Brine Shrimp Feeding Contributes to Fast Growth and Enhanced Immune Capacity of Reattached Polyps of Scleractinian Coral Pocillopora damicornis
Simple Summary
Abstract
1. Introduction
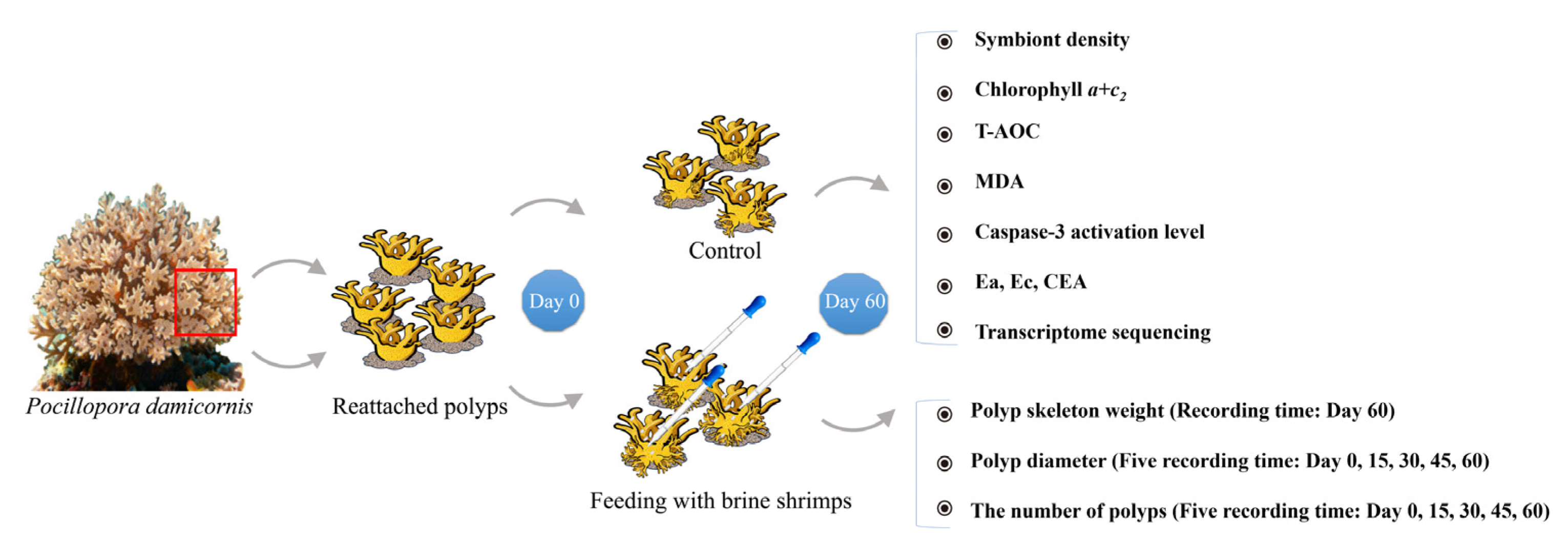
2. Materials and Methods
2.1. Coral Polyp Bailout and Reattachment
2.2. Brine Shrimp Feeding, Observation and Sample Collection
2.3. Determination of Algal Symbiont Density and Chlorophyll Content
2.4. Detection of Caspase-3 Activation Level and Antioxidant Capacity
2.5. Energy Metabolism in Corals
2.6. RNA Extraction and Transcriptome Sequencing
2.7. Gene Co-Expression Network Analysis
2.8. Statistical Analysis
3. Results
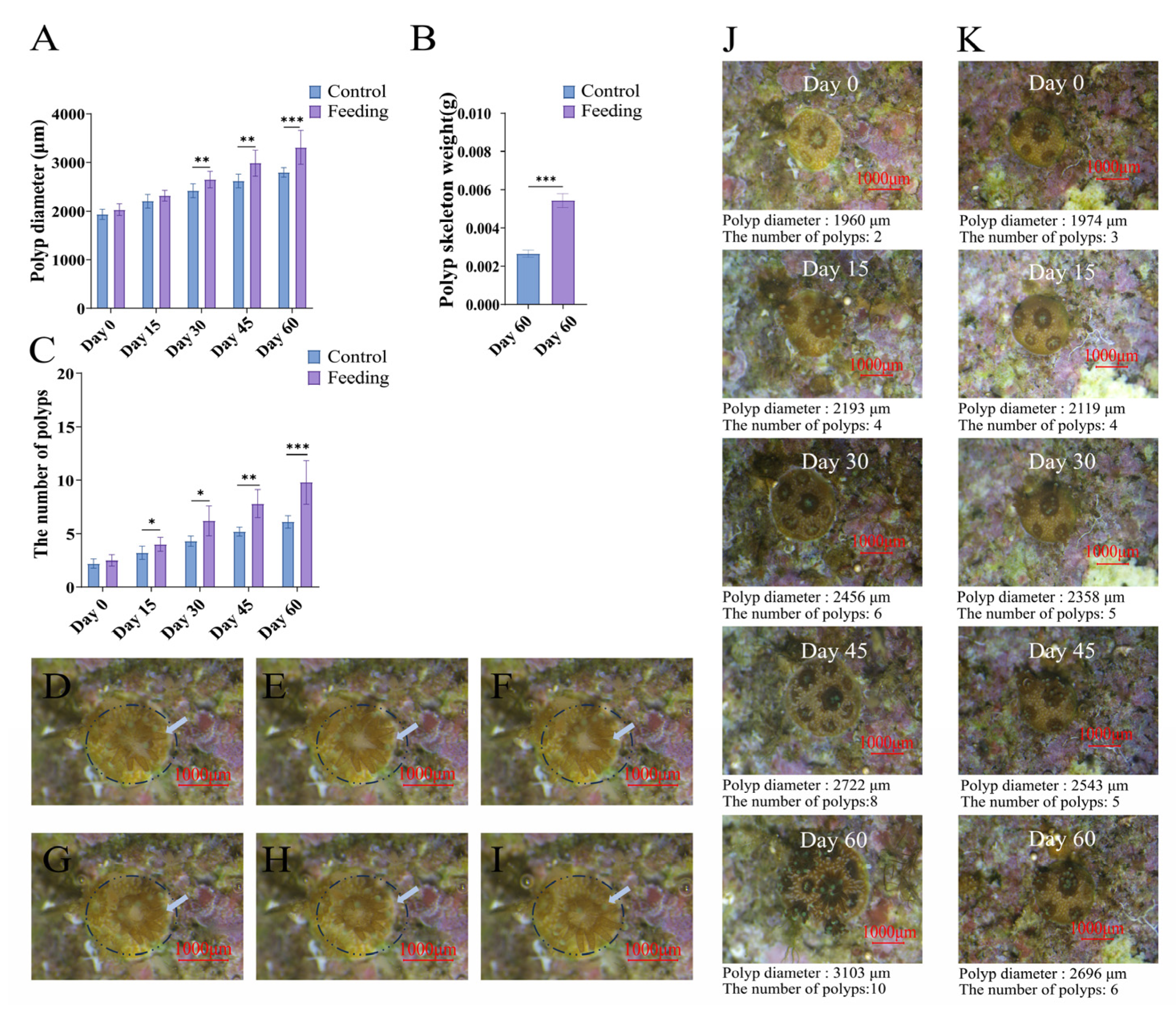
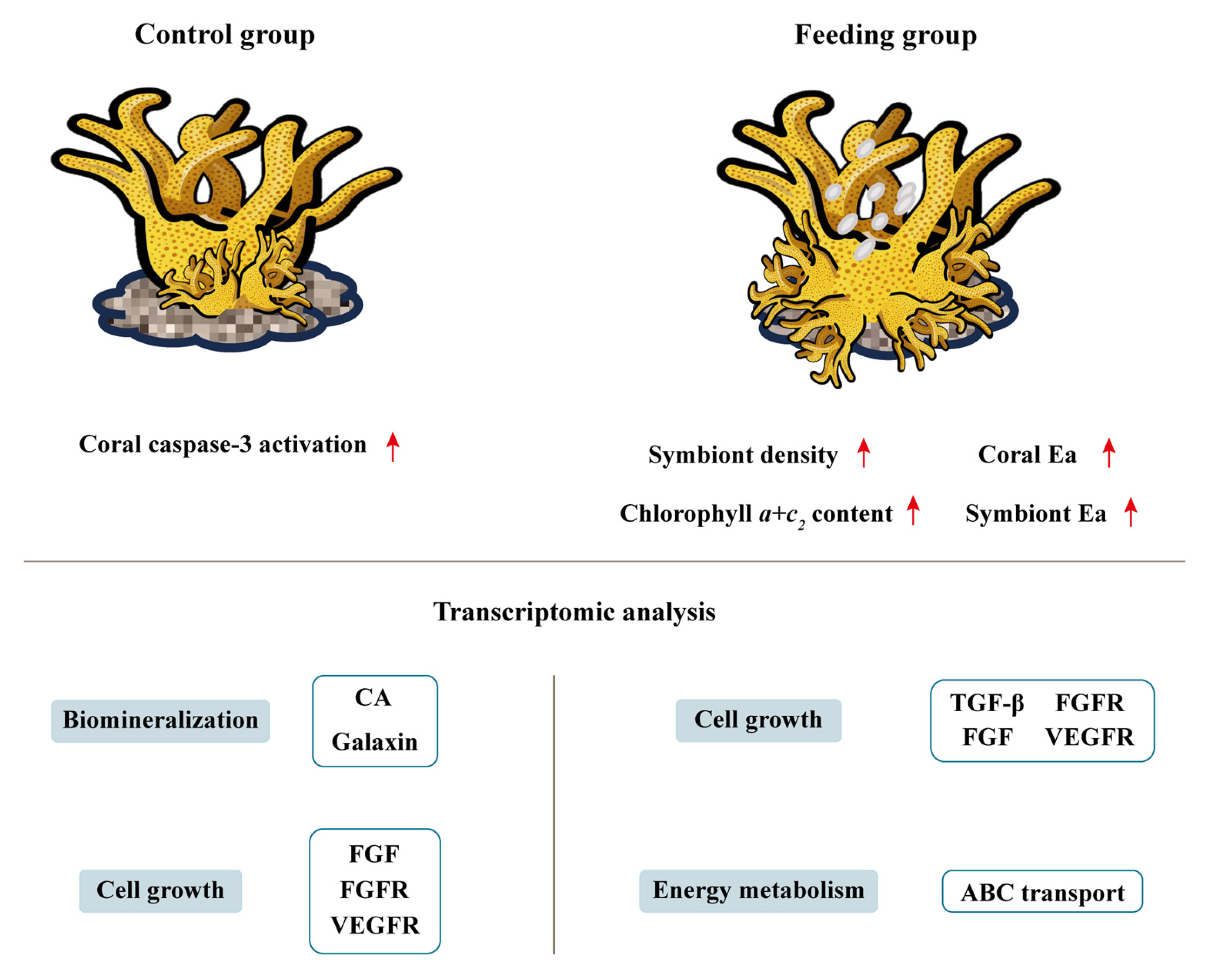
3.1. The Growth of Reattached Polyps After Brine Shrimp Feeding
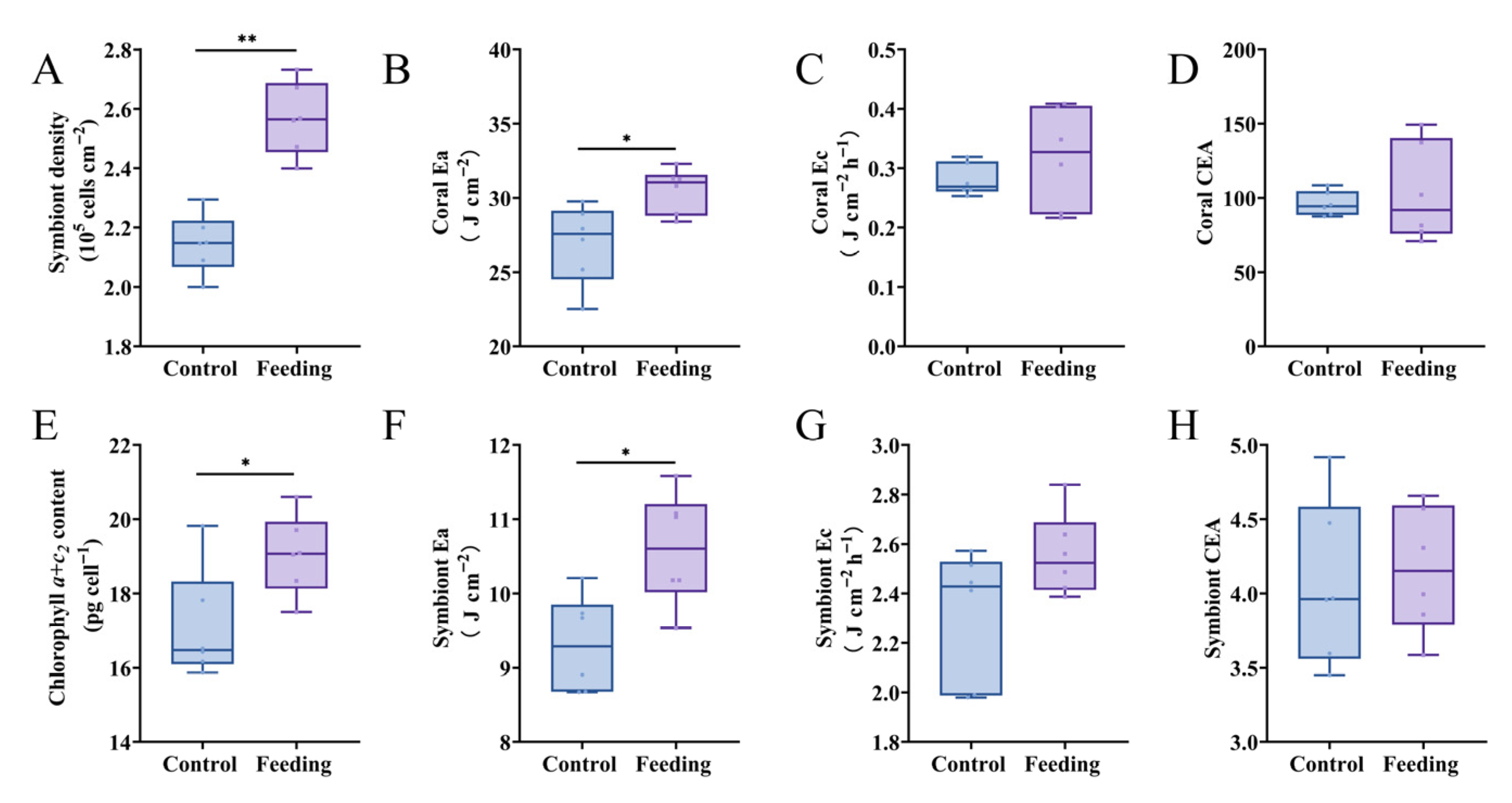
3.2. Energy Metabolism of Reattached Polyps After Brine Shrimp Feeding
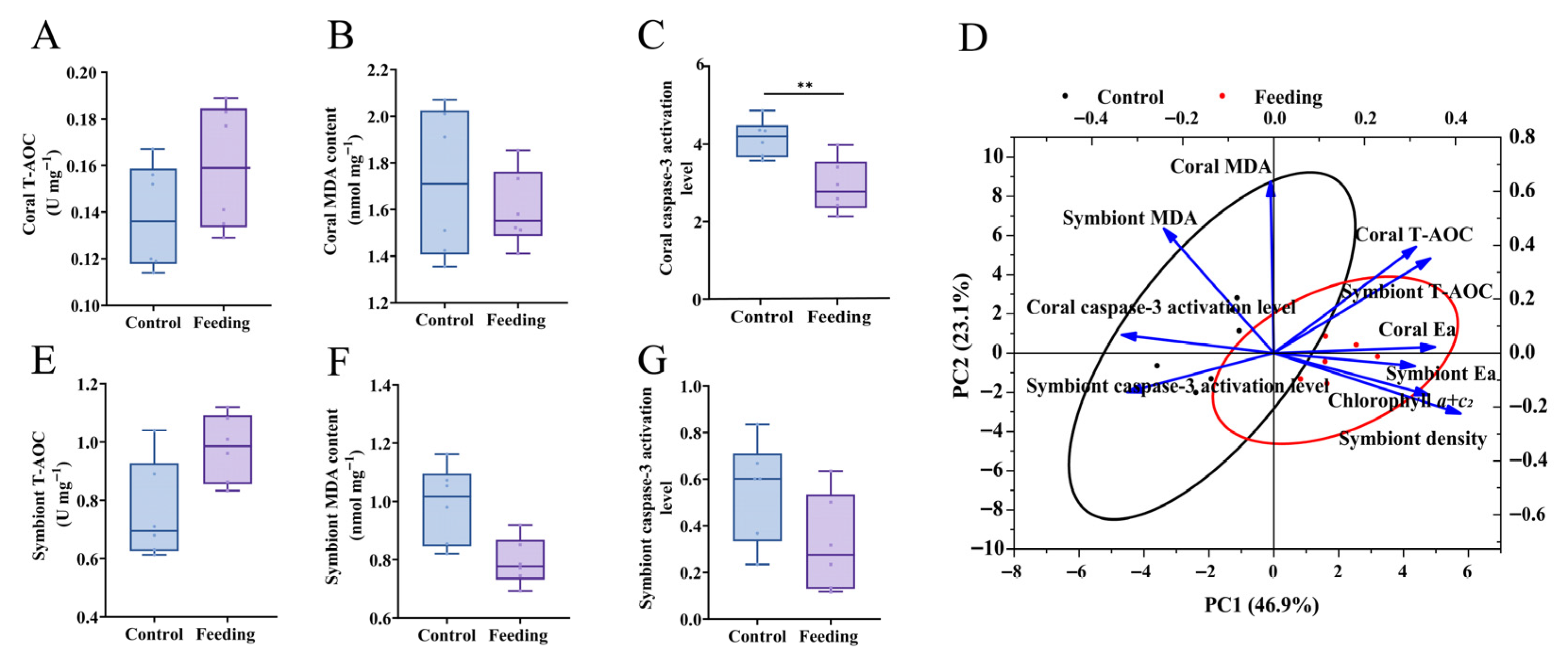
3.3. Changes in Immune Capacity After Brine Shrimp Feeding
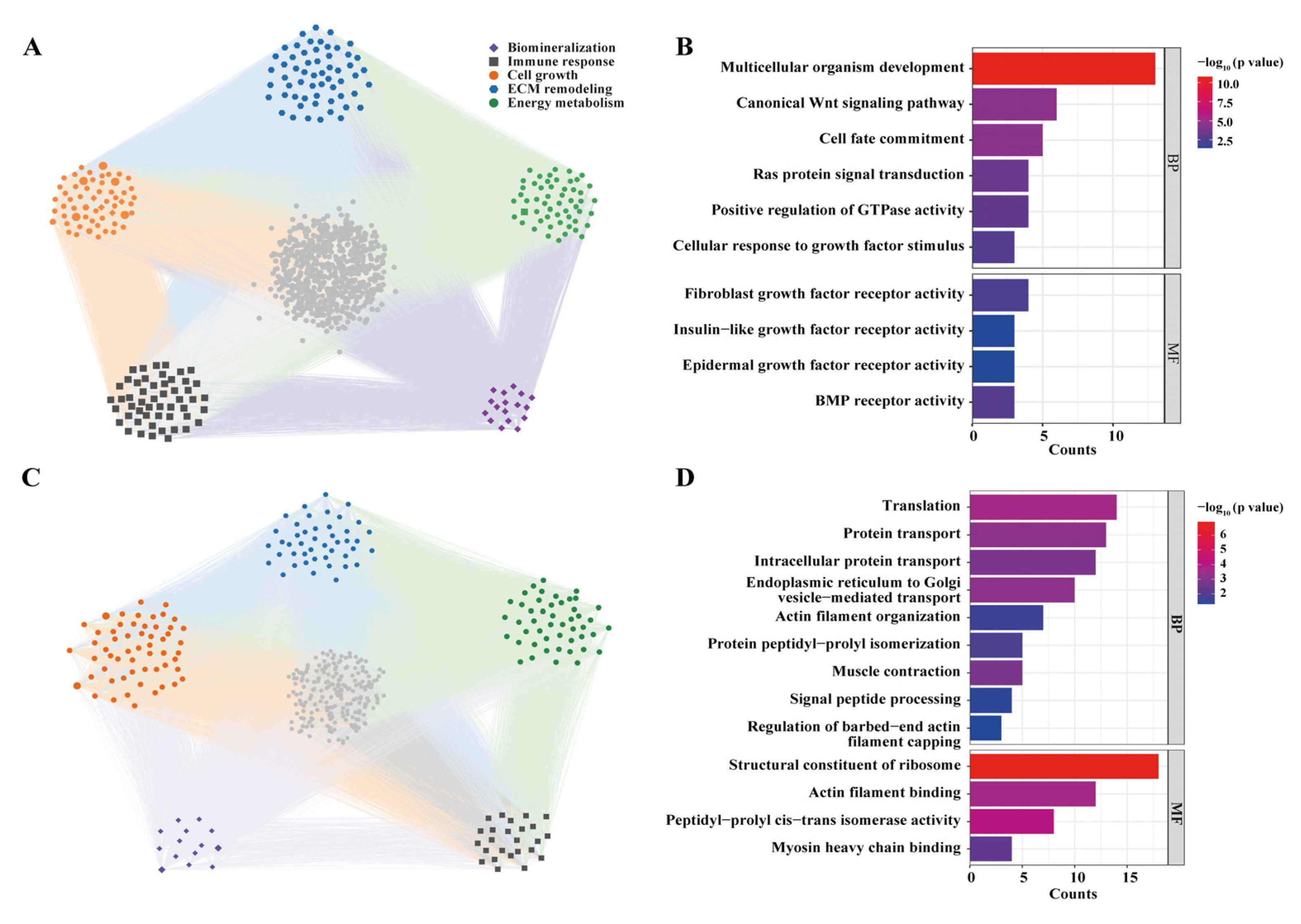
3.4. Differentially Expressed Gene and GO Enrichment
3.5. Co-Expression Network and Functional Module
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Zhou, Z.; Wang, L.; Huang, B. Transcriptome, expression, and activity analyses reveal a vital heat shock protein 70 in the stress response of stony coral Pocillopora damicornis. Cell Stress. Chaperones 2018, 23, 711–721. [Google Scholar] [CrossRef]
- Muniz-Castillo, A.I.; Rivera-Sosa, A.; Chollett, I.; Eakin, C.M.; Andrade-Gomez, L.; McField, M.; Arias-Gonzalez, J.E. Three decades of heat stress exposure in Caribbean coral reefs: A new regional delineation to enhance conservation. Sci. Rep. 2019, 9, 11013. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Kennedy, E.V.; Beyer, H.L.; McClennen, C.; Possingham, H.P. Securing a Long-term Future for Coral Reefs. Trends Ecol. Evol. 2018, 33, 936–944. [Google Scholar] [CrossRef]
- Osborne, K.; Thompson, A.A.; Cheal, A.J.; Emslie, M.J.; Johns, K.A.; Jonker, M.J.; Logan, M.; Miller, I.R.; Sweatman, H.P.A. Delayed coral recovery in a warming ocean. Glob. Change Biol. 2017, 23, 3869–3881. [Google Scholar] [CrossRef]
