrane emp24 domain-containing protein 9 | 3 | 5.391 | 2.6839 × 10−11 | TMED9 | Cell organization and biogenesis |
3.2.2. Low CP Female vs. Control Female Comparison
| Accession Number | Protein Name | Unique Peptides | Abundance Ratio: LF/CF | Adjusted p Value: LF/CF | Gene | Main Biological Process |
|---|---|---|---|---|---|---|
| F1S3E0 | Transmembrane emp24 domain-containing protein 9 | 3 | 0.159 | 2.30539 × 10−16 | TMED9 | Cell organization and biogenesis |
| A7WLH8 | Small ubiquitin-related modifier 1 | 2 | 0.182 | 1.77915 × 10−13 | SUMO1 | Protein metabolism |
| F1SK17 | Perilipin 1 | 8 | 0.313 | 2.39698 × 10−6 | PLIN1 | Other metabolic processes |
| P05207 | cAMP-dependent protein kinase type II-alpha regulatory subunit | 3 | 0.335 | 3.90284 × 10−5 | PRKAR2A | Other biological processes |
| A0A287AIW3 | Dynein light intermediate chain | 7 | 0.377 | 4.59333 × 10−5 | DYNC1LI1 | Cell organization and biogenesis |
| P61013 | Cardiac phospholamban | 2 | 0.396 | 6.02922 × 10−6 | PLN | Cell organization and biogenesis |
| A0A4X1UMD5 | Tubulin polymerization-promoting protein family member 3 | 11 | 0.407 | 6.07621 × 10−5 | TPPP3 | Cell organization and biogenesis |
| F1S6B4 | Prolargin | 16 | 0.429 | 2.10256 × 10−5 | PRELP | Other metabolic processes |
| F1SCC7 | Serpin domain-containing protein | 6 | 0.435 | 0.000105201 | LOC396684 | No biological process assigned |
| A0A287A0I8 | Drebrin 1 | 6 | 0.437 | 0.033932864 | DBN1 | No biological process assigned |
| A0A5G2QKX2 | Ankyrin repeat domain 2 | 16 | 0.446 | 2.48421 × 10−5 | ANKRD2 | Other biological processes |
| F1SN67 | Fibrillin 1 | 47 | 0.475 | 0.000406089 | FBN1 | Cell adhesion |
| A0A287BAW0 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 5 | 7 | 0.479 | 0.002752956 | NDUFA5 | Cell organization and biogenesis |
| A0A5G2R2Y0 | Microfibril-associated protein 5 | 3 | 0.481 | 0.001660983 | MFAP5 | Cell organization and biogenesis |
| F1SP56 | Nipsnap homolog 3B | 8 | 0.491 | 0.001995836 | NIPSNAP3B | No biological process assigned |
| A0A4X1SUH2 | Glutathione peroxidase | 7 | 2.137 | 0.007988995 | GPX1 | Stress response |
| A0A287AI92 | Carbonic anhydrase | 3 | 2.428 | 0.000601199 | CA1 | Other metabolic processes |
| K7GR43 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 1 | 2 | 2.803 | 7.58379 × 10−8 | NDUFA1 | Cell organization and biogenesis |
| F1RVS9 | Peptidase inhibitor 16 | 2 | 3.636 | 4.77915 × 10−7 | PI16 | Other biological processes |
3.2.3. Low CP Males vs. Control Males Comparison
| Accession Number | Protein Name | Unique Peptides | Abundance Ratio: LM/CM | Adjusted p Value: LM/CM | Gene | Main Biological Process |
|---|---|---|---|---|---|---|
| A0A480T8S4 | Mitochondrial-processing peptidase subunit beta | 4 | 0.410 | 0.01510042 | PMPCB | Protein metabolism |
| A0A4X1VYD8 | Protein tyrosine phosphatase 4A2 | 2 | 0.425 | 0.000313165 | PTP4A2 | No biological process assigned |
| O02668 | Inter-alpha-trypsin inhibitor heavy chain H2 | 9 | 0.44 | 0.000589175 | ITIH2 | Other metabolic processes |
| P02769 | Bovine serum albumin precursor | 17 | 2.003 | 8.82799 × 10−5 | ALB | Stress response |
| A0A8W4FDW8 | Sperm-associated antigen 8 | 4 | 2.021 | 0.002867223 | SPAG8 | No role assigned |
| A0A5G2QPJ7 | Fumarylacetoacetase | 5 | 2.070 | 0.005354906 | FAH | Other metabolic processes |
| F1SCC9 | Serpin domain-containing protein | 5 | 2.214 | 0.044908208 | LOC106504545 | |
| A0A4X1W9B8 | Canopy FGF signaling regulator 2 | 2 | 2.274 | 0.040164779 | CNPY2 | Other biological processes |
| F1S9W8 | Kinesin light chain | 9 | 2.587 | 0.001471845 | KLC1 | Other biological processes |
| A0A4X1W2B1 | Tropomyosin 3 | 5 | 3.083 | 8.33538 × 10−11 | TPM3 | No biological process assigned |
| A0A8W4FDQ1 | Myelin protein P0 | 7 | 3.570 | 1.89787 × 10−16 | MPZ | Cell adhesion |
