Tomographic Evaluation of the Bronchial and Pulmonary Vascular Relationships in Cats Naturally Infected with Immature Dirofilaria immitis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Collection and Assays
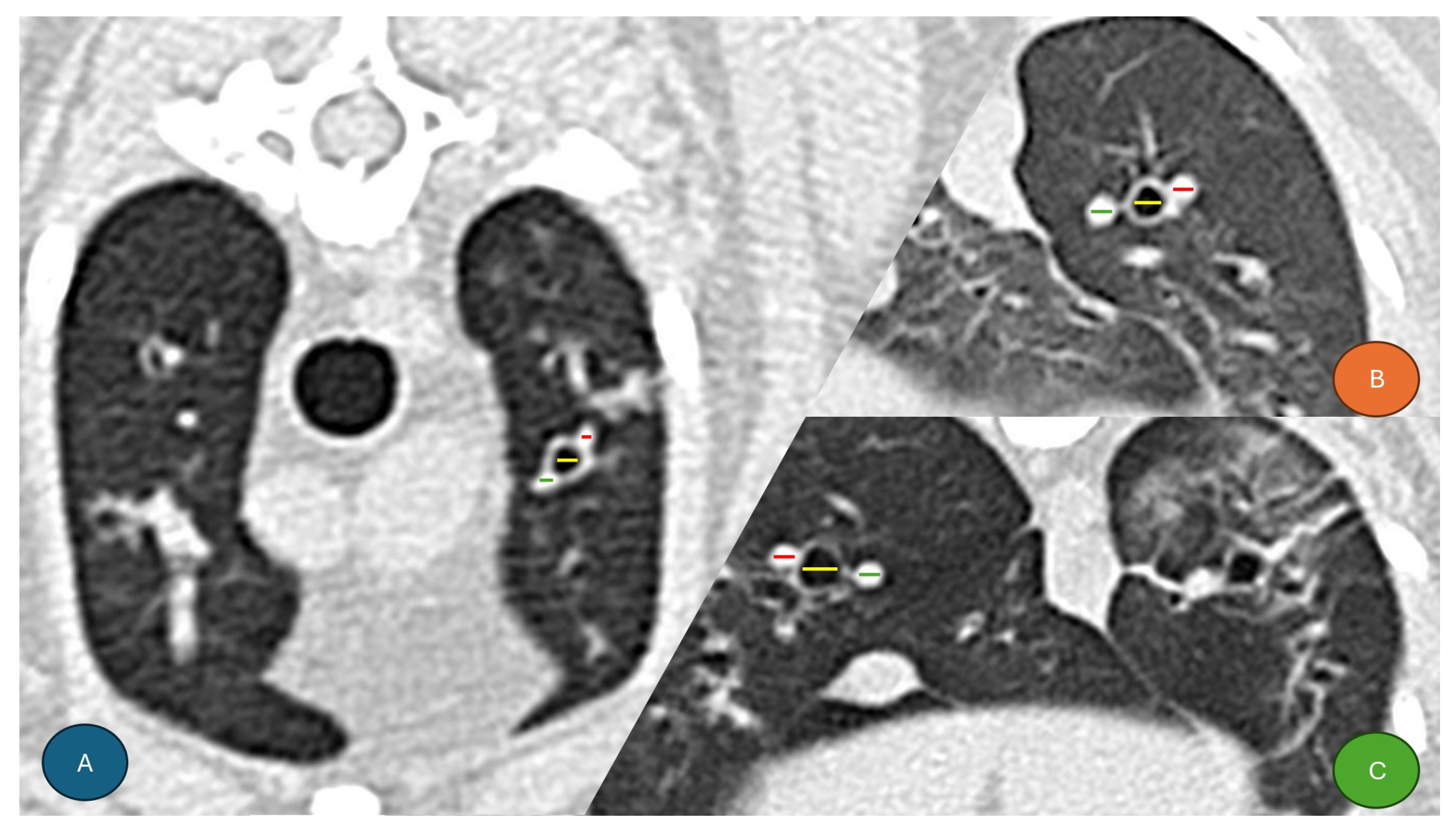
2.3. CT Scan Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Descriptive Study
3.2. Comparison of the Ratios According to the ELISA Technique Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pennisi, M.G.; Tasker, S.; Hartmann, K.; Belák, S.; Addie, D.; Boucraut-Baralon, C.; Herman, E.; Frymus, T.; Hofmann-Lehmann, R.; Hosie, M.; et al. Dirofilarioses in cats: European guidelines from the ABCD on prevention and management. J. Feline Med. Surg. 2020, 22, 442–451. [Google Scholar] [CrossRef]
- Garrity, S.; Lee-Fowler, T.; Reinero, C. Feline asthma and heartworm disease: Clinical features, diagnostics and therapeutics. J. Feline Med. Surg. 2019, 21, 825–834. [Google Scholar] [CrossRef]
- Dillon, A.R.; Blagburn, B.L.; Tillson, M.; Brawner, W.; Welles, B.; Johnson, C.; Cattley, R.; Rynders, P.; Barney, S. The progression of heartworm associated respiratory disease (HARD) in SPF cats 18 months after Dirofilaria immitis infection. Parasit. Vectors 2017, 10, 533–544. [Google Scholar] [CrossRef]
- Dillon, A.R.; Warner, A.E.; Brawner, W.; Hudson, J.; Tillson, M. Activity of pulmonary intravascular macrophages in cats and dogs with and without adult Dirofilaria immitis. Vet. Parasitol. 2008, 158, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Venco, L.; Marchesotti, F.; Manzocchi, S. Feline heartworm disease: A ‘Rubik’s-cube-like’ diagnostic and therapeutic challenge. J. Vet. Cardiol. 2015, 17, S190–S201. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.T. Dirofilaria immitis in Cats: Anatomy of a Disease. Compend. Contin. Educ. Vet. 2008, 30, 382–389. [Google Scholar]
- Stannard, R. The facts about feline heartworm disease. Today’s Vet. Pract. 2015, 5, 93–98. [Google Scholar]
