Heat Shock Differentially Compromises Embryonic Development and Gene Expression in a Mouse Embryoid Body Model System
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Use and Care of Animals
2.2. Preparation of Feeder Cells
2.3. Culture of mESCs and mEBs
2.4. Formation of mEBs
2.5. PCR Analysis
2.6. Real-Time PCR Analysis
2.7. Immunocytochemical Staining for Specific Germ Layer Markers
2.8. Detection of Apoptosis by TUNEL Assay
2.9. Live–Dead Cell (Viability) Staining of mEBs
2.10. Experimental Designs
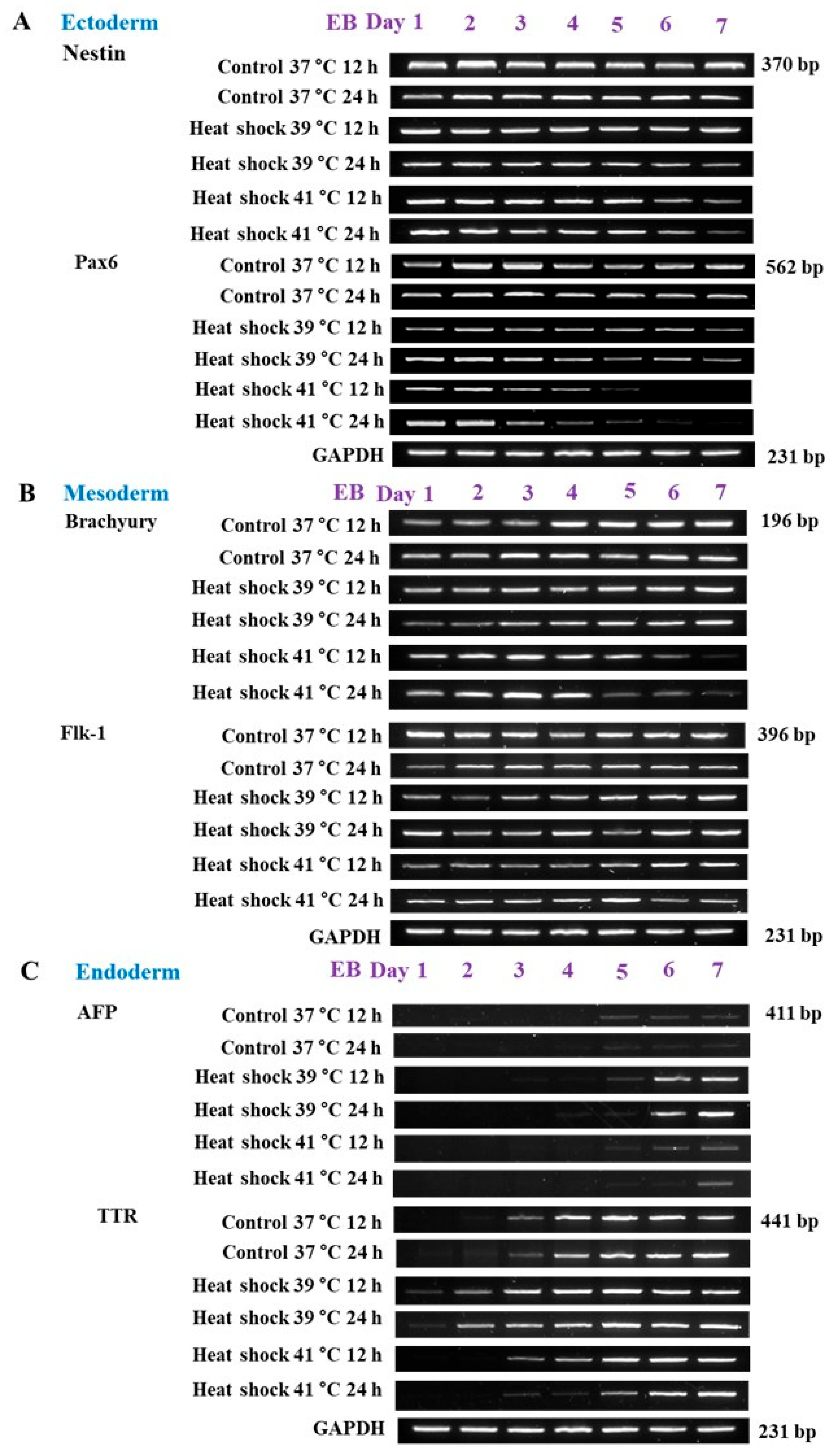
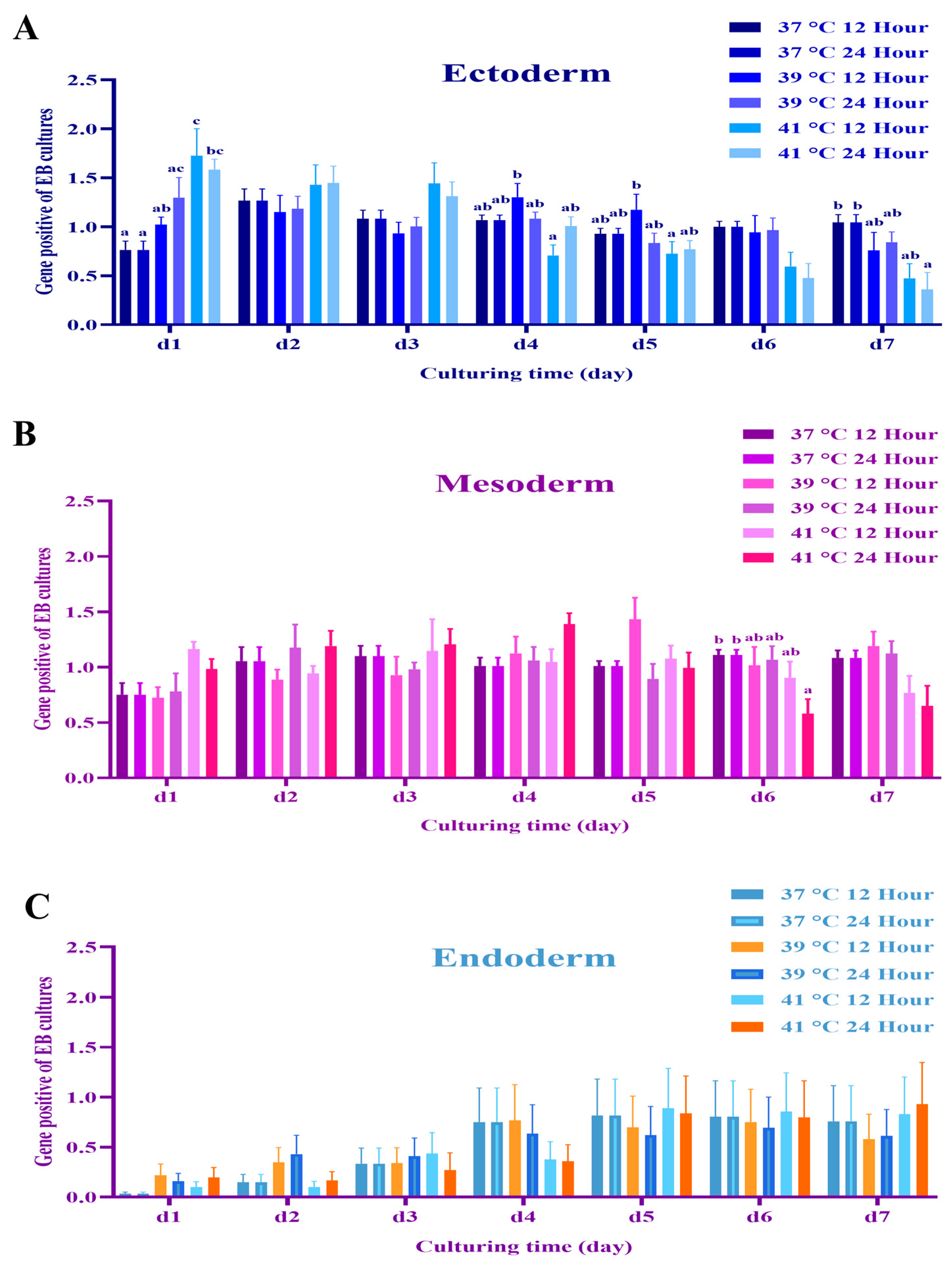