| F1SAR5 | Glutaredoxin 5 | 2 | 5.557 | 1.38727 × 10−10 | GLRX5 | Cell organization and biogenesis |
3.2.4. Low CP Female vs. Low CP Male Comparison
| Accession Number | Protein Name | Unique Peptides | Abundance Ratio: LF/LM | Adjusted p Value: LF/LM | Gene | Main Biological Process |
|---|---|---|---|---|---|---|
| A0A287B4R8 | Acyl-CoA synthetase short-chain family member 3, mitochondrial | 2 | 0.095 | 1.81568E-16 | ACSS3 | Other metabolic processes |
| F2Z5V3 | NHP2-like protein 1 | 3 | 0.234 | 5.71814 × 10−7 | SNU13 | RNA metabolism |
| A0A8W4FDQ1 | Myelin protein P0 | 7 | 0.289 | 1.47771 × 10−10 | MPZ | Cell adhesion |
| A0A287AGW0 | Serpin domain-containing protein | 6 | 0.308 | 2.53406 × 10−11 | LOC106504547 | No biological process assigned |
| A0A4X1W943 | Myosin light chain 6B | 14 | 0.446 | 4.29249 × 10−5 | MYL6B | No biological process assigned |
| F1SIK9 | Septin | 9 | 0.456 | 0.000195907 | SEPTIN7 | Other biological processes |
| A0A4X1SVF9 | Periostin | 9 | 0.469 | 0.044217747 | POSTN | Cell adhesion |
| A0A4X1UMQ6 | Ribosomal protein L37a | 4 | 0.472 | 0.044217747 | RPL37A | Protein metabolism |
| A0A5G2QSD6 | Golgi-associated plant pathogenesis-related protein 1 | 2 | 2.039 | 0.011790049 | GLIPR2 | Other biological processes |
| A0A480PRD9 | Tudor domain-containing protein 3 | 2 | 2.163 | 0.007072509 | TDRD3 | Cell organization and biogenesis |
| A0A287BQ93 | Mitogen-activated protein kinase | 3 | 3.617 | 0.000236537 | MAPK12 | Signal transduction |
4. Discussion
4.1. Control Male vs. Control Female Comparison
4.2. Low CP Female vs. Control Female Comparison
4.3. Low CP Males vs. Control Males Comparison
4.4. Low CP Female vs. Low CP Male Comparison
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Almeida, A.M.; Latorre, M.A.; Alvarez-Rodriguez, J. Productive, Physiological, and Environmental Implications of Reducing Crude Protein Content in Swine Diets: A Review. Animals 2024, 14, 3081. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Lee, S.S.; Kim, Y.H.; Wu, Z.; Kim, I.H. Glutamic acid supplementation recovers the reduced performance of weanling pigs fed reduced crude protein diets. Anim. Nutr. 2022, 8, 249–255. [Google Scholar] [CrossRef]
- Vonderohe, C.E.; Mills, K.M.; Liu, S.; Asmus, M.D.; Otto-Tice, E.R.; Richert, B.T.; Ni, J.Q.; Radcliffe, J.S. The effect of reduced CP, synthetic amino acid supplemented diets on growth performance and nutrient excretion in wean to finish swine. J. Anim. Sci. 2022, 100, skac075. [Google Scholar] [CrossRef]
- Song, W.; Wu, Z.; Li, W.; Li, Y. Multiple amino acid supplementations to reduce dietary protein for pigs during early and late finisher periods under commercial conditions. J. Sci. Food Agric. 2023, 103, 3205–3209. [Google Scholar] [CrossRef]
- Dourmad, J.Y.; Jondreville, C. Impact of nutrition on nitrogen, phosphorus, Cu and Zn in pig manure, and on emissions of ammonia and odours. Liv. Sci. 2007, 112, 192–198. [Google Scholar] [CrossRef]
- Fuentes, V.; Ventanas, S.; Ventanas, J.; Estévez, M. The genetic background affects composition, oxidative stability and qual-ity traits of Iberian dry-cured hams: Purebred Iberian versus reciprocal Iberian × Duroc crossbred pigs. Meat Sci. 2014, 96, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Cilla, I.; Altarriba, J.; Guerrero, L.; Gispert, M.; Martínez, L.; Moreno, C.; Beltrán, J.A.; Guàrdia, M.D.; Diestre, A.; Arnau, J.; et al. Effect of different Duroc line sires on carcass composition, meat quality and dry-cured ham acceptability. Meat Sci. 2006, 72, 252–260. [Google Scholar] [CrossRef] [PubMed]
- FEDNA. Normas FEDNA—Necesidades Nutricionales para Ganado Porcino. Federación Española para el Desarrollo de la Nutrición Animal, Zaragoza (Spain). Available online: https://www.fundacionfedna.org/sites/default/files/Normas%20PORCINO_2013rev2_0.pdf (accessed on 1 October 2025).
