Rhodotorula spp. in Laboratory and Veterinary Clinical Practice: Contamination or an Emerging Problem?
Simple Summary
Abstract
1. Introduction
2. Taxonomic Classification and Revision
3. Key Aspects of Identifying Yeast-like Fungi of the Genus Rhodotorula
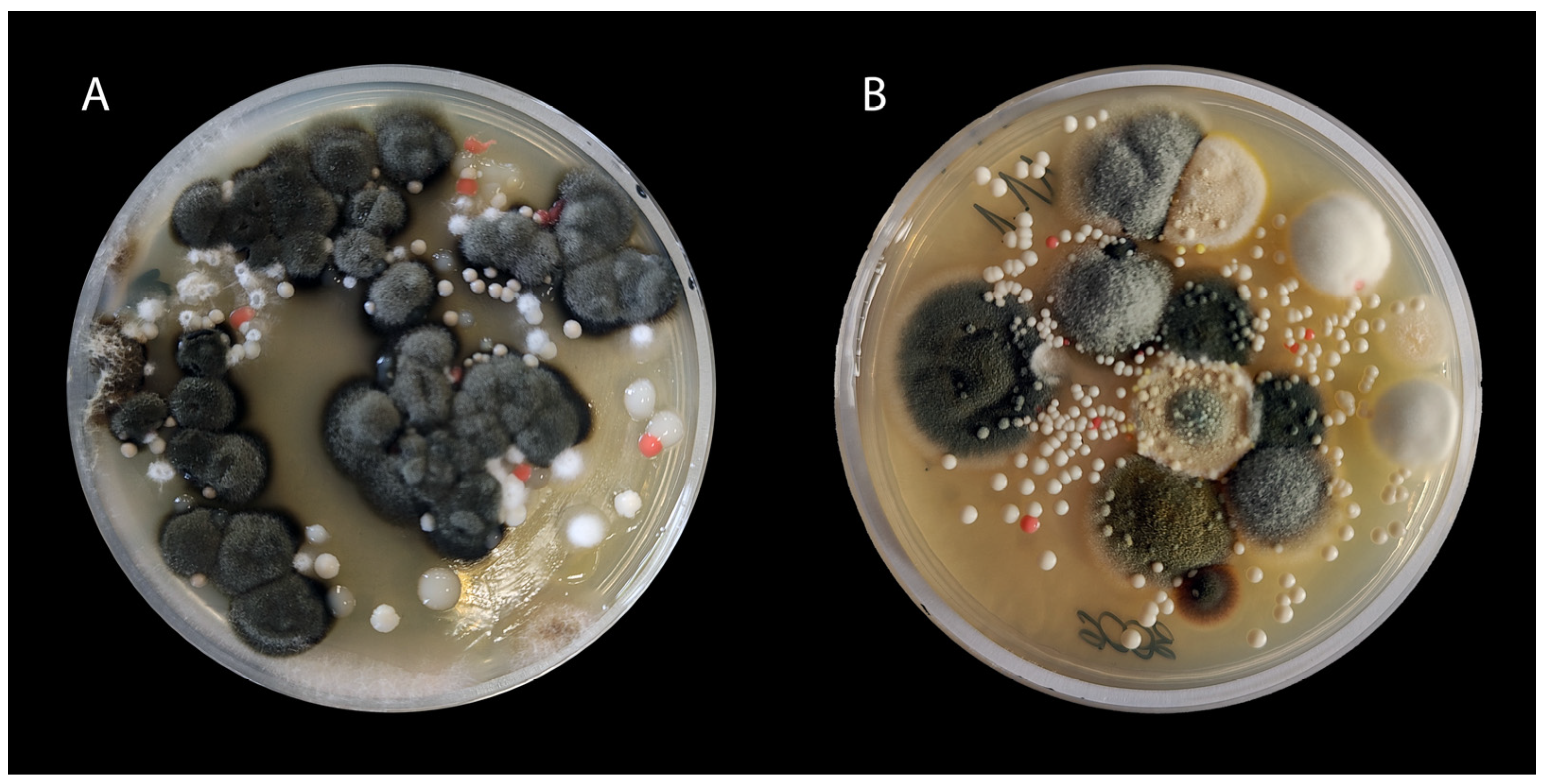
3.1. Culture
3.2. Microscopic Examination
3.3. Biochemical Tests
3.4. Differentiation from Other Species of Yeast-like Fungi
3.5. Automatic and Molecular Methods
3.6. An Integrated Approach to the Diagnosis of Rhodotorula spp. Infections
4. Virulence Factors of Rhodotorula spp. and Their Significance in Pathogenesis
4.1. Pathogenesis
4.2. Virulence Factors
4.2.1. Biofilm Formation
4.2.2. Phospholipases
4.2.3. Hydrolytic Enzymes
4.2.4. Iron Acquisition
4.2.5. Adhesion
4.2.6. Urease Activity
4.2.7. Catalase
4.2.8. Carotenoids
5. Drug Sensitivity and Resistance Mechanisms
5.1. Resistance to Azoles
5.2. Resistance to Echinocandins
5.3. Sensitivity to Polyenes and Flucytosine
6. Veterinary Cases
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Neill, D.G.; Gunn-Moore, D.; Sorrell, S.; McAuslan, H.; Church, D.B.; Pegram, C.; Brodbelt, D.C. Commonly Diagnosed Disorders in Domestic Cats in the UK and Their Associations with Sex and Age. J. Feline Med. Surg. 2023, 25, 1098612X231155016. [Google Scholar] [CrossRef]
- O’Neill, D.G.; James, H.; Brodbelt, D.C.; Church, D.B.; Pegram, C. Prevalence of Commonly Diagnosed Disorders in UK Dogs under Primary Veterinary Care: Results and Applications. BMC Vet. Res. 2021, 17, 69. [Google Scholar] [CrossRef]
- O′Neill, D.G.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Prevalence of Disorders Recorded in Dogs Attending Primary-Care Veterinary Practices in England. PLoS ONE 2014, 9, e90501. [Google Scholar] [CrossRef]
- Summers, J.F.; O’Neill, D.G.; Church, D.; Collins, L.; Sargan, D.; Brodbelt, D.C. Health-Related Welfare Prioritisation of Canine Disorders Using Electronic Health Records in Primary Care Practice in the UK. BMC Vet. Res. 2019, 15, 163. [Google Scholar] [CrossRef]
- Nuttall, T. Managing Recurrent Otitis Externa in Dogs: What Have We Learned and What Can We Do Better? J. Am. Vet. Med. Assoc. 2023, 261, S10–S22. [Google Scholar] [CrossRef]
- Murphy, K.M. A Review of Techniques for the Investigation of Otitis Externa and Otitis Media. Clin. Tech. Small Anim. Pract. 2001, 16, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Dworecka-Kaszak, B.; Bieganska, M.J.; Dabrowska, I. Occurrence of Various Pathogenic and Opportunistic Fungi in Skin Diseases of Domestic Animals: A Retrospective Study. BMC Vet. Res. 2020, 16, 248. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, J. Canine Otitis Externa—Treatment and Complications. Can. Vet. J. 2019, 60, 97–99. [Google Scholar] [PubMed] [PubMed Central]
- Bond, R.; Morris, D.O.; Guillot, J.; Bensignor, E.J.; Robson, D.; Mason, K.V.; Kano, R.; Hill, P.B. Biology, Diagnosis and Treatment of Malassezia Dermatitis in Dogs and Cats Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2020, 31, 28–74. [Google Scholar] [CrossRef]
- Hobi, S.; Bęczkowski, P.M.; Mueller, R.; Tse, M.; Barrs, V.R. Malassezia Dermatitis in Dogs and Cats. Vet. J. 2024, 304, 106084. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, F.M.; Martins, H.M.; Martins, M.L. A Survey of Mycotic Otitis Externa of Dogs in Lisbon. Rev. Iberoam. Micol. 1998, 15, 163–165. [Google Scholar]
- De Bona, E.; Ubiratan Paz Telesca, S.; Meneghello Fuentefria, A. Occurrence and Identification of Yeasts in Dogs External Ear Canal with and without Otitis. Rev. MVZ Córdoba 2012, 17, 3059–3064. [Google Scholar] [CrossRef]
- Aboul-Ella, H.; Mosallam, T.; Samir, O.; Ali, A.; Qasim, A.; Mahmoud, H.E.; Samir, A. Emergence of Rhodotorula mucilaginosa among Pet Animals: A Possible Public Health Risk on the Move. BMC Microbiol. 2025, 25, 273. [Google Scholar] [CrossRef]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Najar, B.; Pistelli, L.; Mancianti, F. Antibacterial and Antifungal Activity of Essential Oils against Pathogens Responsible for Otitis Externa in Dogs and Cats. Medicines 2017, 4, 21. [Google Scholar] [CrossRef]
- García, N.; García, M.; Ferrer, L.; Ordeix, L. Clinical and Pathological Features of Two Cases of Erythematous-Ceruminous Otitis Externa Associated with Candida Spp. Infection in Dogs. Vet. Rec. Case Reports 2025, 13, e70028. [Google Scholar] [CrossRef]
- Siak, M.K.; Paul, A.; Drees, R.; Arthur, I.; Burrows, A.K.; Tebb, A.J.; Malik, R. Otogenic Meningoencephalomyelitis Due to Cryptococcus Gattii (VGII) Infection in a Cat from Western Australia. JFMS Open Rep. 2015, 1, 2055116915585022. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Bosco, S.d.M.G.; de Hoog, S.; Ebel, F.; Elad, D.; Gomes, R.R.; Jacobsen, I.D.; Jensen, H.E.; Martel, A.; Mignon, B.; et al. Fungal Infections in Animals: A Patchwork of Different Situations. Med. Mycol. 2018, 56, S165–S187. [Google Scholar] [CrossRef] [PubMed]
- Juhola, J.; Brennan, E.; Ferguson, E.A.; Loeffler, A.; Hendricks, A.; Frosini, S.M.; Chang, Y.M.; Bond, R. Fungal Dysbiosis Following Antibacterial Monotherapy in Canine Otitis Externa. J. Small Anim. Pract. 2025, 66, 149–157. [Google Scholar] [CrossRef]
- Beatty, J.A.; Barrs, V.R.; Swinney, G.R.; Martin, P.A.; Malik, R. Peripheral Vestibular Disease Associated with Cryptococcosis in Three Cats. J. Feline Med. Surg. 2000, 2, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-M.; Yurkov, A.M.; Göker, M.; Lumbsch, H.T.; Leavitt, S.D.; Groenewald, M.; Theelen, B.; Liu, X.-Z.; Boekhout, T.; Bai, F.-Y. Phylogenetic Classification of Yeasts and Related Taxa within Pucciniomycotina. Stud. Mycol. 2015, 81, 149–189. [Google Scholar] [CrossRef]
- Wirth, F.; Goldani, L.Z. Epidemiology of Rhodotorula: An Emerging Pathogen. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 465717. [Google Scholar] [CrossRef] [PubMed]
- Jarros, I.C.; Veiga, F.F.; Corrêa, J.L.; Barros, I.L.E.; Gadelha, M.C.; Voidaleski, M.F.; Pieralisi, N.; Pedroso, R.B.; Vicente, V.A.; Negri, M.; et al. Microbiological and Virulence Aspects of Rhodotorula mucilaginosa. EXCLI J. 2020, 19, 687–704. [Google Scholar] [CrossRef]
- García-Suárez, J.; Gómez-Herruz, P.; Cuadros, J.A.; Guillén, H.; Burgaleta, C. Rhodotorula mucilaginosa Catheter-Related Fungaemia in a Patient with Multiple Myeloma. Mycoses 2011, 54, e214–e216. [Google Scholar] [CrossRef]
- Menu, E.; Filori, Q.; Dufour, J.-C.; Ranque, S.; L’Ollivier, C. A Repertoire of the Less Common Clinical Yeasts. J. Fungi 2023, 9, 1099. [Google Scholar] [CrossRef]