2.10.1. Experiment 1: Germ Layer Gene Expression Profile
2.10.2. Experiment 2: Effects of Heat Shock on Germ Layer Gene Expression in mEBs
2.10.3. Experiment 3: Effects of Various Heat Shock Regimens on HSP70, HSP72 and Caspase 9 Gene Expression
2.11. Statistical Analysis
3. Results
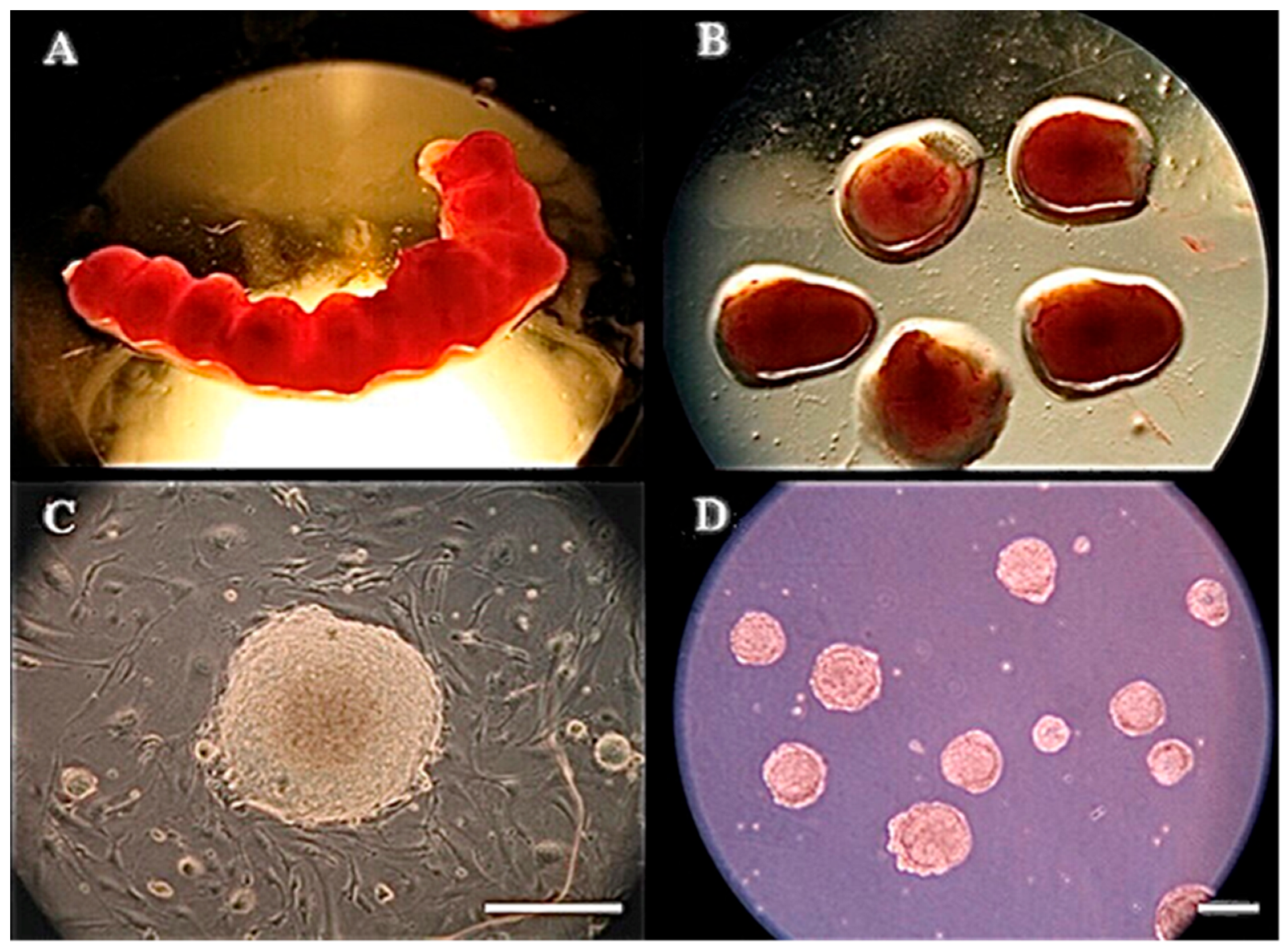
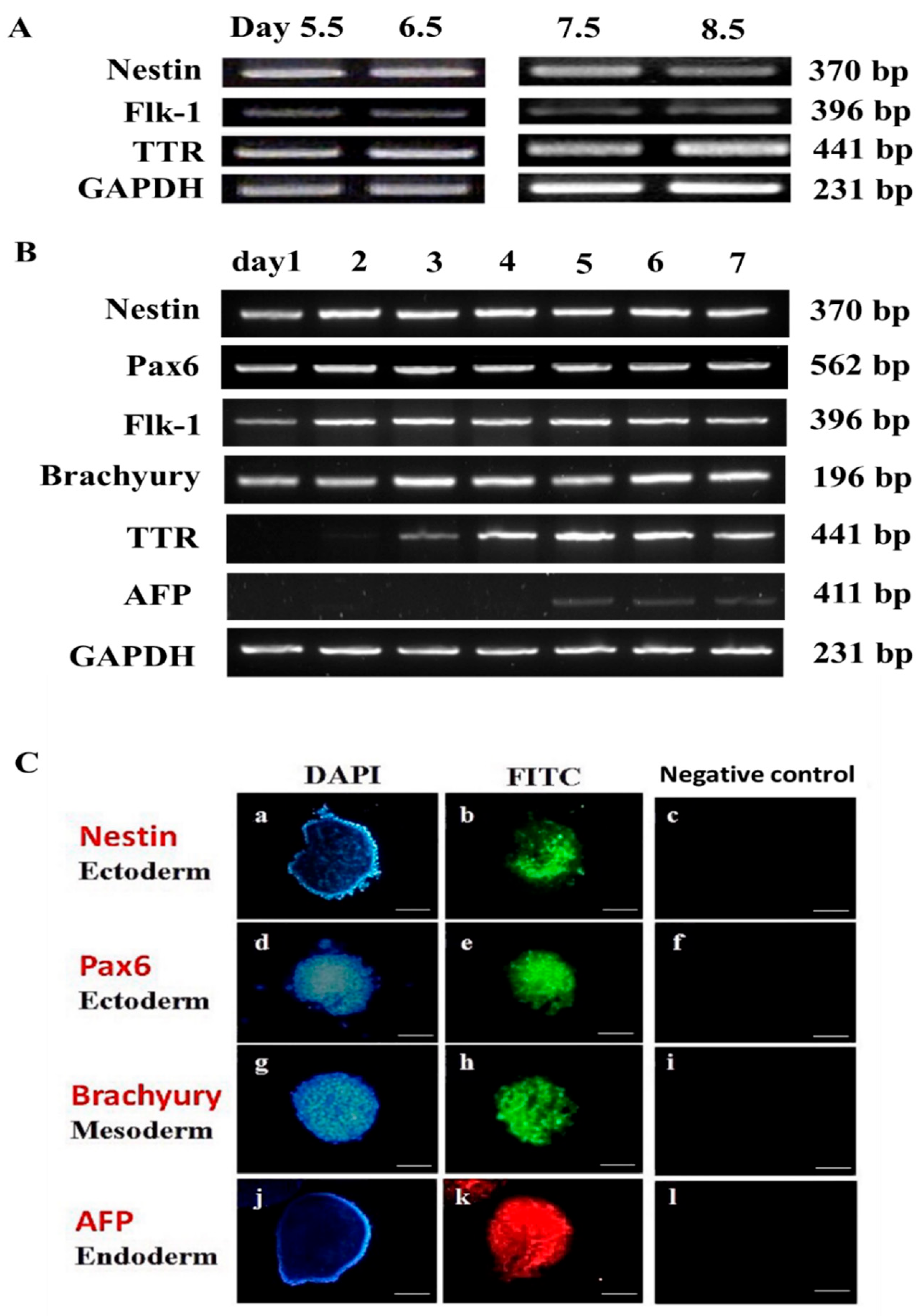
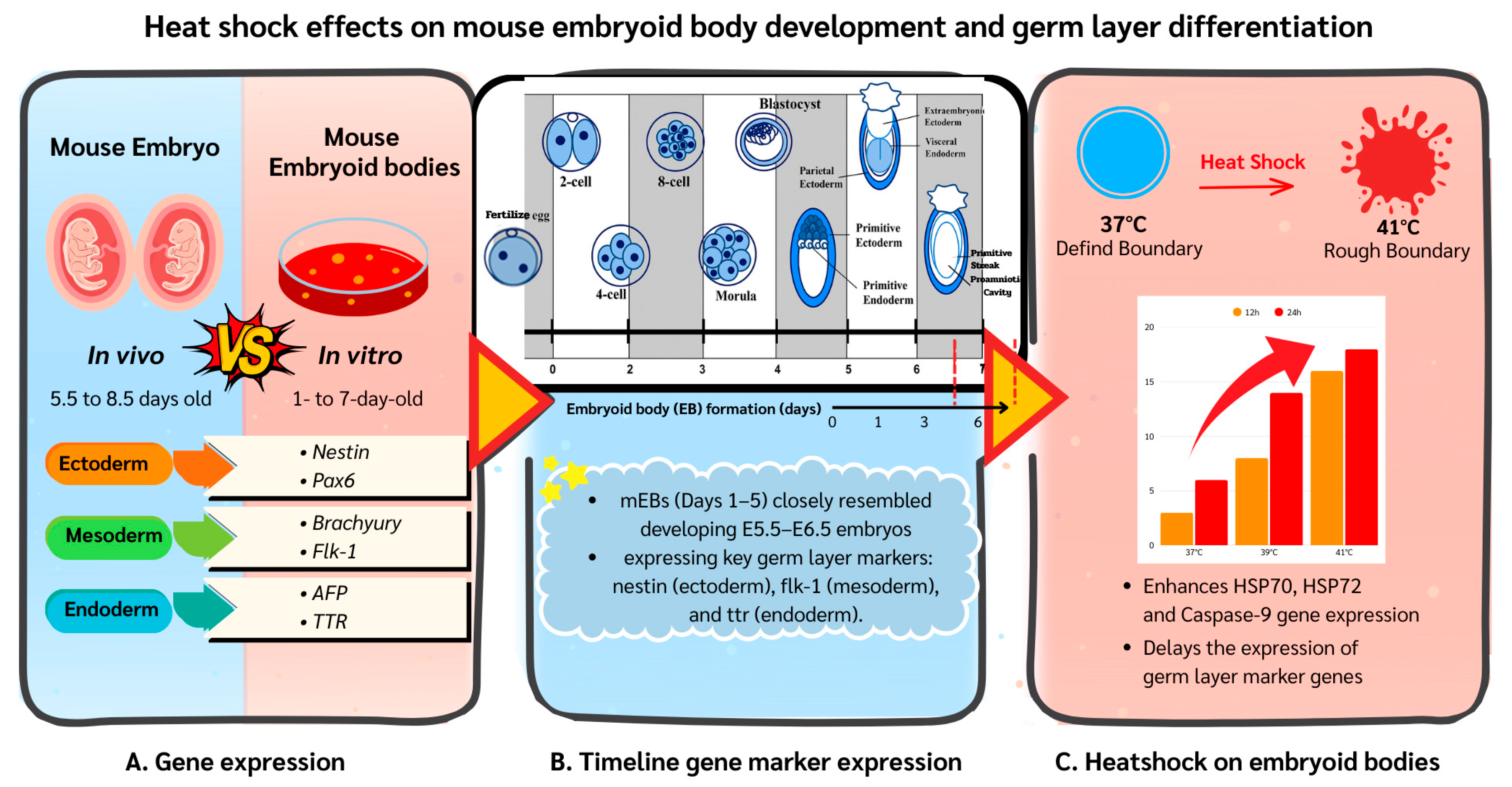