- De Rycke, L.M.; Gielen, I.M.; Simoens, P.J.; van Bree, H. Computed tomography and cross-sectional anatomy of the thorax in clinically normal dogs. Am. J. Vet. Res. 2005, 66, 512–524. [Google Scholar] [CrossRef]
- Drees, R.; François, C.; Saunders, J. Invited review-computed tomographic angiography (CTA) of the thoracic cardiovascular system in companion animals. Vet. Radiol. Ultrasound 2014, 55, 229–240. [Google Scholar] [CrossRef]
- Panopoulos, I.; Auriemma, E.; Specchi, S.; Diana, A.; Pietra, M.; Papastefanou, A.; Zini, E.; Cipone, M. 64-multidetector CT anatomical assessment of the feline bronchial and pulmonary vascular structures. J. Feline Med. Surg. 2019, 21, 893–901. [Google Scholar] [CrossRef]
- Prather, A.; Berry, C.; Thrall, D. Use of radiography in combination with computed tomography for the assessment of noncardiac thoracic disease in the dog and cat. Vet. Radiol. Ultrasound 2005, 46, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Masseau, I.; Reinero, C.R. Thoracic computed tomographic interpretation for clinicians to aid in the diagnosis of dogs and cats with respiratory disease. Vet. J. 2019, 253, 105388. [Google Scholar] [CrossRef]
- Cannon, M.; Johnson, L.; Pesavento, P.; Kass, P.; Wisner, E. Quantitative and qualitative computed tomographic characteristics of bronchiectasis in 12 dogs. Vet. Radiol. Ultrasound 2013, 54, 351–357. [Google Scholar] [CrossRef]
- Sutherland-Smith, J.; Hankin, E.J.; Cunningham, S.M.; Sato, A.F.; Barton, B.A. Comparison of a computed tomographic pulmonary trunk to aorta diameter ratio with echocardiographic indices of pulmonary hypertension in dogs. Vet. Radiol. Ultrasound 2017, 59, 18–26. [Google Scholar] [CrossRef]
- Mortier, J.R.; Mesquita, L.; Ferrandis, I.; McConnell, J.F.; Maddox, T.W. Accuracy of and interobserver agreement regarding thoracic computed tomography for the diagnosis of chronic bronchitis in dogs. J. Am. Vet. Med. Assoc. 2018, 253, 757–762. [Google Scholar] [CrossRef]