- Ioannou, P.; Vamvoukaki, R.; Samonis, G. Rhodotorula Species Infections in Humans: A Systematic Review. Mycoses 2019, 62, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.M.; Bizerra, F.C.; Carmona E Ferreira, R.; Colombo, A.L. Molecular Identification, Antifungal Susceptibility Profile, and Biofilm Formation of Clinical and Environmental Rhodotorula Species Isolates. Antimicrob. Agents Chemother. 2013, 57, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Gattlen, J.; Zinn, M.; Guimond, S.; Körner, E.; Amberg, C.; Mauclaire, L. Biofilm Formation by the Yeast Rhodotorula mucilaginosa: Process, Repeatability and Cell Attachment in a Continuous Biofilm Reactor. Biofouling 2011, 27, 979–991. [Google Scholar] [CrossRef]
- Glushakova, A.; Kachalkin, A. Extracellular Phospholipase, Protease and Hemolysin Production by Strains of Opportunistic Yeasts from the Excreta of Mew Gulls Breeding in Natural and Urban Habitats. Vet. Res. Commun. 2025, 49, 63. [Google Scholar] [CrossRef]
- Lario, L.D.; Pillaca-Pullo, O.S.; Durães Sette, L.; Converti, A.; Casati, P.; Spampinato, C.; Pessoa, A. Optimization of Protease Production and Sequence Analysis of the Purified Enzyme from the Cold Adapted Yeast Rhodotorula mucilaginosa CBMAI 1528. Biotechnol. Rep. 2020, 28, e00546. [Google Scholar] [CrossRef]
- Chen, S.C.A.; Perfect, J.; Colombo, A.L.; Cornely, O.A.; Groll, A.H.; Seidel, D.; Albus, K.; de Almeida, J.N.; Garcia-Effron, G.; Gilroy, N.; et al. Global Guideline for the Diagnosis and Management of Rare Yeast Infections: An Initiative of the ECMM in Cooperation with ISHAM and ASM. Lancet Infect. Dis. 2021, 21, e375–e386. [Google Scholar] [CrossRef]
- Peter Donnelly, J.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Diekema, D.J.; Petroelje, B.; Messer, S.A.; Hollis, R.J.; Pfaller, M.A. Activities of Available and Investigational Antifungal Agents against Rhodotorula Species. J. Clin. Microbiol. 2005, 43, 476–478. [Google Scholar] [CrossRef]
- Duggal, S.; Jain, H.; Tyagi, A.; Sharma, A.; Chugh, T.D. Rhodotorula Fungemia: Two Cases and a Brief Review. Med. Mycol. 2011, 49, 879–882. [Google Scholar] [CrossRef]
- Zaas, A.K.; Boyce, M.; Schell, W.; Lodge, B.A.; Miller, J.L.; Perfect, J.R. Risk of Fungemia Due to Rhodotorula and Antifungal Susceptibility Testing of Rhodotorula Isolates. J. Clin. Microbiol. 2003, 41, 5233–5235. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Boekhout, T.; Akova, M.; Meis, J.F.; Cornely, O.A.; Lortholary, O.; Arikan-Akdagli, S.; Cuenca-Estrella, M.; Dannaoui, E.; van Diepeningen, A.D.; et al. ESCMID and ECMM Joint Clinical Guidelines for the Diagnosis and Management of Rare Invasive Yeast Infections. Clin. Microbiol. Infect. 2014, 20, 76–98. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). EUCAST Definitive Document E.DEF 7.4: Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts; EUCAST: Basel, Switzerland, 2023. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts, 3rd ed.; CLSI guideline M44; CLSI: Wayne, PA, USA, 2018; ISBN 978-1-68440-030-0. [Google Scholar]
- Ahmed, N.H.; Xess, I.; Singh, G.; Satpathy, G.; Sharma, N.; Agarwal, T.; Hussain, T.; Chawla, R.; Tandon, R. Species Identification, Antifungal Susceptibility Profiles and Biofilm Formation Attributes of Rhodotorula Isolates from Ocular Infections. Mycoses 2021, 64, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Wiederhold, N.P.; Ebel, F.; Hedayati, M.T.; Rafati, H.; Verweij, P.E. Antifungal Use in Veterinary Practice and Emergence of Resistance BT-Emerging and Epizootic Fungal Infections in Animals; Seyedmousavi, S., de Hoog, G.S., Guillot, J., Verweij, P.E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 359–402. ISBN 978-3-319-72093-7. [Google Scholar]
- de Hoog, G.S.; Guarro, J.; Gené, J.; Ahmed, S.; Al-Hatmi, A.; Figueras, M.J.; Vitale, R.G. Atlas of Clinical Fungi, 4th ed.; Foundation Atlas of Clinical Fungi: Hilversum, The Netherlands, 2020; ISBN 9070351439. [Google Scholar]
- Borman, A.M.; Johnson, E.M. Changes in Fungal Taxonomy: Mycological Rationale and Clinical Implications. Clin. Microbiol. Rev. 2023, 36, e00099-22. [Google Scholar] [CrossRef]
- Aime, M.C.; Castlebury, L.A.; Abbasi, M.; Begerow, D.; Berndt, R.; Kirschner, R.; Marvanová, L.; Ono, Y.; Padamsee, M.; Scholler, M.; et al. Competing Sexual and Asexual Generic Names in Pucciniomycotina and Ustilaginomycotina (Basidiomycota) and Recommendations for Use. IMA Fungus 2018, 9, 75–89. [Google Scholar] [CrossRef]
- Yurkov, A.M.; Kachalkin, A.V.; Daniel, H.M.; Groenewald, M.; Libkind, D.; de Garcia, V.; Zalar, P.; Gouliamova, D.E.; Boekhout, T.; Begerow, D. Two Yeast Species Cystobasidium psychroaquaticum f.a. Sp. Nov. and Cystobasidium rietchieii f.a. Sp. Nov. Isolated from Natural Environments, and the Transfer of Rhodotorula minuta Clade Members to the Genus Cystobasidium. Antonie Van Leeuwenhoek 2015, 107, 173–185. [Google Scholar] [CrossRef]
- McNeill, J.; Barrie, F.R.; Buck, W.R.; Demoulin, V.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Marhold, K.; Prado, J.; et al. International Code of Nomenclature for algae, fungi, and plants (Melbourne Code). In Proceedings of the Eighteenth International Botanical Congress, Melbourne, Australia, 18–22 July 2011; Koeltz Scientific Books: Königstein, Germany, 2012; Volume 154, ISBN 978-3-87429-425-6. [Google Scholar]
- Ochoa-Viñals, N.; Alonso-Estrada, D.; Pacios-Michelena, S.; García-Cruz, A.; Ramos-González, R.; Faife-Pérez, E.; Michelena-Álvarez, L.G.; Martínez-Hernández, J.L.; Iliná, A. Current Advances in Carotenoid Production by Rhodotorula sp. Fermentation 2024, 10, 190. [Google Scholar] [CrossRef]
- Li, A.-H.; Yuan, F.-X.; Groenewald, M.; Bensch, K.; Yurkov, A.M.; Li, K.; Han, P.-J.; Guo, L.-D.; Aime, M.C.; Sampaio, J.P.; et al. Diversity and Phylogeny of Basidiomycetous Yeasts from Plant Leaves and Soil: Proposal of Two New Orders, Three New Families, Eight New Genera and One Hundred and Seven New Species. Stud. Mycol. 2020, 96, 17–140. [Google Scholar] [CrossRef]
- Liu, X.-Z.; Wang, Q.-M.; Göker, M.; Groenewald, M.; Kachalkin, A.V.; Lumbsch, H.T.; Millanes, A.M.; Wedin, M.; Yurkov, A.M.; Boekhout, T.; et al. Towards an Integrated Phylogenetic Classification of the Tremellomycetes. Stud. Mycol. 2015, 81, 85–147. [Google Scholar] [CrossRef]
- Tiwari, S.; Baghela, A.; Libkind, D. Rhodotorula sampaioana f.a., Sp. Nov., a Novel Red Yeast of the Order Sporidiobolales Isolated from Argentina and India. Antonie Van Leeuwenhoek 2021, 114, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.M.; Sęk, W.; Kieliszek, M.; Błażejak, S.; Pobiega, K.; Brzezińska, R. Diversity of Red Yeasts in Various Regions and Environments of Poland and Biotechnological Potential of the Isolated Strains. Appl. Biochem. Biotechnol. 2024, 196, 3274–3316. [Google Scholar] [CrossRef] [PubMed]
- CDC Identification of Yeasts Using MALDI-TOF. Available online: https://www.cdc.gov/fungal/hcp/laboratories/identification-of-yeasts-using-maldi-tof-1.html (accessed on 6 November 2025).