- de Almeida, A.M.; Latorre, M.A.; Ripoll, G.; Verdú, M.; Alvarez-Rodriguez, J. Reducing Dietary Protein Levels with Amino Acid Supplementation in Duroc Sire Line Finishing Pigs: Growth Performances, Carcass and Meat Traits and Nitrogen Balance in Males and Females. Animals 2024, 14, 3572. [Google Scholar] [CrossRef]
- Almeida, A.M.; Bassols, A.; Bendixen, E.; Bhide, M.; Ceciliani, F.; Cristobal, S.; Eckersall, P.D.; Hollung, K.; Lisacek, F.; Mazzucchelli, G.; et al. Animal board invited review: Advances in proteomics for animal and food sciences. Animal 2015, 9, 1–17. [Google Scholar] [CrossRef]
- Almeida, A.M.; Ali, S.A.; Ceciliani, F.; Eckersall, P.D.; Hernández-Castellano, L.E.; Han, R.; Hodnik, J.J.; Jaswal, S.; Lippolis, J.D.; McLaughlin, M.; et al. Domestic animal proteomics in the 21st century: A global retrospective and viewpoint analysis. J. Proteom. 2021, 241, 104220. [Google Scholar] [CrossRef]
- Paredi, G.; Raboni, S.; Bendixen, E.; de Almeida, A.M.; Mozzarelli, A. “Muscle to meat” molecular events and technological transformations: The proteomics insight. J. Proteom. 2012, 75, 4275–4289. [Google Scholar] [CrossRef]
- Paredi, G.; Sentandreu, M.A.; Mozzarelli, A.; Fadda, S.; Hollung, K.; de Almeida, A.M. Muscle and meat: New horizons and applications for proteomics on a farm to fork perspective. J. Proteom. 2013, 88, 58–82. [Google Scholar] [CrossRef]
- Sierra, V.; González-Blanco, L.; Diñeiro, Y.; Díaz, F.; García-Espina, M.J.; Coto-Montes, A.; Gagaoua, M.; Oliván, M. New Insights on the Impact of Cattle Handling on Post-Mortem Myofibrillar Muscle Proteome and Meat Tenderization. Foods 2021, 10, 3115. [Google Scholar] [CrossRef]
- Ribeiro, D.M.; Planchon, S.; Leclercq, C.C.; Raundrup, K.; Alves, S.P.; Bessa, R.J.B.; Renaut, J.; Almeida, A.M. The muscular, hepatic and adipose tissues proteomes in muskox (Ovibos moschatus): Differences between males and females. J. Proteom. 2019, 208, 103480. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Grossmann, J.; Fortes, C.; Kilminster, T.; Scanlon, T.; Milton, J.; Greeff, J.; Oldham, C.; Nanni, P.; Almeida, A.M. The sheep (Ovis aries) muscle proteome: Decoding the mechanisms of tolerance to Seasonal Weight Loss using label-free proteomics. J. Proteom. 2017, 161, 57–67. [Google Scholar] [CrossRef]
- Ribeiro, D.M.; Planchon, S.; Leclercq, C.C.; Dentinho, M.T.P.; Bessa, R.J.B.; Santos-Silva, J.; Paulos, K.; Jerónimo, E.; Renaut, J.; Almeida, A.M. The effects of improving low dietary protein utilization on the proteome of lamb tissues. J. Proteom. 2020, 223, 103798. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.; Franco, C.; Pires, E.; Ventosa, M.; Palhinhas, R.; Koci, K.; Martinho de Almeida, A.; Varela Coelho, A. Mass spectrometry and animal science: Protein identification strategies and particularities of farm animal species. J. Proteom. 2012, 75, 4190–4206. [Google Scholar] [CrossRef]
- de Almeida, A.M.; Bendixen, E. Pig proteomics: A review of a species in the crossroad between biomedical and food sciences. J. Proteom. 2012, 75, 4296–4314. [Google Scholar] [CrossRef]
- Ribeiro, D.M.; Coelho, D.; Costa, M.; Carvalho, D.F.P.; Leclercq, C.C.; Renaut, J.; Freire, J.P.B.; Almeida, A.M.; Mestre Prates, J.A. Integrated transcriptomics and proteomics analysis reveals muscle metabolism effects of dietary Ulva lactuca and ulvan lyase supplementation in weaned piglets. Sci. Rep. 2024, 14, 4589. [Google Scholar] [CrossRef]
- Ribeiro, D.M.; Martins, C.F.; Kuleš, J.; Horvatić, A.; Guillemin, N.; Freire, J.P.B.; Eckersall, P.D.; Almeida, A.M.; Prates, J.A.M. Influence of dietary Spirulina inclusion and lysozyme supplementation on the longissimus lumborum muscle proteome of newly weaned piglets. J. Proteom. 2021, 244, 104274. [Google Scholar] [CrossRef]
- Osório, H.; Silva, C.; Ferreira, M.; Gullo, I.; Máximo, V.; Barros, R.; Mendonça, F.; Oliveira, C.; Carneiro, F. Proteomics Analysis of Gastric Cancer Patients with Diabetes Mellitus. J. Clin. Med. 2021, 10, 407. [Google Scholar] [CrossRef]