- Oshiro, Y.; Murayama, S.; Sunagawa, U.; Nakamoto, A.; Owan, I.; Kuba, M.; Uehara, T.; Miyahira, T.; Kawabata, T.; Kuniyoshi, M.; et al. Pulmonary dirofilariasis computed tomography findings and correlation with pathologic features. J. Comput. Assist. Tomogr. 2004, 28, 796–800. [Google Scholar] [CrossRef]
- Nikolaou, K.; Thieme, S.; Sommer, W.; Johnson, T.; Reiser, M.F. Diagnosing pulmonary embolism new computed tomography applications. J. Thorac. Imaging 2010, 25, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.I.; Falcón-Cordón, Y.; García-Rodríguez, S.N.; Costa-Rodríguez, N.; Montoya-Alonso, J.A.; Carretón, E. Evaluation of pulmonary hypertension in dogs with heartworm disease using the computed tomographic pulmonary trunk to aorta diameter ratio. Animals 2022, 12, 2441. [Google Scholar] [CrossRef]
- Matos, J.I.; Caro-Vadillo, A.; Falcón-Cordón, Y.; García-Rodríguez, S.N.; Costa-Rodríguez, N.; Carretón, E.; Montoya-Alonso, J.A. Echocardiographic Assessment of the Pulmonary Vein to Pulmonary Artery Ratio in Canine Heartworm Disease. Animals 2023, 13, 703. [Google Scholar] [CrossRef] [PubMed]
- Reid, L.; Ray Dillon, A.; Hathcock, J.; Brown, L.; Tillson, M.; Wooldridge, A. High-resolution computed tomography bronchial lumen to pulmonary artery diameter ratio in anesthetized ventilated cats with normal lungs. Vet. Radiol. Ultrasound 2012, 53, 34–37. [Google Scholar] [CrossRef]
- Lee-Fowler, T.; Cole, R.; Dillon, A.; Tillson, M.; Garbarino, R.; Barney, S. High-resolution computed tomography evaluation of the bronchial lumen to vertebral body diameter and pulmonary artery to vertebral body diameter ratios in anesthetized ventilated normal cats. J. Feline Med. Surg. 2017, 19, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Lee-Fowler, T.; Cole, R.C.; Dillon, A.R.; Graham, S.; Tillson, D.M.; Barney, S. High-resolution CT evaluation of bronchial lumen to vertebral body, pulmonary artery to vertebral body and bronchial lumen to pulmonary artery ratios in Dirofilaria immitis-infected cats with and without selamectin administration. J. Feline Med. Surg. 2018, 20, 928–933. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, S.N.; Costa-Rodríguez, N.; Matos, J.I.; Falcón-Cordón, Y.; Morchón, R.; Carretón, E.; Montoya-Alonso, J.A. Feline heartworm disease and environmental allergens hypersensitivity: Is there a link? Parasit. Vectors 2023, 16, 192. [Google Scholar] [CrossRef]
- Dillon, A.R.; Tillson, D.M.; Wooldridg, A.; Cattley, R.; Hathcock, J.; Brawner, W.R.; Cole, R.; Welles, B.; Christopherson, P.W.; Lee-Fowler, T.; et al. Effect of pre-cardiac and adult stages of Dirofilaria immitis in pulmonary disease of cats: CBC, bronchial lavage cytology, serology, radiographs, CT images, bronchial reactivity, and histopathology. Vet. Parasitol. 2014, 206, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Falcón-Cordón, S.; Falcón-Cordón, Y.; García-Rodríguez, S.N.; Costa-Rodríguez, N.; Vera-Rodríguez, D.J.; Montoya-Alonso, J.A.; Carretón, E. Radiological Evaluation of vascular structures in cats infected with immature worms of Dirofilaria immitis. Animals 2024, 14, 2943. [Google Scholar] [CrossRef]
- Cannon, M.S.; Wisner, E.R.; Johnson, L.R.; Kass, P.H. Computed tomography bronchial lumen to pulmonary artery diameter ratio in dogs without clinical pulmonary disease. Vet. Radiol. Ultrasound 2009, 50, 622–624. [Google Scholar] [CrossRef]
- Hansell, D.M. Bronchiectasis. Radiol. Clin. N. Am. 1998, 36, 107–128. [Google Scholar] [CrossRef]
- McGuinness, G.; Naidich, D.P. CT of airways disease and bronchiectasis. Radiol. Clin. N. Am. 2002, 40, 1–19. [Google Scholar] [CrossRef]
- Won, S.; Yun, S.; Lee, J.; Lee, M.; Choi, M.; Yoon, J. High resolution computed tomographic evaluation of bronchial wall thickness in healthy and clinically asthmatic cats. J. Vet. Med. Sci. 2017, 79, 567–571. [Google Scholar] [CrossRef]