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Bolchacova, E.; Voigt, K.; Crous, P.W.; et al. Nuclear Ribosomal Internal Transcribed Spacer (ITS) Region as a Universal DNA Barcode Marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Kappe, R.; Schulze-Berge, A. New Cause for False-Positive Results with the Pastorex aspergillus Antigen Latex Agglutination Test. J. Clin. Microbiol. 1993, 31, 2489–2490. [Google Scholar] [CrossRef]
- Kidd, S.; Halliday, C.; Ellis, D. Descriptions of Medical Fungi; CABI: Oxfordshire, UK, 2022; ISBN 9781800622326. [Google Scholar]
- Giovannini, J.; Lee, R.; Zhang, S.X.; Jun, A.S.; Bower, K.S. Rhodotorula Keratitis: A Rarely Encountered Ocular Pathogen. Case Rep. Ophthalmol. 2014, 5, 302–310. [Google Scholar] [CrossRef]
- Hof, H. Rhodotorula spp. in the Gut-Foe or Friend? GMS Infect. Dis. 2019, 7, Doc02. [Google Scholar] [CrossRef]
- Yockey, J.; Andres, L.; Carson, M.; Ory, J.J.; Reese, A.J. Cell Envelope Integrity and Capsule Characterization of Rhodotorula mucilaginosa Strains from Clinical and Environmental Sources. mSphere 2019, 4, e00166-19. [Google Scholar] [CrossRef]
- Dornelles, G.; Araújo, G.R.d.S.; Rodrigues, M.; Alves, V.; Almeida-Paes, R.; Frases, S. Comparative Analysis of Capsular and Secreted Polysaccharides Produced by Rhodotorula mucilaginosa and Cryptococcus Neoformans. J. Fungi 2023, 9, 1124. [Google Scholar] [CrossRef]
- Public Health England (PHE). UK Standards for Microbiology Investigations (UK SMI) ID 1: Introduction to the Preliminary Identification of Medically Important Bacteria and Fungi from Culture; PHE: London, UK, 2017; Available online: https://www.rcpath.org/profession/publications/standards-for-microbiology-investigations/identification.html (accessed on 30 September 2025).
- Gomez-Lopez, A.; Mellado, E.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M. Susceptibility Profile of 29 Clinical Isolates of Rhodotorula spp. and Literature Review. J. Antimicrob. Chemother. 2005, 55, 312–316. [Google Scholar] [CrossRef]
- Wadsworth Center, New York State Department of Health. Mycology Proficiency Testing Program: Test Event Critique; Wadsworth Center, New York State Department of Health: Albany, NY, USA, 2015. [Google Scholar]
- Sheppard, D.C.; DeSouza, E.; Hashmi, Z.; Robson, H.G.; René, P. Evaluation of the Auxacolor System for Biochemical Identification of Medically Important Yeasts. J. Clin. Microbiol. 1998, 36, 3726–3727. [Google Scholar] [CrossRef]
- Ramani, R.; Gromadzki, S.; Pincus, D.H.; Salkin, I.F.; Chaturvedi, V. Efficacy of API 20C and ID 32C Systems for Identification of Common and Rare Clinical Yeast Isolates. J. Clin. Microbiol. 1998, 36, 3396–3398. [Google Scholar] [CrossRef]
- Meletiadis, J.; Arabatzis, M.; Bompola, M.; Tsiveriotis, K.; Hini, S.; Petinaki, E.; Velegraki, A.; Zerva, L. Comparative Evaluation of Three Commercial Identification Systems Using Common and Rare Bloodstream Yeast Isolates. J. Clin. Microbiol. 2011, 49, 2722–2727. [Google Scholar] [CrossRef]
- Hopkins, J.M.; Land, G.A. Rapid Method for Determining Nitrate Utilization by Yeasts. J. Clin. Microbiol. 1977, 5, 497–500. [Google Scholar] [CrossRef]
- Morozkina, E.V.; Nosikov, A.N.; Zvyagilskaya, R.A.; L’vov, N.P. Isolation, Purification, and Characterization of Nitrate Reductase from a Salt-Tolerant Rhodotorula glutinis Yeast Strain Grown in the Presence of Tungsten. Biochemistry 2005, 70, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Mannazzu, I.; Landolfo, S.; Lopes da Silva, T.; Buzzini, P. Red Yeasts and Carotenoid Production: Outlining a Future for Non-Conventional Yeasts of Biotechnological Interest. World J. Microbiol. Biotechnol. 2015, 31, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Allahkarami, S.; Sepahi, A.A.; Hosseini, H.; Razavi, M.R. Isolation and Identification of Carotenoid-Producing Rhodotorula sp. from Pinaceae Forest Ecosystems and Optimization of in Vitro Carotenoid Production. Biotechnol. Rep. 2021, 32, e00687. [Google Scholar] [CrossRef]
- Anshi; Kaur, H.; Goswami, L.; Kapil, S.; Sharma, V. Isolation, Optimization and Characterization of Rhodotorula alborubescens for Dietary Pigment β-Carotene Production. Appl. Microbiol. 2025, 5, 54. [Google Scholar] [CrossRef]
- Chreptowicz, K.; Mierzejewska, J.; Tkáčová, J.; Młynek, M.; Čertik, M. Carotenoid-Producing Yeasts: Identification and Characteristics of Environmental Isolates with a Valuable Extracellular Enzymatic Activity. Microorganisms 2019, 7, 653. [Google Scholar] [CrossRef] [PubMed]
- Sipiczki, M. Metschnikowia pulcherrima and Related Pulcherrimin-Producing Yeasts: Fuzzy Species Boundaries and Complex Antimicrobial Antagonism. Microorganisms 2020, 8, 1029. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, B.; Wang, H.; Xiao, S.; Li, Y.; Wang, J. Production of Astaxanthin at Moderate Temperature in Xanthophyllomyces dendrorhous Using a Two-Step Process. Eng. Life Sci. 2018, 18, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-M.; Bai, F.-Y. Four New Yeast Species of the Genus Sporobolomyces from Plant Leaves. FEMS Yeast Res. 2004, 4, 579–586. [Google Scholar] [CrossRef]
- Castro e Silva, D.d.M.; Santos, D.C.S.; Pukinskas, S.R.B.S.; Oshida, J.T.U.; Oliveira, L.; Carvallho, A.F.; Melhem, M.S.C. A New Culture Medium for Recovering the Agents of Cryptococcosis from Environmental Sources. Braz. J. Microbiol. 2015, 46, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. A Moldy Application of MALDI: MALDI-ToF Mass Spectrometry for Fungal Identification. J. Fungi 2019, 5, 4. [Google Scholar] [CrossRef]
- Buchan, B.W.; Ledeboer, N.A. Advances in Identification of Clinical Yeast Isolates by Use of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2013, 51, 1359–1366. [Google Scholar] [CrossRef]
- UK Health Security Agency (UKHSA). Matrix-Assisted Laser Desorption/Ionisation–Time of Flight Mass Spectrometry (MALDI-TOF MS) Test Procedure; UK Health Security Agency: London, UK, 2025. [Google Scholar]
- Kurtzman, C.P.; Robnett, C.J. Identification of Clinically Important Ascomycetous Yeasts Based on Nucleotide Divergence in the 5’ End of the Large-Subunit (26S) Ribosomal DNA Gene. J. Clin. Microbiol. 1997, 35, 1216–1223. [Google Scholar] [CrossRef]
- Aydin, M.; Kustimur, S.; Kalkanci, A.; Duran, T. Identification of Medically Important Yeasts by Sequence Analysis of the Internal Transcribed Spacer and D1/D2 Region of the Large Ribosomal Subunit. Rev. Iberoam. Micol. 2019, 36, 129–138. [Google Scholar] [CrossRef]
- Robert, V.; Vu, D.; Amor, A.B.H.; van de Wiele, N.; Brouwer, C.; Jabas, B.; Szoke, S.; Dridi, A.; Triki, M.; ben Daoud, S.; et al. MycoBank Gearing up for New Horizons. IMA Fungus 2013, 4, 371–379. [Google Scholar] [CrossRef]
- May, T.W. Report of the Nomenclature Committee for Fungi—20. IMA Fungus 2017, 8, 189–203. [Google Scholar] [CrossRef]
- Heaton, S.M.; Weintrob, A.C.; Downing, K.; Keenan, B.; Aggarwal, D.; Shaikh, F.; Tribble, D.R.; Wells, J.; the Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study Group. Histopathological Techniques for the Diagnosis of Combat-Related Invasive Fungal Wound Infections. BMC Clin. Pathol. 2016, 16, 11. [Google Scholar] [CrossRef]
- Garcia-Gutiérrez, C.A.; Cuétara-García, M.S.; Moragues, M.D.; Ligero, J.; Quevedo, S.M.; Buitrago, M.J. Low Sensitivity of Conventional Fungal Agars in Fungemia by Rhodotorula mucilaginosa: Description of Two Cases. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 21. [Google Scholar] [CrossRef]
- Guillot, J.; Bond, R. Malassezia Yeasts in Veterinary Dermatology: An Updated Overview. Front. Cell Infect. Microbiol. 2020, 10, 79. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC), Office of Laboratory Systems and Response, Division of Laboratory Systems. Preventing Adult Blood Culture Contamination: A Quality Tool for Clinical Laboratory Professionals; CDC: Atlanta, GA, USA, 2023. [Google Scholar]
- Epstein, S.E.; Byrne, B.A.; Sykes, J.E. Indications for Blood Cultures in Dogs and Associations With Positive Results in 323 Submissions. J. Vet. Intern. Med. 2025, 39, e70228. [Google Scholar] [CrossRef] [PubMed]
- Neumann, N.; Solis, S.A.F.; Crawford, S.; Rogovskyy, A.S. Are Multiple Blood Cultures Advantageous for Canine Patients? J. Vet. Diagn. Investig. 2023, 35, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Lamy, B.; Dargère, S.; Arendrup, M.C.; Parienti, J.-J.; Tattevin, P. How to Optimize the Use of Blood Cultures for the Diagnosis of Bloodstream Infections? A State-of-the Art. Front. Microbiol. 2016, 7, 697. [Google Scholar] [CrossRef] [PubMed]
- Doern, G.V.; Carroll, K.C.; Diekema, D.J.; Garey, K.W.; Rupp, M.E.; Weinstein, M.P.; Sexton, D.J. Practical Guidance for Clinical Microbiology Laboratories: A Comprehensive Update on the Problem of Blood Culture Contamination and a Discussion of Methods for Addressing the Problem. Clin. Microbiol. Rev. 2019, 33, e00009-19. [Google Scholar] [CrossRef]
- Keighley, C.; Pope, A.L.; Marriott, D.; Chen, S.C.-A.; Slavin, M.A. Time-to-Positivity in Bloodstream Infection for Candida Species as a Prognostic Marker for Mortality. Med. Mycol. 2023, 61, myad028. [Google Scholar] [CrossRef]