- Rinkevich, B. Rebuilding coral reefs: Does active reef restoration lead to sustainable reefs? Curr. Opin. Environ. Sust. 2014, 7, 28–36. [Google Scholar] [CrossRef]
- Twan, W.H.; Hwang, J.S.; Lee, Y.H.; Wu, H.F.; Tung, Y.H.; Chang, C.F. Hormones and reproduction in scleractinian corals. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 247–253. [Google Scholar] [CrossRef]
- Cox, E.F.; Ward, S. Impact of elevated ammonium on reproduction in two Hawaiian scleractinian corals with different life history patterns. Mar. Pollut. Bull. 2002, 44, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Combosch, D.J.; Vollmer, S.V. Mixed asexual and sexual reproduction in the Indo-Pacific reef coral Pocillopora damicornis. Ecol. Evol. 2013, 3, 3379–3387. [Google Scholar] [CrossRef]
- Chauvenet, A.L.M.; Ewen, J.G.; Armstrong, D.P.; Blackburn, T.M.; Pettorelli, N. Maximizing the success of assisted colonizations. Anim. Conserv. 2012, 16, 161–169. [Google Scholar] [CrossRef]
- Rinkevich, B. Novel tradable instruments in the conservation of coral reefs, based on the coral gardening concept for reef restoration. J. Environ. Manag. 2015, 162, 199–205. [Google Scholar] [CrossRef]
- Bostrom-Einarsson, L.; Babcock, R.C.; Bayraktarov, E.; Ceccarelli, D.; Cook, N.; Ferse, S.C.A.; Hancock, B.; Harrison, P.; Hein, M.; Shaver, E.; et al. Coral restoration—A systematic review of current methods, successes, failures and future directions. PLoS ONE 2020, 15, e0226631. [Google Scholar] [CrossRef]
- Lange, I.D.; Razak, T.B.; Perry, C.T.; Maulana, P.B.; Prasetya, M.E.; Irwan; Lamont, T.A.C. Coral restoration can drive rapid reef carbonate budget recovery. Curr. Biol. 2024, 34, 1341–1348.e1343. [Google Scholar] [CrossRef]
- Burdett, H.L.; Albright, R.; Foster, G.L.; Mass, T.; Page, T.M.; Rinkevich, B.; Schoepf, V.; Silverman, J.; Kamenos, N.A. Including environmental and climatic considerations for sustainable coral reef restoration. PLoS Biol. 2024, 22, e3002542. [Google Scholar] [CrossRef]