- Merveille, A.C.; Bolen, G.; Krafft, E.; Roels, E.; Gomart, S.; Etienne, A.L.; Clercx, C.; Mc Entee, K. Pulmonary Vein-to-Pulmonary Artery Ratio is an Echocardiographic Index of Congestive Heart Failure in Dogs with Degenerative Mitral Valve Disease. J. Vet. Intern. Med. 2015, 29, 1502–1509. [Google Scholar] [CrossRef]
- Roels, E.; Merveille, A.C.; Moyse, E.; Gomart, S.; Clercx, C.; Mc Entee, K. Diagnostic value of the pulmonary vein-to-right pulmonary artery ratio in dogs with pulmonary hypertension of precapillary origin. J. Vet. Cardiol. 2019, 24, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Patata, V.; Caivano, D.; Porciello, F.; Rishniw, M.; Domenech, O.; Marchesotti, F.; Giorgi, M.E.; Guglielmini, C.; Poser, H.; Spina, F.; et al. Pulmonary vein to pulmonary artery ratio in healthy and cardiomyopathic cats. J. Vet. Cardiol. 2020, 27, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Makara, M.; Dennler, M.; Schnyder, M.; Bektas, R.; Kircher, P.; Hall, E.; Glaus, T. Effect of ventilation technique and airway diameter on bronchial lumen to pulmonary artery diameter ratios in clinically normal beagle dogs. Vet. Radiol. Ultrasound 2013, 54, 605–609. [Google Scholar] [CrossRef]
- Lee, J.; Chung, J.; Baek, L.; Je, M.; Choi, J.; Yoon, J. Respiratory phase can affect pulmonary vein-to-pulmonary artery ratio measured with CT. Am. J. Vet. Res. 2022, 83, ajvr.22.02.0026. [Google Scholar] [CrossRef] [PubMed]

| Result of the ELISA Technique | Chi2 and p-Value | Cramer’s V | ||||
|---|---|---|---|---|---|---|
| Total (N%) | Seronegative (N%) | Seropositive (N%) | ||||
| SEX | Total | 38 (100.0%) | 8 (100.0%) | 30 (100.0%) | Chi2(1) = 0.088, p = 0.767 | 1.000 |
| Female | 22 | 5 | 17 | |||
| Male | 16 | 3 | 13 | |||
| BREED | Total | 38 (100.0%) | 8 (100.0%) | 30 (100.0%) | Chi2(3) = 4.453, p = 0.217 | |
| Common European | 33 | 6 | 27 | |||
| Persian | 1 | 1 | 0 | |||
| Siamese | 1 | 0 | 1 | |||
| Sphynx | 3 | 1 | 2 | |||