- Hughes, C.S.; Moggridge, S.; Müller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Sacarrão-Birrento, L.; Ribeiro, D.M.; Dittmann, A.; Alves, S.P.; Kunz, L.; Silva, S.; Venâncio, C.A.; de Almeida, A.M. The effect of the production system on the proteomics profiles of the Longissimus thoracis muscle in Arouquesa cattle. J. Proteom. 2024, 307, 105265. [Google Scholar] [CrossRef]
- Hazell, G.G.; Peachey, A.M.; Teasdale, J.E.; Sala-Newby, G.B.; Angelini, G.D.; Newby, A.C.; White, S.J. PI16 is a shear stress and inflammation-regulated inhibitor of MMP2. Sci. Rep. 2016, 6, 39553. [Google Scholar] [CrossRef] [PubMed]
- Regn, M.; Laggerbauer, B.; Jentzsch, C.; Ramanujam, D.; Ahles, A.; Sichler, S.; Calzada-Wack, J.; Koenen, R.R.; Braun, A.; Nieswandt, B.; et al. Peptidase inhibitor 16 is a membrane-tethered regulator of chemerin processing in the myocardium. J. Mol. Cell. Cardiol. 2016, 99, 57–64. [Google Scholar] [CrossRef]
- Kim, N.K.; Park, H.R.; Lee, H.C.; Yoon, D.; Son, E.S.; Kim, Y.S.; Kim, S.R.; Kim, O.H.; Lee, C.S. Comparative studies of skeletal muscle proteome and transcriptome profilings between pig breeds. Mamm. Genome 2010, 5–6, 307–319. [Google Scholar] [CrossRef]
- Saco, Y.; Bassols, A. Acute phase proteins in cattle and swine: A review. Vet. Clin. Pathol. 2023, 52 (Suppl. 1), 50–63. [Google Scholar] [CrossRef]
- Chen, G.F.; Sudhahar, V.; Youn, S.W.; Das, A.; Cho, J.; Kamiya, T.; Urao, N.; McKinney, R.D.; Surenkhuu, B.; Hamakubo, T.; et al. Copper Transport Protein Antioxidant-1 Promotes Inflammatory Neovascularization via Chaperone and Transcription Factor Function. Sci. Rep. 2015, 5, 14780. [Google Scholar] [CrossRef]
- Girbig, M.; Misiaszek, A.D.; Müller, C.W. Structural insights into nuclear transcription by eukaryotic DNA-dependent RNA polymerases. Nat. Rev. Mol. Cell. Biol. 2022, 23, 603–622. [Google Scholar] [CrossRef] [PubMed]
- AZhaTi, B.; Wu, G.; Zhan, H.; Liang, W.; Song, Z.; Lu, L.; Xie, Q. Alternative splicing patterns reveal prognostic indicator in muscle-invasive bladder cancer. World J. Surg. Oncol. 2022, 20, 231. [Google Scholar] [CrossRef]
- Huang, X.; Shi, Z.; Wang, W.; Bai, J.; Chen, Z.; Xu, J.; Zhang, D.; Fu, S. Identification and characterization of a novel protein ISOC2 that interacts with p16INK4a. Biochem. Biophys. Res. Commun. 2007, 361, 287–293. [Google Scholar] [CrossRef]
- Jun, J.H.; Son, M.J.; Lee, H.G.; Shim, K.Y.; Baek, W.K.; Kim, J.Y.; Joo, C.K. Regulation of Ras homolog family member G by microRNA-124 regulates proliferation and migration of human retinal pigment epithelial cells. Sci. Rep. 2020, 10, 15420. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, J.; Liu, H.F.; Zhang, X.N.; Zhan, M.; Chen, F.L. Overexpression of mimecan in human aortic smooth muscle cells inhibits cell proliferation and enhances apoptosis and migration. Exp. Ther. Med. 2015, 10, 187–192. [Google Scholar] [CrossRef][Green Version]
- Yin, H.; Cui, C.; Han, S.; Chen, Y.; Zhao, J.; He, H.; Li, D.; Zhu, Q. Fibromodulin Modulates Chicken Skeletal Muscle Development via the Transforming Growth Factor-beta Signaling Pathway. Animals 2020, 10, 1477. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T.; Lee, E.J.; Choi, I. Fibromodulin: A regulatory molecule maintaining cellular architecture for normal cellular function. Int. J. Biochem. Cell. Biol. 2016, 80, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, J.; Torvaldson, E.; Gullmets, J.; Karvonen, H.; Nagy, A.; Taimen, P.; Eriksson, J.E. Nestin contributes to skeletal muscle homeostasis and regeneration. J. Cell. Sci. 2017, 130, 2833–2842. [Google Scholar] [CrossRef]
- Orgil, B.O.; Spaulding, M.S.; Smith, H.P.; Baba, Z.; Alberson, N.R.; Batsaikhan, E.; Towbin, J.A.; Purevjav, E. Transmembrane Protein 43: Molecular and Pathogenetic Implications in Arrhythmogenic Cardiomyopathy and Various Other Diseases. Int. J. Mol. Sci. 2025, 26, 6856. [Google Scholar] [CrossRef] [PubMed]
- Ohlendieck, K.; Swandulla, D. Molecular pathogenesis of Duchenne muscular dystrophy-related fibrosis. Pathologe 2017, 38, 21–29. [Google Scholar] [CrossRef]
- Renaville, B.; Bacciu, N.; Lanzoni, M.; Mossa, F.; Piasentier, E. Association of single nucleotide polymorphisms in fat metabolism candidate genes with fatty acid profiles of muscle and subcutaneous fat in heavy pigs. Meat Sci. 2018, 139, 220–227. [Google Scholar] [CrossRef]