- Vardi, T.; Hoot, W.C.; Levy, J.; Shaver, E.; Winters, R.S.; Banaszak, A.T.; Baums, I.B.; Chamberland, V.F.; Cook, N.; Gulko, D.; et al. Six priorities to advance the science and practice of coral reef restoration worldwide. Restor. Ecol. 2021, 29, e13498. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Morrison, T.H.; Torda, G. Principles for coral reef restoration in the anthropocene. One Earth 2023, 6, 656–665. [Google Scholar] [CrossRef]
- Chuang, P.; Mitarai, S. Signaling pathways in the coral polyp bail-out response. Coral Reefs 2020, 39, 1535–1548. [Google Scholar] [CrossRef]
- Coppari, M.; Fumarola, L.; Bramanti, L.; Romans, P.; Pillot, R.; Bavestrello, G.; Bo, M. Unveiling asexual reproductive traits in black corals: Polyp bailout in Antipathella subpinnata. Coral Reefs 2020, 39, 1517–1523. [Google Scholar] [CrossRef]
- Shapiro, O.H.; Kramarsky-Winter, E.; Gavish, A.R.; Stocker, R.; Vardi, A. A coral-on-a-chip microfluidic platform enabling live-imaging microscopy of reef-building corals. Nat. Commun. 2016, 7, 10860. [Google Scholar] [CrossRef] [PubMed]
- Serrano, E.; Coma, R.; Inostroza, K.; Serrano, O. Polyp bail-out by the coral Astroides calycularis (Scleractinia, Dendrophylliidae). Mar. Biodivers. 2018, 48, 1661–1665. [Google Scholar] [CrossRef]
- Capel, K.; Migotto, A.; Zilberberg, C.; Kitahara, M. Another tool towards invasion? Polyp “bail-out” in Tubastraea coccinea. Coral Reefs 2014, 33, 1165. [Google Scholar] [CrossRef]
- Sammarco, P. Polyp bail-out: An escape response to environmental stress and a new means of reproduction in corals. Mar. Ecol. Prog. Ser. 1982, 10, 57–65. [Google Scholar] [CrossRef]
- Piraino, S.; De Vito, D.; Schmich, J.; Bouillon, J.; Boero, F. Reverse development in Cnidaria. Can. J. Zool. 2004, 82, 1748–1754. [Google Scholar] [CrossRef]
- Kariyazono, S.; Hatta, M. Bail-out of the polyp from the skeleton of spats in the scleractinian coral Acropora tenuis. Galaxea J. Coral Reef. Stud. 2015, 17, 19–20. [Google Scholar] [CrossRef]