| Result of the ELISA Technique | Standardized Statistical and Mann–Whitney p-Value | |||||
| Total (N%) | Seronegative (N%) | Seropositive (N%) | ||||
| BODY WEIGHT (kg) | Valid N | 38 | 8 | 30 | U = 1.272, p = 0.208 | |
| Median | 3.56 | 3.27 | 3.95 | |||
| Percentile 25 | 3.00 | 2.80 | 3.00 | |||
| Percentile 75 | 4.80 | 3.93 | 5.00 | |||
| AGE | Valid N | 38 | 8 | 30 | U = 0.859, p = 0.407 | |
| Median | 4.32 | 3.56 | 4.68 | |||
| Percentile 25 | 2.56 | 1.64 | 2.66 | |||
| Percentile 75 | 8.36 | 6.36 | 9.62 | |||
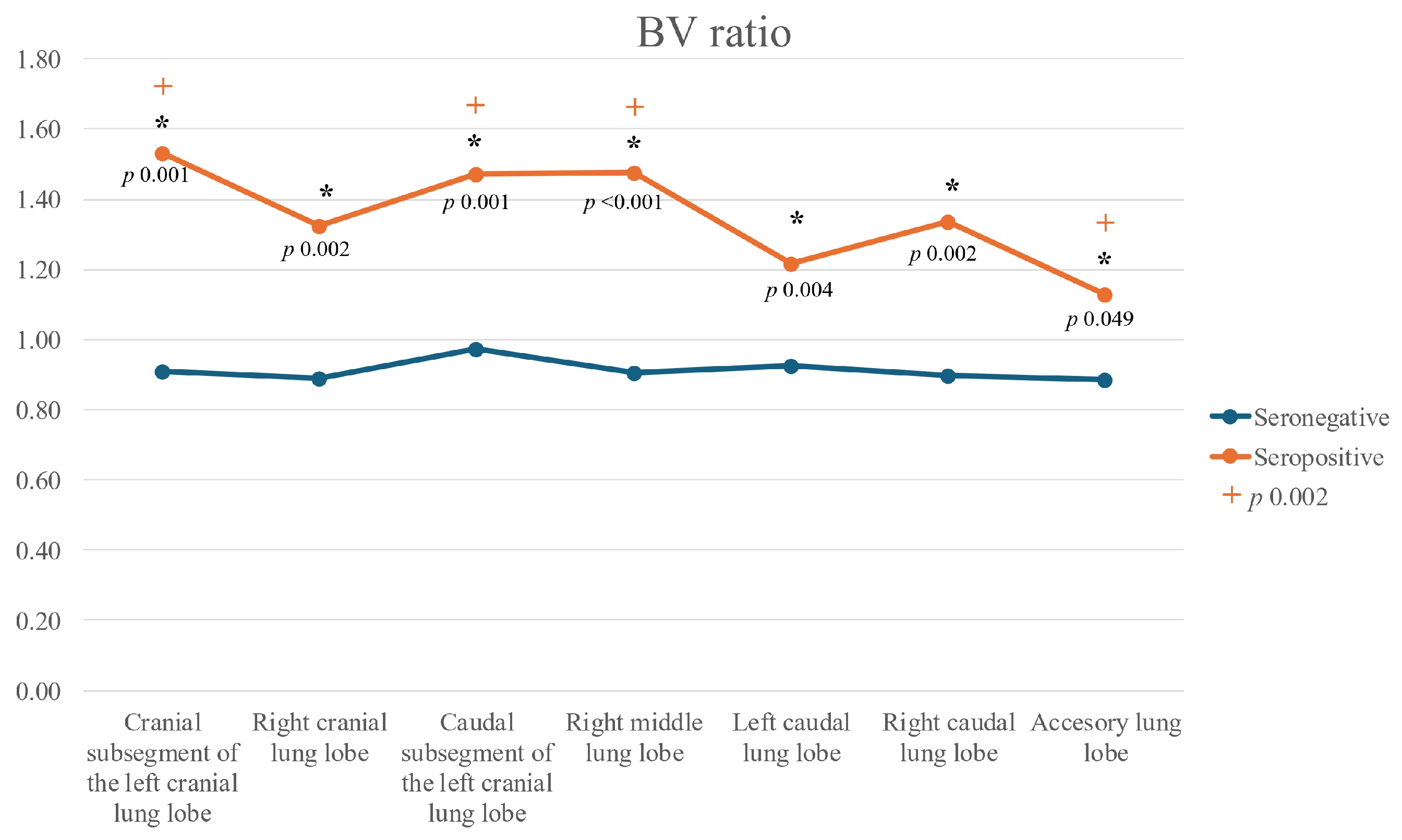
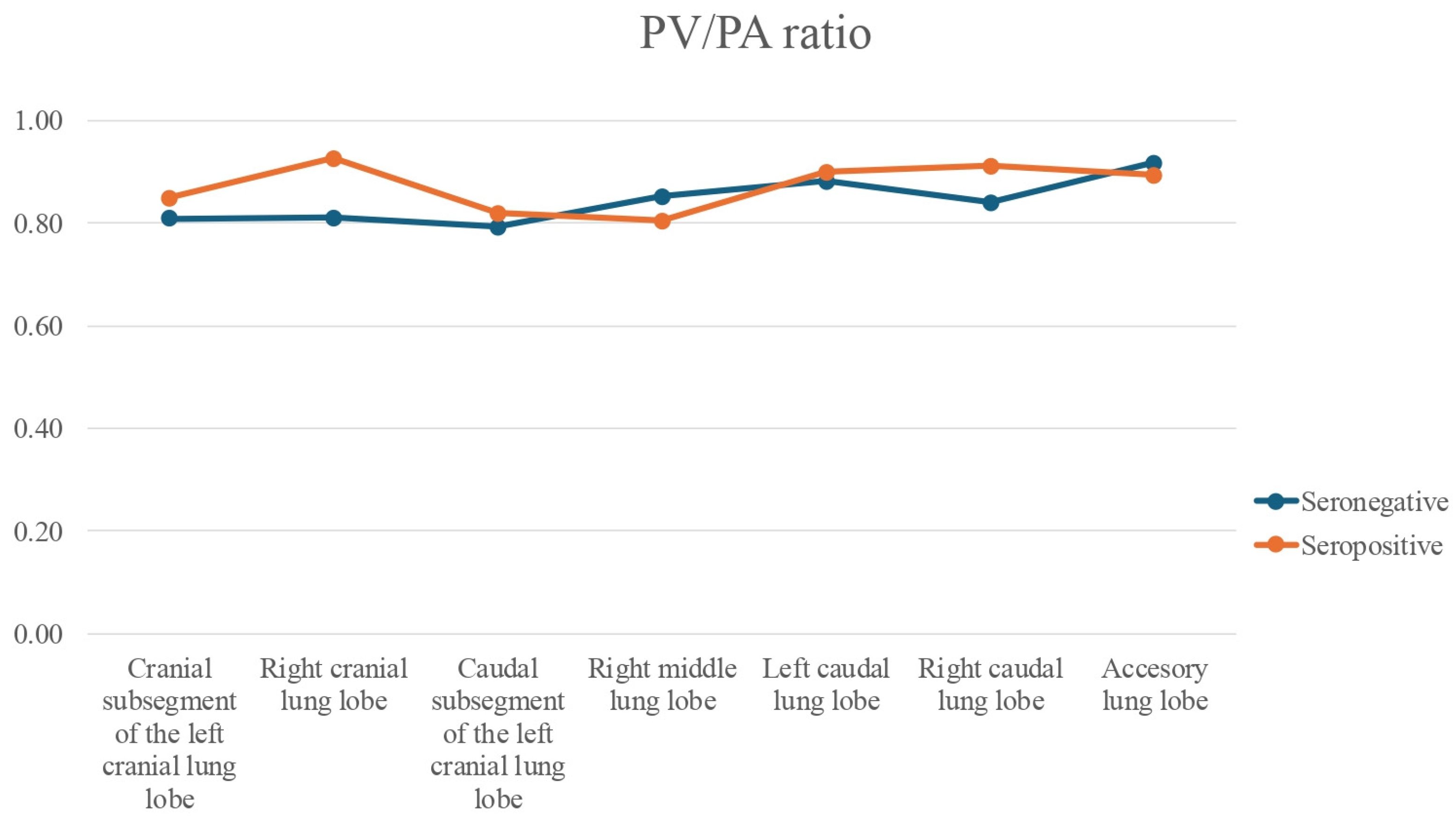
| Lung Lobe | Variable | Median (Group A) [RI] | Median (Group B) [RI] | U-Value | p-Value | Effect Size (r) | Magnitude |
|---|---|---|---|---|---|---|---|
| Left cranial (cranial subsegment) | BA | 1.20 [0.96–1.48] | 0.73 [0.65–0.79] | 3.795 | <0.001 *** | >0.5 | Large |
| BV | 1.53 [1.03–1.90] | 0.91 [0.81–1.04] | 3.115 | 0.001 *** | >0.5 | Large | |
| Bronchus | 1.25 [1.11–1.71] | 0.91 [0.83–1.26] | 2.148 | 0.031 ** | 0.3–0.5 | Medium | |
| Left cranial (caudal subsegment) | BA | 0.99 [0.79–1.48] | 0.76 [0.70–0.83] | 2.864 | 0.003 *** | 0.3–0.5 | Medium |
| BV | 1.47 [1.16–1.69] | 0.97 [0.89–1.09] | 3.043 | 0.001 *** | 0.3–0.5 | Medium | |
| Bronchus | 1.21 [1.02–1.71] | 1.02 [0.71–1.62] | 1.021 | 0.314 | |||
| Left caudal | BA | 1.11 [0.86–1.33] | 0.74 [0.71–0.81] | 3.079 | 0.001 *** | 0.3–0.5 | Medium |
| BV | 1.22 [1.06–1.67] | 0.92 [0.83–0.99] | 2.757 | 0.004 *** | 0.3–0.5 | Medium | |
| Bronchus | 2.32 [1.80–2.75] | 1.81 [1.63–1.92] | 2.077 | 0.038 ** | 0.3–0.5 | Medium | |
| Right cranial | BA | 1.16 [0.98–1.55] | 0.70 [0.65–0.77] | 3.294 | <0.001 *** | >0.5 | Large |
| BV | 1.33 [1.05–1.62] | 0.89 [0.74–1.07] | 2.936 | 0.002 *** | 0.3–0.5 | Medium | |
| Bronchus | 1.39 [0.98–1.72] | 0.96 [0.80–1.32] | 1.647 | 0.104 | |||
| Right middle | BA | 1.11 [0.94–1.47] | 0.72 [0.67–0.77] | 3.760 | <0.001 *** | >0.5 | Large |
| BV | 1.48 [1.08–1.85] | 0.91 [0.80–1.02] | 3.545 | <0.001 *** | >0.5 | Large | |