- Nunes, C.Z.; Marra, A.R.; Edmond, M.B.; da Silva Victor, E.; Pereira, C.A.P. Time to Blood Culture Positivity as a Predictor of Clinical Outcome in Patients with Candida Albicansbloodstream Infection. BMC Infect. Dis. 2013, 13, 486. [Google Scholar] [CrossRef]
- Ransom, E.M.; Alipour, Z.; Wallace, M.A.; Burnham, C.A. Evaluation of Optimal Blood Culture Incubation Time To Maximize Clinically Relevant Results from a Contemporary Blood Culture Instrument and Media System. J. Clin. Microbiol. 2021, 59, e02459-20. [Google Scholar] [CrossRef]
- Sophia, U.P.; Bastian, I.N.; Chen, D.J. Clinical Significance of BD Bactec FX Blood Culture Incubation Beyond 96 Hours (4 Days). J. Clin. Microbiol. 2022, 60, e00549-22. [Google Scholar] [CrossRef]
- Lamoth, F.; Nucci, M.; Fernandez-Cruz, A.; Azoulay, E.; Lanternier, F.; Bremerich, J.; Einsele, H.; Johnson, E.; Lehrnbecher, T.; Mercier, T.; et al. Performance of the Beta-Glucan Test for the Diagnosis of Invasive Fusariosis and Scedosporiosis: A Meta-Analysis. Med. Mycol. 2023, 61, myad061. [Google Scholar] [CrossRef] [PubMed]
- Christner, M.; Abdennadher, B.; Wichmann, D.; Kluge, S.; Pepić, A.; Aepfelbacher, M.; Rohde, H.; Olearo, F. The Added Value of (1,3)-β-D-Glucan for the Diagnosis of Invasive Candidiasis in ICU Patients: A Prospective Cohort Study. Infection 2024, 52, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Asensi-Díaz, E.; Barbero Del Olmo, L.; Urrutia, P.; Lario, A.; Gómez-G de la Pedrosa, E.; García-Ruiz de Morales, A.G.; Martín-Dávila, P.; Fortún, J. When Fungal Prophylaxis Fails: A Rare Case of Rhodotorula mucilaginosa Fungemia with Suspected Abdominal Origin. J. Fungi 2025, 11, 723. [Google Scholar] [CrossRef]
- Biegańska, M.J.; Rzewuska, M.; Dąbrowska, I.; Malewska-Biel, B.; Ostrzeszewicz, M.; Dworecka-Kaszak, B. Mixed Infection of Respiratory Tract in a Dog Caused by Rhodotorula mucilaginosa and Trichosporon jirovecii: A Case Report. Mycopathologia 2018, 183, 637–644. [Google Scholar] [CrossRef]
- Filigenzi, M.S. Mass Spectrometry in Animal Health Laboratories: Recent History, Current Applications, and Future Directions. J. Vet. Diagn. Investig. 2024, 36, 777–789. [Google Scholar] [CrossRef]
- Thompson, J.E. Matrix-Assisted Laser Desorption Ionization-Time-of-Flight Mass Spectrometry in Veterinary Medicine: Recent Advances (2019-Present). Vet. World 2022, 15, 2623–2657. [Google Scholar] [CrossRef] [PubMed]
- Sadat Naghavi, F.; Hanachi, P.; Soudi, M.R.; Saboora, A.; Ghorbani, A. Evaluation of the Relationship between the Incubation Time and Carotenoid Production in Rhodotorula slooffiae and R. mucilaginosa Isolated from Leather Tanning Wastewater. Iran. J. Basic. Med. Sci. 2013, 16, 1114–1118. [Google Scholar]
- Benvenuti, M.; Burlando, M.; Cozzani, E.C. Rhodotorula mucilaginosa (A. Jörg.) F.C. Harrison 1928 and Onychomycosis: Three Case Reports for an Unusual and Underestimated Combo. FEMS Microbiol. Lett. 2025, 372, fnaf089. [Google Scholar] [CrossRef]
- Bentz, M.L.; Le, N.; Min, B.; Nunnally, N.S.; Sullivan, V.; Tran, M.; Lockhart, S.R.; Litvintseva, A.; Berkow, E.L.; Sexton, D.J. Evaluation of CHROMagar Candida Plus for the Detection of C. auris with a Panel of 206 Fungal Isolates and 83 Colonization Screening Skin-Swabs. Microbiol. Spectr. 2024, 12, e0356423. [Google Scholar] [CrossRef]
- Bellanger, A.-P.; Grenouillet, F.; François, N.; Skana, F.; Millon, L. Inhibitory Effect of Chromogenic Culture Media on the Growth of Rhodotorula: Relevance to the Diagnosis of Rhodotorula spp. Infections. APMIS 2013, 121, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Daneshnia, F.; Kord, M.; Roudbary, M.; Zarrinfar, H.; Fang, W.; Hashemi, S.J.; Najafzadeh, M.J.; Khodavaisy, S.; Pan, W.; et al. Comparison of 21-Plex PCR and API 20C AUX, MALDI-TOF MS, and RDNA Sequencing for a Wide Range of Clinically Isolated Yeast Species: Improved Identification by Combining 21-Plex PCR and API 20C AUX as an Alternative Strategy for Developing Countries. Front. Cell. Infect. Microbiol. 2019, 9, 21. [Google Scholar] [CrossRef]
- De Almeida, G.M.D.; Costa, S.F.; Melhem, M.; Motta, A.L.; Szeszs, M.W.; Miyashita, F.; Pierrotti, L.C.; Rossi, F.; Burattini, M.N. Rhodotorula spp. Isolated from Blood Cultures: Clinical and Microbiological Aspects. Med. Mycol. 2008, 46, 547–556. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Tsang, C.-C.; Xiao, M.; Chan, J.F.W.; Lau, S.K.P.; Kong, F.; Xu, Y.; Woo, P.C.Y. Yeast Identification by Sequencing, Biochemical Kits, MALDI–TOF MS and Rep-PCR DNA Fingerprinting. Med. Mycol. 2018, 56, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.-G.; Cornet, M.; Hennebique, A.; Rasamoelina, T.; Caspar, Y.; Pondérand, L.; Bidart, M.; Durand, H.; Jacquet, M.; Garnaud, C.; et al. MALDI-TOF MS in a Medical Mycology Laboratory: On Stage and Backstage. Microorganisms 2021, 9, 1283. [Google Scholar] [CrossRef]
- Wickes, B.L.; Wiederhold, N.P. Molecular Diagnostics in Medical Mycology. Nat. Commun. 2018, 9, 5135. [Google Scholar] [CrossRef]
- Idris, N.F.B.; Jia, Q.; Lu, H.; Guo, Y.; Wang, Y.; Hao, R.; Tu, Z. Reduced Survival and Resistance of Rhodotorula mucilaginosa Following Inhibition of Pigment Production by Naftifine. Curr. Microbiol. 2023, 80, 285. [Google Scholar] [CrossRef]
- Tuon, F.F.; Costa, S.F. Rhodotorula Infection. A Systematic Review of 128 Cases from Literature. Rev. Iberoam. Micol. 2008, 25, 135–140. [Google Scholar] [CrossRef]
- Pérez Pintado, E.; Fernández García, L.M.; Pérez Pintado, E. Fungemia Causada Por Rhodotorula En Un Lactante Crítico. Rev. Cuba. pediatr. 2019, 91, e639. [Google Scholar]
- Vasquez, A.M.; Lake, J.; Ngai, S.; Halbrook, M.; Vallabhaneni, S.; Keckler, M.S.; Moulton-Meissner, H.; Lockhart, S.R.; Lee, C.T.; Perkins, K.; et al. Notes from the Field: Fungal Bloodstream Infections Associated with a Compounded Intravenous Medication at an Outpatient Oncology Clinic—New York City, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 1274–1275. [Google Scholar] [CrossRef] [PubMed]
- Melly, M.A.; Meng, H.C.; Schaffner, W. Microbial Growth in Lipid Emulsions Used in Parenteral Nutrition. Arch. Surg. 1975, 110, 1479–1481. [Google Scholar] [CrossRef]
- Kim, H.; Byeon, S.J.; Kim, C.H.; Bae, Y.A.; Lee, H.; Kim, H.S. A Case of Localized Fungal Pneumonia Caused by Rhodotorula mucilaginosa in an Immunocompetent Patient. Ann. Lab. Med. 2021, 41, 120–122. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Drummond, R.A.; Hohl, T.M. Immune Responses to Human Fungal Pathogens and Therapeutic Prospects. Nat. Rev. Immunol. 2023, 23, 433–452. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. Β-Glucan Recognition By the Innate Immune System. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef]
- Sonnberger, J.; Kasper, L.; Lange, T.; Brunke, S.; Hube, B. “We’ve Got to Get out”—Strategies of Human Pathogenic Fungi to Escape from Phagocytes. Mol. Microbiol. 2024, 121, 341–358. [Google Scholar] [CrossRef]
- Mosqueda-Martínez, E.; Chiquete-Félix, N.; Castañeda-Tamez, P.; Ricardez-García, C.; Gutiérrez-Aguilar, M.; Uribe-Carvajal, S.; Mendez-Romero, O. In Rhodotorula mucilaginosa, Active Oxidative Metabolism Increases Carotenoids to Inactivate Excess Reactive Oxygen Species. Front. Fungal Biol. 2024, 5, 1378590. [Google Scholar] [CrossRef]
- Moore, M.M.; Breedveld, M.W.; Autor, A.P. The Role of Carotenoids in Preventing Oxidative Damage in the Pigmented Yeast, Rhodotorula mucilaginosa. Arch. Biochem. Biophys. 1989, 270, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.D.; Brownlie, C.E.D.; Shankland, G.S. Enhanced Phagocytosis and Intracellular Killing of Candida albicans by GM-CSF-Activated Human Neutrophils. Med. Mycol. 1992, 30, 433–441. [Google Scholar] [CrossRef]
- Kitazawa, T.; Ishigaki, S.; Seo, K.; Yoshino, Y.; Ota, Y. Catheter-Related Bloodstream Infection Due to Rhodotorula mucilaginosa with Normal Serum (1→3)-β-D-Glucan Level. J. Mycol. Med. 2018, 28, 393–395. [Google Scholar] [CrossRef]
- Lamoth, F.; Akan, H.; Andes, D.; Cruciani, M.; Marchetti, O.; Ostrosky-Zeichner, L.; Racil, Z.; Clancy, C.J. Assessment of the Role of 1,3-β-d-Glucan Testing for the Diagnosis of Invasive Fungal Infections in Adults. Clin. Infect. Dis. 2021, 72, S102–S108. [Google Scholar] [CrossRef]
- Rimek, D.; Singh, J.; Kappe, R. Cross-Reactivity of the Platelia candida Antigen Detection Enzyme Immunoassay with Fungal Antigen Extracts. J. Clin. Microbiol. 2003, 41, 3395–3398. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. Immunoglobulins in Defense, Pathogenesis, and Therapy of Fungal Diseases. Cell Host Microbe 2012, 11, 447–456. [Google Scholar] [CrossRef]
- Farmakiotis, D.; Kontoyiannis, D.P. Epidemiology of Antifungal Resistance in Human Pathogenic Yeasts: Current Viewpoint and Practical Recommendations for Management. Int. J. Antimicrob. Agents 2017, 50, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, P. Ocena Wybranych Determinant Patogenności Grzybów z Rodzaju Rhodotorula. Ph.D. Thesis, Wydział Lekarski, Uniwersytet Jagielloński–Collegium Medicum, Kraków, Polska, 2010. Available online: https://portalwiedzy.cm-uj.krakow.pl/docstore/download.seam?entityType=phd&entityId=UJCM4307f3d8b85f4530b6758ca5f4555a9f&fileId=UJCM5cd9797be52f4bfd8c430c29abbb8596 (accessed on 30 September 2025).