- Fordyce, A.J.; Camp, E.F.; Ainsworth, T.D. Polyp bailout in Pocillopora damicornis following thermal stress. F1000Research 2017, 6, 687. [Google Scholar] [CrossRef]
- Schweinsberg, M.; Gosser, F.; Tollrian, R. The history, biological relevance, and potential applications for polyp bailout in corals. Ecol. Evol. 2021, 11, 8424–8440. [Google Scholar] [CrossRef]
- Lam, K.W.; McRae, C.J.; Liu, Z.T.; Zhang, X.C.; Fan, T.Y. Effective Techniques for the Feeding and Ex Situ Culture of a Brooding Scleractinian Coral, Pocillopora acuta. J. Vis. Exp. 2023. [Google Scholar] [CrossRef]
- Goldberg, W.M. Gastrodermal structure and feeding responses in the scleractinian Mycetophyllia reesi, a coral with novel digestive filaments. Tissue Cell 2002, 34, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Wijgerde, T.; Diantari, R.; Lewaru, M.W.; Verreth, J.A.; Osinga, R. Extracoelenteric zooplankton feeding is a key mechanism of nutrient acquisition for the scleractinian coral Galaxea fascicularis. J. Exp. Biol. 2011, 214, 3351–3357. [Google Scholar] [CrossRef] [PubMed]
- Huffmyer, A.S.; Johnson, C.J.; Epps, A.M.; Lemus, J.D.; Gates, R.D. Feeding and thermal conditioning enhance coral temperature tolerance in juvenile Pocillopora acuta. R. Soc. Open Sci. 2021, 8, 210644. [Google Scholar] [CrossRef] [PubMed]
- Crabbe, M.J.; Smith, D.J. Modelling variations in corallite morphology of Galaxea fascicularis coral colonies with depth and light on coastal fringing reefs in the Wakatobi Marine National Park (S.E. Sulawesi, Indonesia). Comput. Biol. Chem. 2006, 30, 155–159. [Google Scholar] [CrossRef]
- Conlan, J.A.; Humphrey, C.A.; Severati, A.; Francis, D.S. Influence of different feeding regimes on the survival, growth, and biochemical composition of Acropora coral recruits. PLoS ONE 2017, 12, e0188568. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.Y.; Hu, H.Y.; Zhao, X.; Li, Y. Effects of chlorination on the properties of dissolved organic matter and its genotoxicity in secondary sewage effluent under two different ammonium concentrations. Chemosphere 2010, 80, 941–946. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, J.; Cao, X.; Wu, C.; Cai, W.; Lin, S. High Heterotrophic Plasticity of Massive Coral Porites pukoensis Contributes to Its Tolerance to Bioaccumulated Microplastics. Environ. Sci. Technol. 2023, 57, 3391–3401. [Google Scholar] [CrossRef]