- Reiter, S.S.; Halsey, C.H.; Stronach, B.M.; Bartosh, J.L.; Owsley, W.F.; Bergen, W.G. Lipid metabolism related gene-expression profiling in liver, skeletal muscle and adipose tissue in crossbred Duroc and Pietrain Pigs. Comp. Biochem. Physiol. Part D Genom. Proteom. 2007, 2, 200–206. [Google Scholar] [CrossRef]
- Qiu, Y.Q.; Yang, X.F.; Ma, X.Y.; Xiong, Y.X.; Tian, Z.M.; Fan, Q.L.; Wang, L.; Jiang, Z.Y. CIDE gene expression in adipose tissue, liver, and skeletal muscle from obese and lean pigs. J. Zhejiang Univ. Sci. B 2017, 18, 492–500. [Google Scholar] [CrossRef]
- Wang, W.; Xu, M.; Diao, H.; Long, Q.; Gan, F.; Mao, Y. Effects of grape seed proanthocyanidin extract on cholesterol metabolism and antioxidant status in finishing pigs. Sci. Rep. 2024, 14, 21117. [Google Scholar] [CrossRef]
- Yao, Y.C.; Cai, Z.W.; Zhao, C.J.; Wu, K.L.; Wu, C.X.; Han, W.P.; Xu, N.Y. Influence of castration-induced sex hormone deficiency on serum lipid levels and the genes expression in male pigs. Horm. Metab. Res. 2011, 43, 674–680. [Google Scholar] [CrossRef]
- Ramdhave, A.S.; Ojha, S.; Nandave, M. Energy intake correlates with the levels of fatty acid synthase and insulin-like growth factor-1 in male and female C57BL/6 mice. Am. J. Transl. Res. 2017, 9, 830–844. [Google Scholar]
- An, Q.; Zeng, L.; Wang, W.; Yang, J.; Meng, J.; Zhao, Y.; Song, X. Identification of FASN Gene Polymorphisms, Expression and Their Relationship with Body Size Traits in Guizhou White Goat (Capra hircus) with Different Genders. Genes 2024, 15, 656. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.; Holman, B.W.; Nichols, P.D.; Malau-Aduli, A.E. Effect of dietary supplementation with Spirulina on the expressions of AANAT, ADRB3, BTG2 and FASN genes in the subcutaneous adipose and Longissimus dorsi muscle tissues of purebred and crossbred Australian sheep. J. Anim. Sci. Technol. 2015, 57, 8. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Leonardo, T.R.; Romana-Souza, B.; Shi, J.; Keiser, S.; Yuan, H.; Altakriti, M.; Ranzer, M.J.; Ferri-Borgogno, S.; Mok, S.C.; et al. Microfibril-associated protein 5 and the regulation of skin scar formation. Sci. Rep. 2023, 13, 8728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, H.; Sun, S.; Zhou, W.; Zhang, T.; Yu, Y.; Wang, Q.; Wang, M. Microfibrillar-associated protein 5 suppresses adipogenesis by inhibiting essential coactivator of PPARγ. Sci. Rep. 2023, 13, 5589. [Google Scholar] [CrossRef]
- Romao, J.M.; He, M.L.; McAllister, T.A.; Guan, L.L. Effect of age on bovine subcutaneous fat proteome: Molecular mechanisms of physiological variations during beef cattle growth. J. Anim. Sci. 2014, 92, 3316–3327. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, G.; Lanfranchi, G.; Valle, G. Telethonin and other new proteins of the Z-disc of skeletal muscle. IUBMB Life 2001, 51, 275–282. [Google Scholar] [CrossRef]
- Lambert, M.R.; Gussoni, E. Tropomyosin 3 (TPM3) function in skeletal muscle and in myopathy. Skelet. Muscle 2023, 13, 18. [Google Scholar] [CrossRef]
- Dorbic, T.; Wittig, B. Chromatin from transcribed genes contains HMG17 only downstream from the starting point of transcription. EMBO J. 1987, 6, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Dai, R.; Ma, X.; Huang, C.; Ma, X.; Li, X.; La, Y.; Dingkao, R.; Renqing, J.; Guo, X.; et al. Proteomic Analysis Reveals the Effects of Different Dietary Protein Levels on Growth and Development of Jersey-Yak. Animals 2024, 14, 406. [Google Scholar] [CrossRef]
- Zhou, L.; Li, H.; Yao, H.; Dai, X.; Gao, P.; Cheng, H. TMED family genes and their roles in human diseases. Int. J. Med. Sci. 2023, 20, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Lazzarato, L.; Bianchi, L.; Andolfo, A.; Granata, A.; Lombardi, M.; Sinelli, M.; Rolando, B.; Carini, M.; Corsini, A.; Fruttero, R.; et al. Proteomics Studies Suggest That Nitric Oxide Donor Furoxans Inhibit In Vitro Vascular Smooth Muscle Cell Proliferation by Nitric Oxide-Independent Mechanisms. Molecules 2023, 28, 5724. [Google Scholar] [CrossRef]