- Seifi, Z.; Mahmoudabadi, A.Z.; Hydrinia, S.; Halvaeezadeh, M. Extracellular Enzymes Production and Biofilm Formation in Rhodotorula Species. Curr. Enzym. Inhib. 2015, 12, 155–160. [Google Scholar] [CrossRef]
- Ramage, G.; Mowat, E.; Jones, B.; Williams, C.; Lopez-Ribot, J. Our Current Understanding of Fungal Biofilms Fungal Biofilms. Crit. Rev. Microbiol. 2009, 35, 340–355. [Google Scholar] [CrossRef]
- Reynolds, T.B.; Fink, G.R. Bakers’ yeast, a model for fungal biofilm formation. Science 2001, 291, 878–881. [Google Scholar] [CrossRef]
- Vila, T.V.M.; Rozental, S.; de Sá Guimarães, C.M.D. A New Model of in Vitro Fungal Biofilms Formed on Human Nail Fragments Allows Reliable Testing of Laser and Light Therapies against Onychomycosis. Lasers Med. Sci. 2015, 30, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Silva, S.; Henriques, M. Effect of Voriconazole on Candida Tropicalis Biofilms: Relation with ERG Genes Expression. Mycopathologia 2016, 181, 643–651. [Google Scholar] [CrossRef]
- Hirano, R.; Mitsuhashi, T.; Osanai, K. Rhodotorula mucilaginosa Fungemia, a Rare Opportunistic Infection without Central Venous Catheter Implantation, Successfully Treated by Liposomal Amphotericin B. Case Rep. Infect. Dis. 2022, 2022, 7830126. [Google Scholar] [CrossRef]
- Goravey, W.; Ali, G.A.; Abid, F.; Ibrahim, E.B.; Al Maslamani, M.A.; Abdel Hadi, H. Central Line-Associated Rhodotorula mucilaginosa Fungemia in an Immunocompetent Host: Case Report and Review of the Literature. Clin. Case Rep. 2021, 9, 2158–2161. [Google Scholar] [CrossRef]
- Cabral, A.M.; da Siveira Rioja, S.; Brito-Santos, F.; Peres da Silva, J.R.; MacDowell, M.L.; Melhem, M.S.C.; Mattos-Guaraldi, A.L.; Hirata Junior, R.; Damasco, P.V. Endocarditis Due to Rhodotorula mucilaginosa in a Kidney Transplanted Patient: Case Report and Review of Medical Literature. JMM Case Rep. 2017, 4, e005119. [Google Scholar] [CrossRef]
- Thompson, G.R., 3rd; Jenks, J.D.; Baddley, J.W.; Lewis, J.S., 2nd; Egger, M.; Schwartz, I.S.; Boyer, J.; Patterson, T.F.; Chen, S.C.-A.; Pappas, P.G.; et al. Fungal Endocarditis: Pathophysiology, Epidemiology, Clinical Presentation, Diagnosis, and Management. Clin. Microbiol. Rev. 2023, 36, e0001923. [Google Scholar] [CrossRef] [PubMed]
- Elavarashi, E.; Kindo, A.J.; Rangarajan, S. Enzymatic and Non-Enzymatic Virulence Activities of Dermatophytes on Solid Media. J. Clin. Diagn. Res. 2017, 11, DC23–DC25. [Google Scholar] [CrossRef]
- White, T.C.; Findley, K.; Dawson, T.L.; Scheynius, A.; Boekhout, T.; Cuomo, C.A.; Xu, J.; Saunders, C.W. Fungi on the Skin: Dermatophytes and Malassezia. Cold Spring Harb. Perspect. Med. 2014, 4, a019802. [Google Scholar] [CrossRef]
- Ellepola, A.N.B.; Samaranayake, L.P.; Khan, Z.U. Extracellular Phospholipase Production of Oral Candida Albicans Isolates from Smokers, Diabetics, Asthmatics, Denture Wearers and Healthy Individuals Following Brief Exposure to Polyene, Echinocandin and Azole Antimycotics. Braz. J. Microbiol. 2016, 47, 911–916. [Google Scholar] [CrossRef]
- Cafarchia, C.; Romito, D.; Coccioli, C.; Camarda, A.; Otranto, D. Phospholipase Activity of Yeasts from Wild Birds and Possible Implications for Human Disease. Med. Mycol. 2008, 46, 429–434. [Google Scholar] [CrossRef]
- Pumeesat, P.; Nasom, L.; Thongdee, K.; Damnak, T.; Sirikhetkorn, T.; Unwong, P.; Benjamat, S.; Suwanmanee, S.; Wongsuk, T. Molecular Identification and Extracellular Enzyme Production of Rhodotorula spp. Ital. J. Mycol. 2024, 53, 147–159. [Google Scholar] [CrossRef]
- Troska, P.; Sucharzewska, E.; Dynowska, M.; Ejdys, E. Fungi of the Genus Rhodotorula Isolated from the Oral Cavity of Oncologic Patients with Colorectal Cancer. Ann. Parasitol. 2017, 63, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, P.; Halska, A.; Macura, A.B. Occurrence and Pathogenicity of the Fungi Rhodotorula spp. Postep. Mikrobiol. 2007, 46, 291–300. [Google Scholar]
- Monod, M.; Capoccia, S.; Léchenne, B.; Zaugg, C.; Holdom, M.; Jousson, O. Secreted Proteases from Pathogenic Fungi. Int. J. Med. Microbiol. 2002, 292, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.C.; Silva, L.A.D.E.; Tichota, D.M.; Daroit, D.J.; Velho, R.V.; Pereira, J.Q.; Corrêa, A.P.F.; Brandelli, A. Production of Proteolytic Enzymes by a Keratin-Degrading Aspergillus Niger. Enzyme Res. 2011, 2011, 487093. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, A.; Gupta, P. Secreted Aspartyl Proteases Family: A Perspective Review on the Regulation of Fungal Pathogenesis. Futur. Microbiol. 2023, 18, 295–309. [Google Scholar] [CrossRef]
- Melville, P.A.; Benites, N.R.; Ruz-Peres, M.; Yokoya, E. Proteinase and Phospholipase Activities and Development at Different Temperatures of Yeasts Isolated from Bovine Milk. J. Dairy Res. 2011, 78, 385–390. [Google Scholar] [CrossRef]
- Valderrama, B.; Ruiz, J.J.; Gutiérrez, M.S.; Alveal, K.; Caruffo, M.; Oliva, M.; Flores, H.; Silva, A.; Toro, M.; Reyes-Jara, A.; et al. Cultivable Yeast Microbiota from the Marine Fish Species Genypterus chilensis and Seriolella violacea. J. Fungi 2021, 7, 515. [Google Scholar] [CrossRef]
- Ghannoum, M.A. Potential Role of Phospholipases in Virulence and Fungal Pathogenesis. Clin. Microbiol. Rev. 2000, 13, 122–143. [Google Scholar] [CrossRef]
- Döğen, A.; Gümral, R.; Ilkit, M. Haemolytic and Co-Haemolytic (CAMP-like) Activity in Dermatophytes. Mycoses 2015, 58, 40–47. [Google Scholar] [CrossRef]
- Kronstad, J.W.; Caza, M. Shared and Distinct Mechanisms of Iron Acquisition by Bacterial and Fungal Pathogens of Humans. Front. Cell Infect. Microbiol. 2013, 4, 80. [Google Scholar] [CrossRef]
- Takemura, K.; Kolasinski, V.; de Sa, N.F.V.; Garg, A.; Ojima, I.; Del Poeta, M.; de Sa, N.P. Iron Acquisition Strategies in Pathogenic Fungi. MBio 2025, 16, e0121125. [Google Scholar] [CrossRef]
- Favero, D.; Furlaneto-Maia, L.; França, E.J.G.; Góes, H.P.; Furlaneto, M.C. Hemolytic Factor Production by Clinical Isolates of Candida Species. Curr. Microbiol. 2014, 68, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Perini, L.; Mogrovejo, D.C.; Tomazin, R.; Gostinčar, C.; Brill, F.H.H.; Gunde-Cimerman, N. Phenotypes associated with pathogenicity: Their expression in Arctic fungal isolates. Microorganisms 2019, 7, 600. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Samaranayake, L.P.; Yau, J.Y.Y. Candida Species Exhibit Differential in Vitro Hemolytic Activities. J. Clin. Microbiol. 2001, 39, 2971–2974. [Google Scholar] [CrossRef] [PubMed]
- Andrawes, N.; Weissman, Z.; Pinsky, M.; Moshe, S.; Berman, J.; Kornitzer, D. Regulation of Heme Utilization and Homeostasis in Candida albicans. PLoS Genet. 2022, 18, e1010390. [Google Scholar] [CrossRef]