- Ding, D.S.; Sun, W.T.; Pan, C.H. Feeding of a Scleractinian Coral, Goniopora columna, on Microalgae, Yeast, and Artificial Feed in Captivity. Animals 2021, 11, 3009. [Google Scholar] [CrossRef]
- Forsman, Z.H.; Rinkevich, B.; Hunter, C.L. Investigating fragment size for culturing reef-building corals (Porites lobata and P. compressa) in ex situ nurseries. Aquaculture 2006, 261, 89–97. [Google Scholar] [CrossRef]
- Cardoso, P.M.; Alsaggaf, A.A.; Villela, H.M.; Peixoto, R.S. Inducing Polyp Bail-out in Coral Colonies to Obtain Individualized Micropropagates for Laboratory Experimental Use. J. Vis. Exp. 2022. [Google Scholar] [CrossRef]
- Chuang, P.S.; Yamada, Y.; Liu, P.Y.; Tang, S.L.; Mitarai, S. Bacterial Community Shifts during Polyp Bail-Out Induction in Pocillopora Corals. Microbiol. Spectr. 2023, 11, e0025723. [Google Scholar] [CrossRef]
- Wecker, P.; Lecellier, G.; Guibert, I.; Zhou, Y.; Bonnard, I.; Berteaux-Lecellier, V. Exposure to the environmentally-persistent insecticide chlordecone induces detoxification genes and causes polyp bail-out in the coral P. damicornis. Chemosphere 2018, 195, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; An, M.; Geng, X.; Wu, Z.; Cai, W.; Tang, J.; Zhang, K.; Zhou, Z. The scleractinian coral Pocillopora damicornis relies on neuroendocrine regulation to cope with polycyclic aromatic hydrocarbons under heat stress. Environ. Pollut. 2023, 316, 120565. [Google Scholar] [CrossRef] [PubMed]
- John, C.J.A.; Abatzopoulos, T.J.; Marian, P.M. Characterization of a new parthenogenetic Artemia population from Thamaraikulam, India. J. Biol. Res. 2004, 2, 63–74. [Google Scholar]
- Tang, J.; Wu, Z.; Wan, L.; Cai, W.; Chen, S.; Wang, X.; Luo, J.; Zhou, Z.; Zhao, J.; Lin, S. Differential enrichment and physiological impacts of ingested microplastics in scleractinian corals in situ. J. Hazard. Mater. 2021, 404, 124205. [Google Scholar] [CrossRef]