| Bronchus | 1.10 [0.91–1.41] | 0.71 [0.61–0.84] | 2.524 | 0.010 ** | 0.3–0.5 | Medium | |
| Right caudal | BA | 1.12 [1.01–1.38] | 0.71 [0.62–0.79] | 3.330 | <0.001 *** | >0.5 | Large |
| BV | 1.34 [1.07–1.53] | 0.90 [0.70–0.96] | 3.008 | 0.002 *** | >0.5 | Large | |
| Bronchus | 2.49 [2.93–1.90] | 2.03 [2.54–1.46] | 1.647 | 0.104 | |||
| Accessory | BA | 1.00 [0.85–1.17] | 0.79 [0.68–1.04] | 1.826 | 0.070 * | ||
| BV | 1.13 [0.91–1.32] | 0.88 [0.79–0.96] | 1.969 | 0.049 ** | 0.3–0.5 | Medium | |
| Bronchus | 0.89 [0–75–1.15] | 0.82 [0.70–0.97] | 0.895 | 0.388 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Rodríguez, S.N.; Matos, J.I.; García-Guasch, L.; Mohr-Peraza, E.; Montoya-Alonso, J.A.; Carretón, E. Tomographic Evaluation of the Bronchial and Pulmonary Vascular Relationships in Cats Naturally Infected with Immature Dirofilaria immitis. Animals 2025, 15, 3320. https://doi.org/10.3390/ani15223320
García-Rodríguez SN, Matos JI, García-Guasch L, Mohr-Peraza E, Montoya-Alonso JA, Carretón E. Tomographic Evaluation of the Bronchial and Pulmonary Vascular Relationships in Cats Naturally Infected with Immature Dirofilaria immitis. Animals. 2025; 15(22):3320. https://doi.org/10.3390/ani15223320
Chicago/Turabian StyleGarcía-Rodríguez, Sara Nieves, Jorge Isidoro Matos, Laín García-Guasch, Eva Mohr-Peraza, José Alberto Montoya-Alonso, and Elena Carretón. 2025. "Tomographic Evaluation of the Bronchial and Pulmonary Vascular Relationships in Cats Naturally Infected with Immature Dirofilaria immitis" Animals 15, no. 22: 3320. https://doi.org/10.3390/ani15223320
APA StyleGarcía-Rodríguez, S. N., Matos, J. I., García-Guasch, L., Mohr-Peraza, E., Montoya-Alonso, J. A., & Carretón, E. (2025). Tomographic Evaluation of the Bronchial and Pulmonary Vascular Relationships in Cats Naturally Infected with Immature Dirofilaria immitis. Animals, 15(22), 3320. https://doi.org/10.3390/ani15223320