- Lipke, P.N. What We Do Not Know about Fungal Cell Adhesion Molecules. J. Fungi 2018, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- de Groot, P.W.J.; Bader, O.; de Boer, A.D.; Weig, M.; Chauhan, N. Adhesins in Human Fungal Pathogens: Glue with Plenty of Stick. Eukaryot. Cell 2013, 12, 470–481. [Google Scholar] [CrossRef]
- Hoyer, L.L.; Cota, E. Candida albicans Agglutinin-like Sequence (Als) Family Vignettes: A Review of Als Protein Structure and Function. Front. Microbiol. 2016, 7, 280. [Google Scholar] [CrossRef]
- Buck, J.W.; Andrews, J.H. Localized, Positive Charge Mediates Adhesion of Rhodosporidium toruloides to Barley Leaves and Polystyrene. Appl. Environ. Microbiol. 1999, 65, 2179–2183. [Google Scholar] [CrossRef]
- Buck, J.W.; Andrews, J.H. Attachment of the Yeast Rhodosporidium toruloides Is Mediated by Adhesives Localized at Sites of Bud Cell Development. Appl. Environ. Microbiol. 1999, 65, 465–471. [Google Scholar] [CrossRef]
- Fu, M.S.; Coelho, C.; De Leon-Rodriguez, C.M.; Rossi, D.C.P.; Camacho, E.; Jung, E.H.; Kulkarni, M.; Casadevall, A. Cryptococcus neoformans Urease Affects the Outcome of Intracellular Pathogenesis by Modulating Phagolysosomal PH. PLOS Pathog. 2018, 14, e1007144. [Google Scholar] [CrossRef]
- Baker, L.B. Physiology of Sweat Gland Function: The Roles of Sweating and Sweat Composition in Human Health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef]
- Singh, A.; Panting, R.J.; Varma, A.; Saijo, T.; Waldron, K.J.; Jong, A.; Ngamskulrungroj, P.; Chang, Y.C.; Rutherford, J.C.; Kwon-Chung, K.J. Factors Required for Activation of Urease as a Virulence Determinant in Cryptococcus neoformans. MBio 2013, 4, e00220-13. [Google Scholar] [CrossRef] [PubMed]
- Landolfo, S.; Chessa, R.; Zara, G.; Zara, S.; Budroni, M.; Mannazzu, I. Rhodotorula mucilaginosa C2.5t1 Modulates Carotenoid Content and CAR Genes Transcript Levels to Counteract the Pro-Oxidant Effect of Hydrogen Peroxide. Microorganisms 2019, 7, 316. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Herrero-de-Dios, C.; Belmonte, R.; Budge, S.; Lopez Garcia, A.; Kolmogorova, A.; Lee, K.K.; Martin, B.D.; Ribeiro, A.; Bebes, A.; et al. Elevated Catalase Expression in a Fungal Pathogen Is a Double-Edged Sword of Iron. PLoS Pathog. 2017, 13, e1006405. [Google Scholar] [CrossRef]
- Unlü, A.E.; Takaç, S. Investigation of the Simultaneous Production of Superoxide Dismutase and Catalase Enzymes from Rhodotorula glutinis under Different Culture Conditions. Artif. Cells. Blood Substit. Immobil. Biotechnol. 2012, 40, 338–344. [Google Scholar] [CrossRef]
- Koleva, D.I.; Petrova, V.Y.; Kujumdzieva, A. V Comparison of Enzymatic Antioxidant Defence Systems in Different Metabolic Types of Yeasts. Can. J. Microbiol. 2008, 54, 957–963. [Google Scholar] [CrossRef]
- Wu, C.-C.; Ohashi, T.; Misaki, R.; Limtong, S.; Fujiyama, K. Ethanol and H2O2 Stresses Enhance Lipid Production in an Oleaginous Rhodotorula toruloides Thermotolerant Mutant L1-1. FEMS Yeast Res. 2020, 20, foaa030. [Google Scholar] [CrossRef]
- Giles, S.S.; Stajich, J.E.; Nichols, C.; Gerrald, Q.D.; Alspaugh, J.A.; Dietrich, F.; Perfect, J.R. The Cryptococcus neoformans Catalase Gene Family and Its Role in Antioxidant Defense. Eukaryot. Cell 2006, 5, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Hansberg, W.; Salas-Lizana, R.; Domínguez, L. Fungal Catalases: Function, Phylogenetic Origin and Structure. Arch. Biochem. Biophys. 2012, 525, 170–180. [Google Scholar] [CrossRef]
- Moliné, M.; Libkind, D.; Van Broock, M. Production of Torularhodin, Torulene, and β-Carotene by Rhodotorula Yeasts. Methods Mol. Biol. 2012, 898, 275–283. [Google Scholar] [CrossRef]
- Kot, A.M.; Błazejak, S.; Gientka, I.; Kieliszek, M.; Bryś, J. Torulene and Torularhodin: “New” Fungal Carotenoids for Industry? Microb. Cell Fact. 2018, 17, 49. [Google Scholar] [CrossRef]
- Moliné, M.; Flores, M.R.; Libkind, D.; Del Carmen Diéguez, M.; Farías, M.E.; Van Broock, M. Photoprotection by Carotenoid Pigments in the Yeast Rhodotorula mucilaginosa: The Role of Torularhodin. Photochem. Photobiol. Sci. 2010, 9, 1145–1151. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Cheng, Y.; Pi, F.; Yao, W.; Xie, Y.; Qian, H. Determination of the Molecular Mechanism of Torularhodin against Hepatic Oxidative Damage by Transcriptome Analysis. Oxid. Med. Cell. Longev. 2019, 2019, 7417263. [Google Scholar] [CrossRef]
- Wang, Y.; Aisen, P.; Casadevall, A. Cryptococcus neoformans Melanin and Virulence: Mechanism of Action. Infect. Immun. 1995, 63, 3131–3136. [Google Scholar] [CrossRef]
- Struyfs, C.; Cammue, B.P.A.; Thevissen, K. Membrane-Interacting Antifungal Peptides. Front. Cell Dev. Biol. 2021, 9, 649875. [Google Scholar] [CrossRef]
- Krzyściak, P.; Macura, A.B. Drug Susceptibility of 64 Strains of Rhodotorula sp. Wiad. Parazytol. 2010, 56, 167–170. Available online: https://annals-parasitology.eu/archive_2001_2022/2010-56-2_167.pdf (accessed on 30 September 2025). [PubMed]
- Lunardi, L.W.; Aquino, V.R.; Zimerman, R.A.; Goldani, L.Z. Epidemiology and Outcome of Rhodotorula Fungemia in a Tertiary Care Hospital. Clin. Infect. Dis. 2006, 43, e60–e63. [Google Scholar] [CrossRef][Green Version]
- Lortholary, O.; Dannaoui, E.; Raoux, D.; Hoinard, D.; Datry, A.; Paugam, A.; Poirot, J.L.; Lacroix, C.; Dromer, F.; Bouges-Michel, C.; et al. In Vitro Susceptibility to Posaconazole of 1,903 Yeast Isolates Recovered in France from 2003 to 2006 and Tested by the Method of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob. Agents Chemother. 2007, 51, 3378–3380. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Desnos-Ollivier, M.; Bretagne, S.; Boullié, A.; Gautier, C.; Dromer, F.; Lortholary, O. Isavuconazole MIC Distribution of 29 Yeast Species Responsible for Invasive Infections (2015–2017). Clin. Microbiol. Infect. 2019, 25, 634.e1–634.e4. [Google Scholar] [CrossRef]
- Spiliopoulou, A.; Anastassiou, E.D.; Christofidou, M. Rhodotorula Fungemia of an Intensive Care Unit Patient and Review of Published Cases. Mycopathologia 2012, 174, 301–309. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, Q.; Zhao, L.; Apaliya, M.T.; Zhang, X.; Zhang, H. Crosstalk between Proteins Expression and Lysine Acetylation in Response to Patulin Stress in Rhodotorula mucilaginosa. Sci. Rep. 2017, 7, 13490. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Mota, M.N.; Sá-Correia, I. Cell Envelope and Stress-Responsive Pathways Underlie an Evolved Oleaginous Rhodotorula toruloides Strain Multi-Stress Tolerance. Biotechnol. Biofuels Bioprod. 2024, 17, 71. [Google Scholar] [CrossRef]
- Prasad, R.; Rawal, M.K. Efflux Pump Proteins in Antifungal Resistance. Front. Pharmacol. 2014, 5, 202. [Google Scholar] [CrossRef]
- Castanheira, M.; Deshpande, L.M.; Davis, A.P.; Rhomberg, P.R.; Pfaller, M.A. Monitoring Antifungal Resistance in a Global Collection of Invasive Yeasts and Molds: Application of CLSI Epidemiological Cutoff Values and Whole-Genome Sequencing Analysis for Detection of Azole Resistance in Candida albicans. Antimicrob. Agents Chemother. 2017, 61, e00906-17. [Google Scholar] [CrossRef]