- Tang, J.; Ni, X.; Zhou, Z.; Wang, L.; Lin, S. Acute microplastic exposure raises stress response and suppresses detoxification and immune capacities in the scleractinian coral Pocillopora damicornis. Environ. Pollut. 2018, 243, 66–74. [Google Scholar] [CrossRef]
- Raz-Bahat, M.; Hanny Faibish, T.M.; Rinkevich, B. Three-dimensional laser scanning as an efficient tool for coralsurface area measurements. Limnol. Oceanogr. Methods 2009, 7, 657–663. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Und Physiol. Der Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Xiao, N.; Wang, X.C.; Diao, Y.F.; Liu, R.; Tian, K.L. Effect of initial fluid resuscitation on subsequent treatment in uncontrolled hemorrhagic shock in rats. Shock 2004, 21, 276–280. [Google Scholar] [CrossRef] [PubMed]
- De Coen, W.M.; Janssen, C.R.; Persoone, G. Biochemical assessment of cellular energy allocation in Daphnia magna exposed to toxic stress as an alternative to the conventional “scope for growth” methodology. In Proceedings of the International Symposium on Biological Markers of Pollution, Chinon, France, 21–22 September 1995; pp. 163–170. [Google Scholar]
- De Coen, W.M.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing. IV. Cellular energy allocation: A new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J. Aquat. Ecosyst. Stress. Recovery 1997, 6, 43–55. [Google Scholar] [CrossRef]
- Rodrigues, L.J.; Grottoli, A.G. Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnol. Oceanogr. 2007, 52, 1874–1882. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernandez-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Conti-Jerpe, I.E.; Thompson, P.D.; Wong, C.W.M.; Oliveira, N.L.; Duprey, N.N.; Moynihan, M.A.; Baker, D.M. Trophic strategy and bleaching resistance in reef-building corals. Sci. Adv. 2020, 6, eaaz5443. [Google Scholar] [CrossRef]