- Purohit, A.; Tynan, S.H.; Vallee, R.; Doxsey, S.J. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J. Cell Biol. 1999, 147, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Li, Y.; Zhao, W.; Wu, N.; Liu, N.; Li, R.; Chen, L.; He, M.; Lu, B.; Wang, X.; et al. Knockdown of Tubulin Polymerization Promoting Protein Family Member 3 inhibits cell proliferation and invasion in human colorectal cancer. J. Cancer 2017, 8, 1750–1758. [Google Scholar] [CrossRef]
- Krauss, R.S. Regulation of Skeletal Myoblast Differentiation by Drebrin. Adv. Exp. Med. Biol. 2017, 1006, 361–373. [Google Scholar] [CrossRef]
- Zhang, Q.; Lee, H.G.; Han, J.A.; Kang, S.K.; Lee, N.K.; Baik, M.; Choi, Y.J. Differentially expressed proteins associated with myogenesis and adipogenesis in skeletal muscle and adipose tissue between bulls and steers. Mol. Biol. Rep. 2012, 39, 953–960. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.; Li, B.; Huang, Z.; Zhao, S.; Chen, S.; Lan, T.; Ren, S.; Wu, F.; Tan, J.; et al. Cancer-associated fibroblasts in the invasive tumour front promote the metastasis of oral squamous cell carcinoma through MFAP5 upregulation. Gene 2023, 876, 147504. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Chen, L.; Cai, Z.; Tang, J.; Zhu, Y.; Li, Y.; Wang, X.; Ruan, Y.; Han, Q. NIPSNAP3A regulates cellular homeostasis by modulating mitochondrial dynamics. Gene 2025, 933, 148976. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Wang, J.; Harlina, P.W.; Geng, F. Quantitative N-Glycoproteomic Analysis of Cattle-Yak and Yak Longissimus thoracis. J. Agric. Food Chem. 2023, 71, 11740–11750. [Google Scholar] [CrossRef]
- Frank, K.; Kranias, E.G. Phospholamban and cardiac contractility. Ann. Med. 2000, 32, 572–578. [Google Scholar] [CrossRef]
- Gandolfi, G.; Mazzoni, M.; Zambonelli, P.; Lalatta-Costerbosa, G.; Tronca, A.; Russo, V.; Davoli, R. Perilipin 1 and perilipin 2 protein localization and gene expression study in skeletal muscles of European cross-breed pigs with different intramuscular fat contents. Meat Sci. 2011, 88, 631–637. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Hou, L.; Wu, W.; Zhao, S.; Xiong, Y. Fibroblast Growth Factor 21 Suppresses Adipogenesis in Pig Intramuscular Fat Cells. Int. J. Mol. Sci. 2015, 17, 11. [Google Scholar] [CrossRef]
- Park, J.C.; Kim, S.C.; Lee, S.D.; Jang, H.C.; Kim, N.K.; Lee, S.H.; Jung, H.J.; Kim, I.C.; Seong, H.H.; Choi, B.H. Effects of Dietary Fat Types on Growth Performance, Pork Quality, and Gene Expression in Growing-finishing Pigs. Asian-Australas. J. Anim. Sci. 2012, 25, 1759–1767. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Z.; Sun, H.; Huang, Y.; Albrecht, E.; Zhao, R.; Yang, X. Lipopolysaccharide challenge significantly influences lipid metabolism and proteome of white adipose tissue in growing pigs. Lipids Health Dis. 2015, 14, 68. [Google Scholar] [CrossRef]
- van Milgen, J.; Noblet, J.; Dubois, S. Energetic efficiency of starch, protein and lipid utilization in growing pigs. J. Nutr. 2001, 131, 1309–1318. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, H.L.; Shi, Y.; Zhao, M.M.; Wang, H.; Zeng, Y.Q. Comparative Analysis on Antioxidative Ability of Muscle between Laiwu Pig and Large White. Asian-Australas. J. Anim. Sci. 2012, 25, 1190–1196. [Google Scholar] [CrossRef][Green Version]
- Yang, T.; Feng, F.; Zhan, K.; Ma, X.; Jiang, M.; Datsomor, O.; Zhu, X.; Huo, Y.; Zhao, G. Effect of the Tea Tree Oil on Growth Performance, Meat Quality, Serum Biochemical Indices, and Antioxidant Capacity in Finishing Pigs. Front. Vet. Sci. 2022, 9, 916625. [Google Scholar] [CrossRef]
- Xu, M.; Chen, X.; Huang, Z.; Chen, D.; Li, M.; He, J.; Chen, H.; Zheng, P.; Yu, J.; Luo, Y.; et al. Effects of dietary grape seed proanthocyanidin extract supplementation on meat quality, muscle fiber characteristics and antioxidant capacity of finishing pigs. Food Chem. 2022, 367, 130781. [Google Scholar] [CrossRef]
- Hunter, E.A.; Grimble, R.F. Dietary sulphur amino acid adequacy influences glutathione synthesis and glutathione-dependent enzymes during the inflammatory response to endotoxin and tumour necrosis factor-alpha in rats. Clin. Sci. 1997, 92, 297–305. [Google Scholar] [CrossRef]