- Ide-Pérez, M.R.; Iza-Arteaga, M.L.; Sánchez-Carbente, M.D.R.; Balcázar-López, E.; Sánchez-Reyes, A. Long-Read Sequencing and de Novo Genome Assembly Data of Candida parapsilosis HMC1 and Rhodotorula mucilaginosa LBMH1012, Two Novel Isolates with Antifungal Resistance Signatures. Data Br. 2024, 56, 110808. [Google Scholar] [CrossRef]
- Wiederhold, N.P. Pharmacodynamics, Mechanisms of Action and Resistance, and Spectrum of Activity of New Antifungal Agents. J. Fungi 2022, 8, 857. [Google Scholar] [CrossRef]
- Johnson, V.; Singh, M.; Saini, V.S.; Sista, V.R.; Yadav, N.K. Effect of PH on Lipid Accumulation by an Oleaginous Yeast: Rhodotorula glutinis IIP-30. World J. Microbiol. Biotechnol. 1992, 8, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Maligie, M.A.; Selitrennikoff, C.P. Cryptococcus neoformans Resistance to Echinocandins: (1,3)β-Glucan Synthase Activity Is Sensitive to Echinocandins. Antimicrob. Agents Chemother. 2005, 49, 2851–2856. [Google Scholar] [CrossRef] [PubMed]
- Szymański, M.; Chmielewska, S.; Czyżewska, U.; Malinowska, M.; Tylicki, A. Echinocandins–Structure, Mechanism of Action and Use in Antifungal Therapy. J. Enzyme Inhib. Med. Chem. 2022, 37, 876–894. [Google Scholar] [CrossRef]
- Cuenca-Estrella, M.; Gomez-Lopez, A.; Mellado, E.; Monzon, A.; Buitrago, M.J.; Rodriguez-Tudela, J.L. Activity Profile In Vitro of Micafungin against Spanish Clinical Isolates of Common and Emerging Species of Yeasts and Molds. Antimicrob. Agents Chemother. 2009, 53, 2192–2195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gray, K.C.; Palacios, D.S.; Dailey, I.; Endo, M.M.; Uno, B.E.; Wilcock, B.C.; Burke, M.D. Amphotericin Primarily Kills Yeast by Simply Binding Ergosterol. Proc. Natl. Acad. Sci. USA 2012, 109, 2234–2239. [Google Scholar] [CrossRef]
- Huang, J.J.; Chen, X.F.; Tsui, C.K.M.; Pang, C.J.; Hu, Z.D.; Shi, Y.; Wang, W.P.; Cui, L.Y.; Xiao, Y.L.; Gong, J.; et al. Persistence of an Epidemic Cluster of Rhodotorula mucilaginosa in Multiple Geographic Regions in China and the Emergence of a 5-Flucytosine Resistant Clone. Emerg. Microbes Infect. 2022, 11, 1079–1089. [Google Scholar] [CrossRef]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.H.; et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef]
- Duarte, E.R.; Resende, J.C.P.; Rosa, C.A.; Hamdan, J.S. Prevalence of Yeasts and Mycelial fungi in Bovine Parasitic Otitis in the State of Minas Gerais, Brazil. J. Vet. Med. Ser. B 2001, 48, 631–635. [Google Scholar] [CrossRef]
- Kadota, K.; Uchida, K.; Nagatomo, T.; Goto, Y.; Shinjo, T.; Hasegawa, T.; Ogawa, H.; Yamaguchi, R.; Tateyama, S. Granulomatous Epididymitis Related to Rhodotorula glutinis Infection in a Dog. Vet. Pathol. 1995, 32, 716–718. [Google Scholar] [CrossRef]
- Bourdeau, P.; Hubert, B.; Magnol, J.P. Suspected dermatosis due to Rhodotorula mucilaginosa [syn. Rhodotorula rubra] in an adult cat infected by FeLV and FIV [ketoconazole, acquired immunodeficiency]. Rec. Med. Vet. Ec. Alfort. 1992, 168, 91–96. [Google Scholar]
- Alvarez-Perez, S.; Mateos, A.; Dominguez, L.; Martinez-Nevado, E.; Blanco, J.L.; Garcia, M.E. Isolation of Rhodotorula mucilaginosa from Skin Lesions in a Southern Sea Lion (Otaria Flavescens): A Case Report. Vet. Med. 2010, 55, 297–301. [Google Scholar] [CrossRef]
- Aruo, S.K. Necrotizing Cutaneous Rhodotorulosis in Chickens in Uganda. Avian Dis. 1980, 24, 1038–1043. [Google Scholar] [CrossRef]
- Monga, D.P.; Garg, D.N. Ovine Pulmonary Infection Caused by Rhodotorula rubra. Mykosen 1980, 23, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.O.; Gandra, C.R.; Pires, M.F.; Coutinho, S.D.; Castilho, W.; Teixeira, C.M. Survey of Bovine Mycotic Mastitis in Dairy Herds in the State of São Paulo, Brazil. Mycopathologia 1993, 124, 13–17. [Google Scholar] [CrossRef]
- Wirth, F.; Goldani, L.Z. Experimental Rhodotorulosis Infection in Rats. Apmis 2012, 120, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Niae, S.; Yurayart, C.; Thengchaisri, N.; Sattasathuchana, P. Prevalence and in Vitro Antifungal Susceptibility of Commensal Yeasts in the External Ear Canal of Cats. BMC Vet. Res. 2021, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Pressler, B.M. Candidiasis and Rhodotorulosis. In Infectious Diseases of the Dog and Cat, 4th ed.; Greene, C.E., Ed.; Elsevier/Saunders: St. Louis, MO, USA, 2012; pp. 666–672. ISBN 978-1-4160-6130-4. [Google Scholar]
- Zhou, Y.; Ren, Y.; Fan, C.; Shao, H.; Zhang, Z.; Mao, W.; Wei, C.; Ni, H.; Zhu, Z.; Hou, X.; et al. Survey of Mycotic Mastitis in Dairy Cows from Heilongjiang Province, China. Trop. Anim. Health Prod. 2013, 45, 1709–1714. [Google Scholar] [CrossRef]
- Dworecka-Kaszak, B.; Krutkiewicz, A.; Szopa, D.; Kleczkowski, M.; Biegańska, M. High Prevalence of Candida Yeast in Milk Samples from Cows Suffering from Mastitis in Poland. Sci. World J. 2012, 2012, 196347. [Google Scholar] [CrossRef]
- Wawron, W.; Bochniarz, M.; Piech, T. Yeast Mastitis in Dairy Cows in the Middle-Eastern Part of Poland. Bull. Vet. Inst. Pulawy 2010, 54, 201–204. [Google Scholar]
- Page, R.K.; Fletcher, O.J.; Eidson, C.S.; Michaels, G.E. Dermatitis Produced by Rhodotorula glutins in Broiler-Age Chickens. Avian Dis. 1976, 20, 416–421. [Google Scholar] [CrossRef]
- Beemer, A.M.; Schneerson-Porat, S.; Kuttin, E.S. Rhodotorula mucilaginosa Dermatitis on Feathered Parts of Chickens: An Epizootic on a Poultry Farm. Avian Dis. 1970, 14, 234–239. [Google Scholar] [CrossRef] [PubMed]
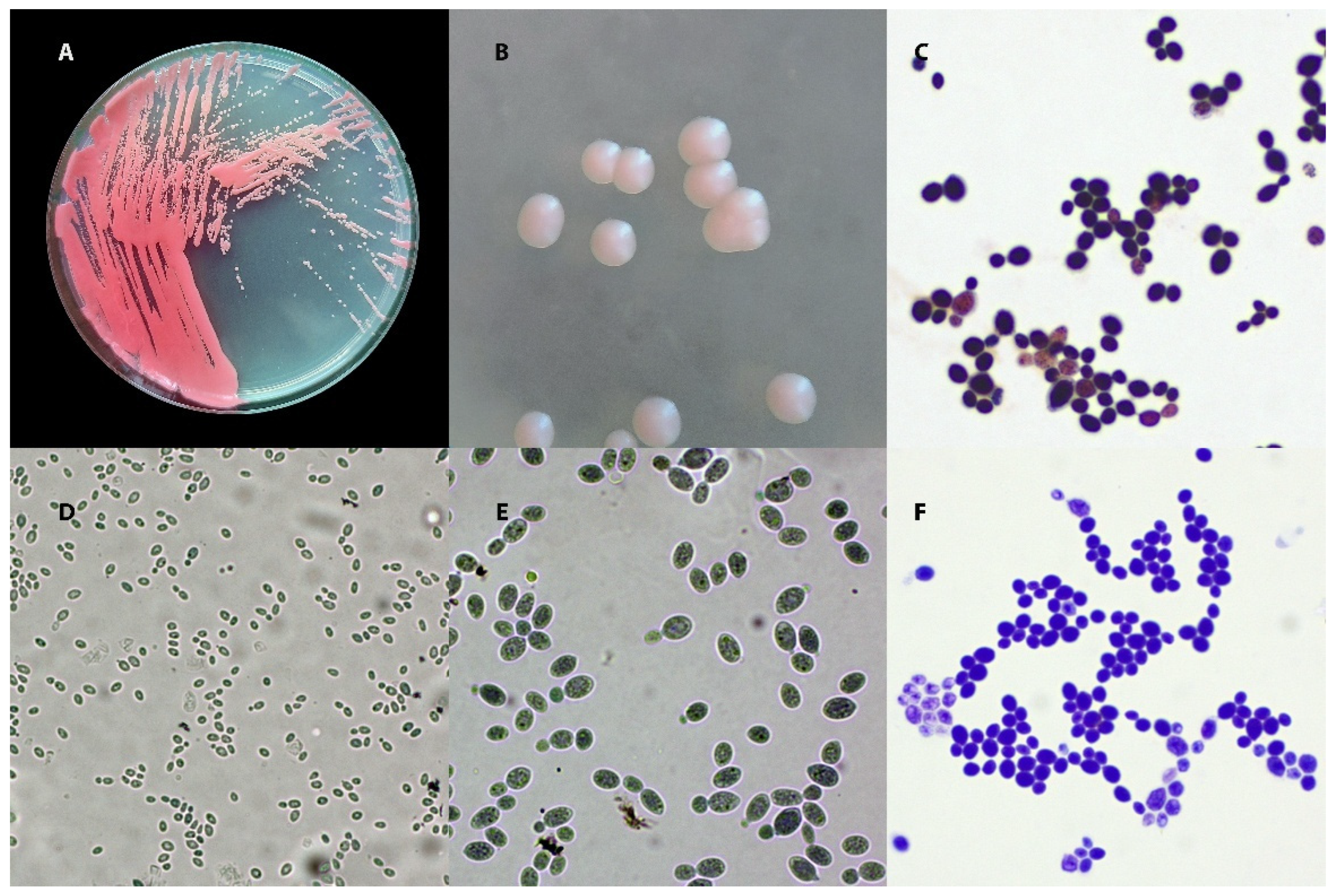
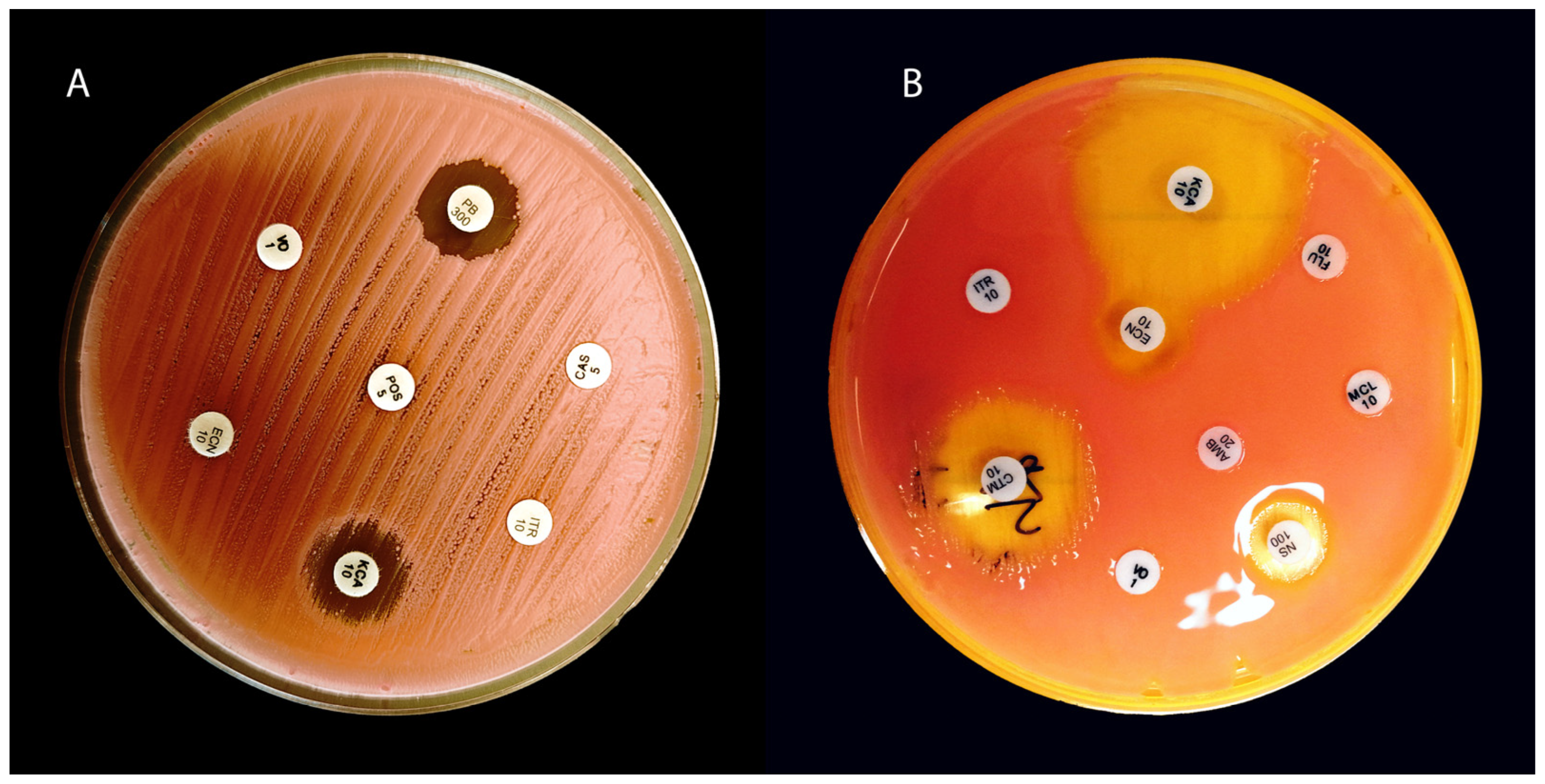
| Feature/Test | Rhodotorula mucilaginosa | Rhodotorula glutinis | Cryptococcus neoformans | Candida albicans |
|---|---|---|---|---|
| Nitrate (NO3−) assimilation | No—does not assimilate | Yes—present (requires specific culture conditions) | No—does not assimilate | No—does not assimilate |
| Growth at 37 °C | Yes—grows (some strains up to 40 °C) | Weak or absent—variable (usually limited) | Yes—grows at 37 °C | Yes—grows (many strains up to 42 °C) |
| Tolerance to 0.1% cycloheximide | No growth—susceptible | Variable—some strains grow weakly | No growth—susceptible | No growth—susceptible (most strains) |
| Maltose assimilation | Variable—strain-dependent | Positive—assimilates | Positive—assimilates | Positive—assimilates |
| Raffinose assimilation | Positive—assimilates raffinose | Variable—strain-dependent | Variable—most strains positive, some weakly | Negative—does not assimilate |
| Melezitose assimilation | Weak or strain-dependent | Positive—clearly assimilates | Positive—assimilates melezitose (typical trait) | Variable—strain-dependent (partial) |
| D-glucosamine assimilation | Variable/weak—partial | Negative—does not assimilate this sugar | Negative—does not assimilate this sugar | Positive |