- Chen, B.; Wei, Y.; Yu, K.; Liang, Y.; Yu, X.; Liao, Z.; Qin, Z.; Xu, L.; Bao, Z. The microbiome dynamics and interaction of endosymbiotic Symbiodiniaceae and fungi are associated with thermal bleaching susceptibility of coral holobionts. Appl. Environ. Microbiol. 2024, 90, e0193923. [Google Scholar] [CrossRef]
- Webster, N.S.; Reusch, T.B.H. Microbial contributions to the persistence of coral reefs. ISME J. 2017, 11, 2167–2174. [Google Scholar] [CrossRef]
- Wiederkehr, F.; Engelhardt, K.E.; Vetter, J.; Ruscheweyh, H.J.; Salazar, G.; O’Brien, J.; Priest, T.; Ziegler, M.; Sunagawa, S. Host-level biodiversity shapes the dynamics and networks within the coral reef microbiome. ISME Commun. 2025, 5, ycaf097. [Google Scholar] [CrossRef]
- Yan, Z.; Cao, X.; Su, H.; Li, C.; Lin, J.; Tang, K.; Zhang, J.; Fan, H.; Chen, Q.; Tang, J.; et al. Coral-Symbiodiniaceae symbiotic associations under antibiotic stress: Accumulation patterns and potential physiological effects in a natural reef. J. Hazard. Mater. 2025, 486, 137039. [Google Scholar] [CrossRef]
- Claar, D.C.; Starko, S.; Tietjen, K.L.; Epstein, H.E.; Cunning, R.; Cobb, K.M.; Baker, A.C.; Gates, R.D.; Baum, J.K. Dynamic symbioses reveal pathways to coral survival through prolonged heatwaves. Nat. Commun. 2020, 11, 6097. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Shi, S.H.; Li, H.H.; Lin, C.Y.; Wang, W.H. Lipid profiling of coral symbiosomes in response to copper-induced carbon limitation: A metabolic effect of algal symbionts on the host immune status. Chemosphere 2022, 293, 133673. [Google Scholar] [CrossRef]
- Roach, T.N.F.; Dilworth, J.; H, C.M.; Jones, A.D.; Quinn, R.A.; Drury, C. Metabolomic signatures of coral bleaching history. Nat. Ecol. Evol. 2021, 5, 495–503. [Google Scholar] [CrossRef]
- Leinbach, S.E.; Speare, K.E.; Rossin, A.M.; Holstein, D.M.; Strader, M.E. Energetic and reproductive costs of coral recovery in divergent bleaching responses. Sci. Rep. 2021, 11, 23546. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.D.; Grottoli, A.G. Heterotrophic compensation: A possible mechanism for resilience of coral reefs to global warming or a sign of prolonged stress? PLoS ONE 2013, 8, e81172. [Google Scholar] [CrossRef] [PubMed]