- Yin, J.; Ren, W.; Duan, J.; Wu, L.; Chen, S.; Li, T.; Yin, Y.; Wu, G. Dietary arginine supplementation enhances intestinal expression of SLC7A7 and SLC7A1 and ameliorates growth depression in mycotoxin-challenged pigs. Amino Acids 2014, 46, 883–892. [Google Scholar] [CrossRef]
- Holanda, D.M.; Marcolla, C.S.; Guimarães, S.E.F.; Neves, M.M.; Hausman, G.J.; Duarte, M.S.; Abreu, M.L.T.; Saraiva, A. Dietary L-arginine supplementation increased mammary gland vascularity of lactating sows. Animal 2019, 13, 790–798. [Google Scholar] [CrossRef]
- Wang, Z.; Shao, D.; Kang, K.; Wu, S.; Zhong, G.; Song, Z.; Shi, S. Low protein with high amino acid diets improves the growth performance of yellow feather broilers by improving intestinal health under cyclic heat stress. J. Therm. Biol. 2022, 105, 103219. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.S. The Role of Carbonic Anhydrase in Hepatic Glucose Production. Curr. Diabetes Rev. 2018, 14, 108–112. [Google Scholar] [CrossRef]
- Zhong, W.; Jiang, Z.; Zheng, C.; Lin, Y.; Yang, L.; Zou, S. Relationship between proteome changes of Longissimus muscle and intramuscular fat content in finishing pigs fed conjugated linoleic acid. Br. J. Nutr. 2011, 105, 1–9. [Google Scholar] [CrossRef]
- Gakh, O.; Cavadini, P.; Isaya, G. Mitochondrial processing peptidases. Biochim. Biophys. Acta 2002, 1592, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, M.; Zheng, Z.; He, Y.; Zhu, Y.; Cheng, Q.; Rong, J.; Weng, H.; Chen, C.; Xu, Y.; et al. Over-expression of protein tyrosine phosphatase 4A2 correlates with tumor progression and poor prognosis in nasopharyngeal carcinoma. Oncotarget 2017, 8, 77527–77539. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Cho, S.; Kim, E.J.; Oh, S.; Lee, Y.; Park, S.; Kang, K.; Shin, D.H.; Ko, S.Y.; et al. Hyaluronan network remodeling by ZEB1 and ITIH2 enhances the motility and invasiveness of cancer cells. J. Clin. Investig. 2025, 135, e180570. [Google Scholar] [CrossRef]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef]
- Mackenzie, M.L.; Warren, M.R.; Wykes, L.J. Colitis increases albumin synthesis at the expense of muscle protein synthesis in macronutrient-restricted piglets. J. Nutr. 2003, 133, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Holme, E.; Lindstedt, S. Tyrosinaemia type I and NTBC (2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione). J. Inherit. Metab. Dis. 1998, 21, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.H.; Zhang, Y.; Cui, J.; Liu, Y.; McAllan, B.M.; Liao, C.C.; Zhang, S. Adaptation of phenylalanine and tyrosine catabolic pathway to hibernation in bats. PLoS ONE 2013, 8, e62039. [Google Scholar] [CrossRef] [PubMed]
- Kakehashi, A.; Suzuki, S.; Shiota, M.; Raymo, N.; Gi, M.; Tachibana, T.; Stefanov, V.; Wanibuchi, H. Canopy Homolog 2 as a Novel Molecular Target in Hepatocarcinogenesis. Cancers 2021, 13, 3613. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Ma, L.; Li, X.; Jin, W.; Yang, Y. CNPY2 governs PDGF-BB-treated vascular smooth muscle cell proliferation, migration and phenotypic transformation via the Akt/mTOR/GSK-3beta signaling pathway. Exp. Ther. Med. 2024, 27, 197. [Google Scholar] [CrossRef]
- Qu, Z.; Shi, L.; Wu, Z.; Lin, P.; Zhang, G.; Cong, X.; Zhao, X.; Ge, H.; Yan, S.; Jiang, L.; et al. Kinesin light chain 1 stabilizes insulin receptor substrate 1 to regulate the IGF-1-AKT signaling pathway during myoblast differentiation. FASEB J. 2024, 38, e23432. [Google Scholar] [CrossRef]
- Ropka-Molik, K.; Zukowski, K.; Eckert, R.; Gurgul, A.; Piórkowska, K.; Oczkowicz, M. Comprehensive analysis of the whole transcriptomes from two different pig breeds using RNA-Seq method. Anim. Genet. 2014, 45, 674–684. [Google Scholar] [CrossRef]
- Wang, Z.; Shang, P.; Li, Q.; Wang, L.; Chamba, Y.; Zhang, B.; Zhang, H.; Wu, C. iTRAQ-based proteomic analysis reveals key proteins affecting muscle growth and lipid deposition in pigs. Sci. Rep. 2017, 7, 46717. [Google Scholar] [CrossRef]
- Wang, W.; Ma, C.; Zhang, Q.; Jiang, Y. TMT-labeled quantitative malonylome analysis on the longissimus dorsi muscle of Laiwu pigs reveals the role of ACOT7 in fat deposition. J. Proteom. 2024, 298, 105129. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Liu, M.; Liu, Y.; Nshogoza, G.; Gao, J.; Ma, R.; Yang, Y.; Wu, J.; Zhang, J.; et al. Structural plasticity of the TDRD3 Tudor domain probed by a fragment screening hit. FEBS J. 2018, 285, 2091–2103. [Google Scholar] [CrossRef]