| Erythritol assimilation | Variable—strain-dependent | Negative—does not assimilate | Negative—does not assimilate | Negative—does not assimilate |
| Urease (urea hydrolysis) | Positive—urease-producing | Positive—urease-producing | Positive—strong urease activity (test positive) | Negative—no urease |
| Catalase | Positive—present | Positive—present | Positive—present | Positive—present |
| Hemolysis (blood agar) | Variable—many strains show β-hemolysis (enhanced at 37 °C) | Variable—β-hemolysis reported in some strains (weaker than R. mucilaginosa) | Negative—no hemolysis (no hemolysins) | Positive—hemolysis present |
| Myo-inositol assimilation | Negative—does not assimilate | Negative—does not assimilate | Positive—assimilates inositol | Negative—does not assimilate |
| Colony pigmentation | Pink to coral, smooth, often mucoid | Salmon-pink to orange; smooth or wrinkled (glossy to matte) | Cream to white, mucoid; dark brown on Staib medium (melanin) | White to cream, smooth, shiny |
| Advantages | Limitations | Turnaround Time | Typical Applications | Citations | |
|---|---|---|---|---|---|
| Culture (SDA/YGC) | recovery of live pure isolate; low cost and wide availability; starting point for MALDI/PCR/AFST; pigmented colonies support genus-level suspicion | colony appearance ≠ species ID; potential confusion with other “red yeasts”; full pigmentation delayed | colonies: 48–72 h; full pigmentation: 72–120 h | routine plating; obtaining pure culture for downstream testing | [30,67,100,101] |
| Chromogenic media | rapid detection of mixed cultures; visual segregation of yeasts by color | platforms designed for Candida spp.; for Rhodotorula spp. colors are non-specific; growth inhibited on some media | typically 48 h readout | screening for yeasts -like fungi | [102,103] |
| Direct microscopy | immediate result; assessment of clinical relevance (yeast cells + inflammatory context); very low cost | no species ID; lower sensitivity in cell-poor specimens; expertise required | minutes | triage of ear/skin specimens; rapid yeast confirmation | [31,84] |
| Biochemical tests (API, Vitek) | commercial panels; genus/species ID for common yeasts; no specialized instrumentation | lower accuracy for rare yeasts; “no-ID”/mis-ID possible; time-consuming | +18–72 h post-isolate (API ~48–72 h; Vitek ~18–24 h) | settings without MALDI/PCR; genus-level verification | [104,105,106] |
| MALDI-TOF MS | rapid species-level ID after colony; low per-test cost; high throughput; libraries continuously updated | requires pure culture; library gaps for very rare species; indeterminate results need confirmation | hours (same day as colony) | routine and urgent ID; surveillance/epidemiology | [104,107] |
| PCR + sequencing (ITS) | highest specificity; resolves rare/spurious cases; PCR directly from specimen possible | cost/know-how; sequence analysis required; not always within 1 day | 24–72 h (≈24 h expedited) | species confirmation; taxonomy/phylogeny; when culture is negative | [30,51,108] |
| Histopathology (H-E, PAS, GMS) | evidence of tissue invasion; distinguishes colonization vs. infection; assesses complications (e.g., endocardial involvement) | species ID usually not possible; tissue required; correlation with culture/MALDI/PCR needed | frozen: hours; routine: 24–48 h | suspected IFD; endocarditis/tissue lesions | [30,31] |
| AFST (MIC; EUCAST BMD) | quantitative in vitro susceptibility profile; aids severe/failing cases; surveillance data | no EUCAST clinical breakpoints for Rhodotorula spp.; adds 1–2 days to TAT; cost/labor of reference methods | +24–48 h post-ID | therapy selection in severe infections; research/monitoring | [82,109] |
| Case (Animal Species) | Rhodotorula Species | Therapy and Treatment Outcome | Citation |
|---|---|---|---|
| Dog, 6 years (male, mixed breed)—chronic tracheobronchitis | R. mucilaginosa | Marbofloxacin (antibiotic) + azole antifungal (Canizol®); clinical improvement; follow-up cultures negative (complete cure). | [97] |
| Dog, 4 years (male, mixed breed)—scrotal lesions; epididymitis | R. glutinis | Surgical removal of the affected epididymides; no detailed data on antifungal therapy—case cured surgically. | [196] |
| Cat, 3 years (female)—chronic skin lesions | R. mucilaginosa | No complete treatment data; skin lesions resolved after topical azole (ketoconazole), according to the case authors. | [197] |
| South American fur seal (female, in a zoo)—skin lesions on the trunk | R. mucilaginosa | Topical azole therapy (sertaconazole) for several weeks; marked improvement and healing of lesions (confirmed by negative follow-up testing). | [198] |
| Cow—otitis externa with mite infestation | R. mucilaginosa | No specific treatment targeting Rhodotorula spp. (therapy was directed against parasites and bacteria); yeasts eliminated after addressing the primary cause. | [195] |
| Chickens (Uganda)—necrotic skin lesions | R. mucilaginosa | No treatment data (descriptive outbreak report). | [199] |
| Sheep—mycotic pneumonia | R. mucilaginosa | No treatment data (case report—infection diagnosed at necropsy after death). | [200] |
| Cow—mycotic mastitis | R. mucilaginosa | No data on specific antifungal therapy (cases noted in a study of dairy herds; mastitis treatment not described in the context of Rhodotorulav spp.). | [201] |
| Laboratory rats (experimental model)—disseminated fungal infection (generalized rhodotorulosis) | R. mucilaginosa | No treatment (experimental model under immunosuppression; severe lesions observed in internal organs—lungs, liver and spleen—caused by the infection). | [202] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wykrętowicz, K.; Czyżewska-Dors, E.; Dors, A.; Pomorska-Mól, M.; Augustyniak, A.; Łagowski, D. Rhodotorula spp. in Laboratory and Veterinary Clinical Practice: Contamination or an Emerging Problem? Animals 2025, 15, 3299. https://doi.org/10.3390/ani15223299
Wykrętowicz K, Czyżewska-Dors E, Dors A, Pomorska-Mól M, Augustyniak A, Łagowski D. Rhodotorula spp. in Laboratory and Veterinary Clinical Practice: Contamination or an Emerging Problem? Animals. 2025; 15(22):3299. https://doi.org/10.3390/ani15223299
Chicago/Turabian StyleWykrętowicz, Kacper, Ewelina Czyżewska-Dors, Arkadiusz Dors, Małgorzata Pomorska-Mól, Agata Augustyniak, and Dominik Łagowski. 2025. "Rhodotorula spp. in Laboratory and Veterinary Clinical Practice: Contamination or an Emerging Problem?" Animals 15, no. 22: 3299. https://doi.org/10.3390/ani15223299
APA StyleWykrętowicz, K., Czyżewska-Dors, E., Dors, A., Pomorska-Mól, M., Augustyniak, A., & Łagowski, D. (2025). Rhodotorula spp. in Laboratory and Veterinary Clinical Practice: Contamination or an Emerging Problem? Animals, 15(22), 3299. https://doi.org/10.3390/ani15223299