- Ferrier-Pagès, C.; Rottier, C.; Beraud, E.; Levy, O. Experimental assessment of the feeding effort of three scleractinian coral species during a thermal stress: Effect on the rates of photosynthesis. J. Exp. Mar. Biol. Ecol. 2010, 390, 118–124. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Zoccola, D.; Moya, A.; Beranger, G.E.; Tambutte, E.; Allemand, D.; Carle, G.F. Specific expression of BMP2/4 ortholog in biomineralizing tissues of corals and action on mouse BMP receptor. Mar. Biotechnol. 2009, 11, 260–269. [Google Scholar] [CrossRef] [PubMed]

| Sample | Total Mapping Gene Ratio | Uniquely Mapping Gene Ratio |
|---|---|---|
| Control-1 | 41.35 | 39.53 |
| Control-2 | 42.44 | 40.43 |
| Control-3 | 42.3 | 40.46 |
| Control-4 | 40.01 | 38.19 |
| Control-5 | 40.93 | 39.12 |
| Feeding-1 | 43.72 | 41.79 |
| Feeding-2 | 38.62 | 36.87 |
| Feeding-3 | 40.94 | 39.13 |
| Feeding-4 | 42.49 | 40.61 |
| Feeding-5 | 41.33 | 39.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Wang, Y.; Liu, Z. Brine Shrimp Feeding Contributes to Fast Growth and Enhanced Immune Capacity of Reattached Polyps of Scleractinian Coral Pocillopora damicornis. Animals 2025, 15, 3318. https://doi.org/10.3390/ani15223318
Huang H, Wang Y, Liu Z. Brine Shrimp Feeding Contributes to Fast Growth and Enhanced Immune Capacity of Reattached Polyps of Scleractinian Coral Pocillopora damicornis. Animals. 2025; 15(22):3318. https://doi.org/10.3390/ani15223318
Chicago/Turabian StyleHuang, Haifeng, Yi Wang, and Zhaoqun Liu. 2025. "Brine Shrimp Feeding Contributes to Fast Growth and Enhanced Immune Capacity of Reattached Polyps of Scleractinian Coral Pocillopora damicornis" Animals 15, no. 22: 3318. https://doi.org/10.3390/ani15223318
APA StyleHuang, H., Wang, Y., & Liu, Z. (2025). Brine Shrimp Feeding Contributes to Fast Growth and Enhanced Immune Capacity of Reattached Polyps of Scleractinian Coral Pocillopora damicornis. Animals, 15(22), 3318. https://doi.org/10.3390/ani15223318