- Qi, X.; Yin, N.; Ma, S.; Lepp, A.; Tang, J.; Jing, W.; Johnson, B.; Dwinell, M.B.; Chitambar, C.R.; Chen, G. p38γ MAPK Is a Therapeutic Target for Triple-Negative Breast Cancer by Stimulation of Cancer Stem-Like Cell Expansion. Stem Cells 2015, 33, 2738–2747. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, Z.; Sun, D.; Li, Y.; Sinha, S.C.; Yu, L.; Bennett, L.; Levine, B. GLIPR2 is a negative regulator of autophagy and the BECN1-ATG14-containing phosphatidylinositol 3-kinase complex. Autophagy 2021, 17, 2891–2904. [Google Scholar] [CrossRef]
- Zhou, L.; Song, Z.; Hu, J.; Liu, L.; Hou, Y.; Zhang, X.; Yang, X.; Chen, K. ACSS3 represses prostate cancer progression through downregulating lipid droplet-associated protein PLIN3. Theranostics 2021, 11, 841–860. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, H.; Li, W.; Yan, P.; Zhao, M.; Li, Z.; Zhao, H.; Wang, S.; Wan, R.; Li, Y.; et al. ACSS3 regulates the metabolic homeostasis of epithelial cells and alleviates pulmonary fibrosis. Biochim. Biophys. Acta Mol. Basis. Dis. 2024, 1870, 166960. [Google Scholar] [CrossRef]
- Zequan, X.; Yonggang, S.; Heng, X.; Yaodong, W.; Xin, M.; Dan, L.; Li, Z.; Tingting, D.; Zirong, W. Transcriptome-based analysis of early post-mortem formation of pale, soft, and exudative (PSE) pork. Meat Sci. 2022, 194, 108962. [Google Scholar] [CrossRef]
- Galindo, C.L.; Nguyen, V.T.; Hill, B.; Easterday, E.; Cleator, J.H.; Sawyer, D.B. Neuregulin (NRG-1beta) Is Pro-Myogenic and Anti-Cachectic in Respiratory Muscles of Post-Myocardial Infarcted Swine. Biology 2022, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- Gönczi, M.; Ráduly, Z.; Szabó, L.; Fodor, J.; Telek, A.; Dobrosi, N.; Balogh, N.; Szentesi, P.; Kis, G.; Antal, M.; et al. Septin7 is indispensable for proper skeletal muscle architecture and function. eLife 2022, 11, e75863. [Google Scholar] [CrossRef]
- Ráduly, Z.; Szabó, L.; Dienes, B.; Szentesi, P.; Bana, Á.V.; Hajdú, T.; Kókai, E.; Hegedűs, C.; Csernoch, L.; Gönczi, M. Migration of Myogenic Cells Is Highly Influenced by Cytoskeletal Septin7. Cells 2023, 12, 1825. [Google Scholar] [CrossRef]
- Szabó, L.; Telek, A.; Fodor, J.; Dobrosi, N.; Dócs, K.; Hegyi, Z.; Gönczi, M.; Csernoch, L.; Dienes, B. Reduced Expression of Septin7 Hinders Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2023, 24, 13536. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Ma, H.M.; Xiao, D.F. Molecular characterization, tissue expression profile, and single nucleotide polymorphism analysis of the periostin gene in swine. Genet. Mol. Res. 2016, 15, 15017187. [Google Scholar] [CrossRef] [PubMed]
- Mo, D.; Yu, K.; Chen, H.; Chen, L.; Liu, X.; He, Z.; Cong, P.; Chen, Y. Transcriptome Landscape of Porcine Intramuscular Adipocytes during Differentiation. J. Agric. Food Chem. 2017, 65, 6317–6328. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida, A.M.; Osório, H.; Latorre, M.Á.; Álvarez-Rodríguez, J. Effect of a Reduced-Protein Diet Supplemented with Essential Amino Acids on the Muscle Proteome of Female and Entire Male Finishing Pigs. Animals 2025, 15, 3325. https://doi.org/10.3390/ani15223325
de Almeida AM, Osório H, Latorre MÁ, Álvarez-Rodríguez J. Effect of a Reduced-Protein Diet Supplemented with Essential Amino Acids on the Muscle Proteome of Female and Entire Male Finishing Pigs. Animals. 2025; 15(22):3325. https://doi.org/10.3390/ani15223325
Chicago/Turabian Stylede Almeida, André M., Hugo Osório, María Ángeles Latorre, and Javier Álvarez-Rodríguez. 2025. "Effect of a Reduced-Protein Diet Supplemented with Essential Amino Acids on the Muscle Proteome of Female and Entire Male Finishing Pigs" Animals 15, no. 22: 3325. https://doi.org/10.3390/ani15223325
APA Stylede Almeida, A. M., Osório, H., Latorre, M. Á., & Álvarez-Rodríguez, J. (2025). Effect of a Reduced-Protein Diet Supplemented with Essential Amino Acids on the Muscle Proteome of Female and Entire Male Finishing Pigs. Animals, 15(22), 3325. https://doi.org/10.3390/ani15223325