3.1. Experiment 1: Germ Layer Gene Expression in Mouse Embryos and mEBs
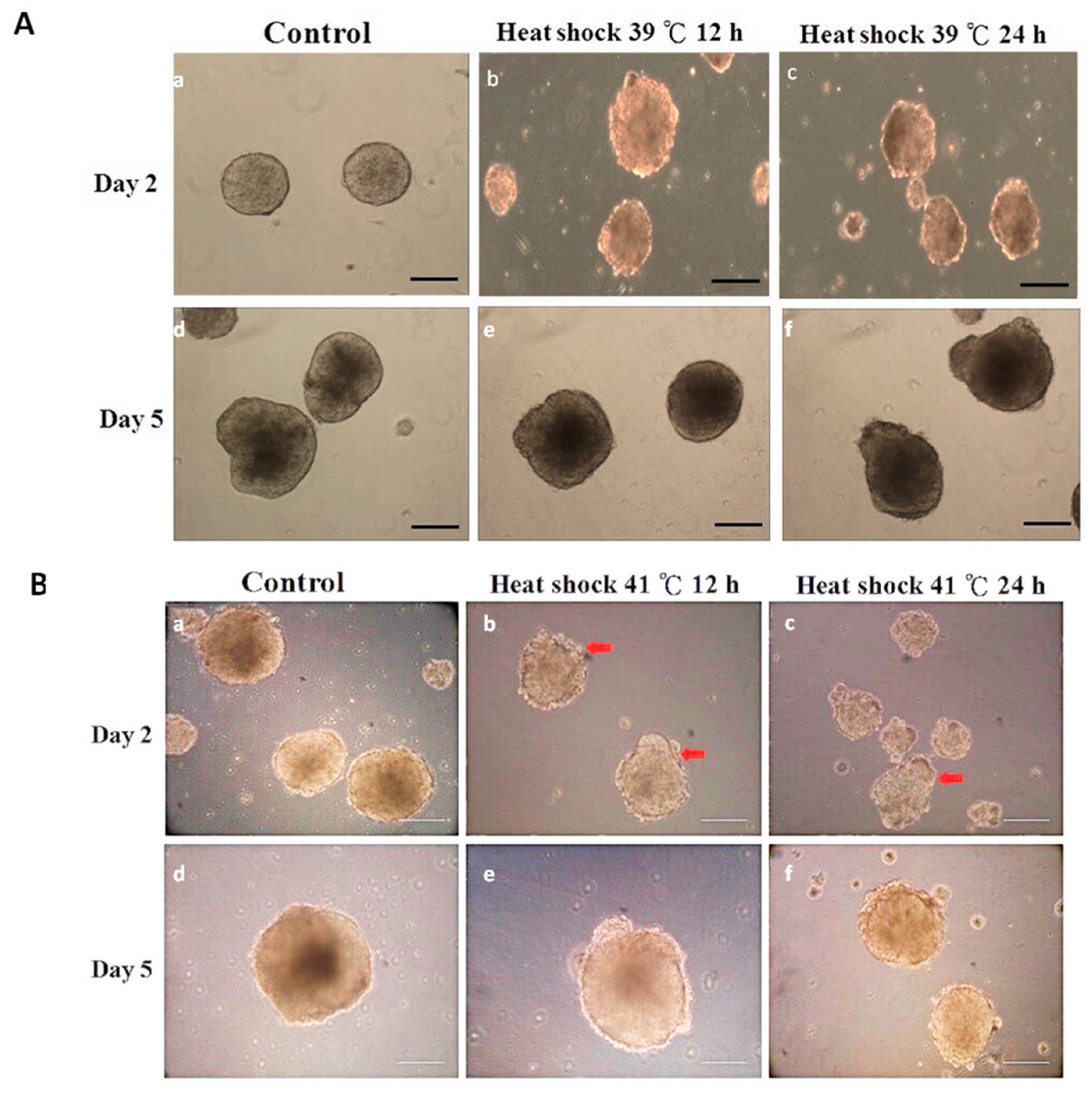
3.2. Experiment 2: Expression of Germ Layer Marker Genes in Heat-Shocked mEBs
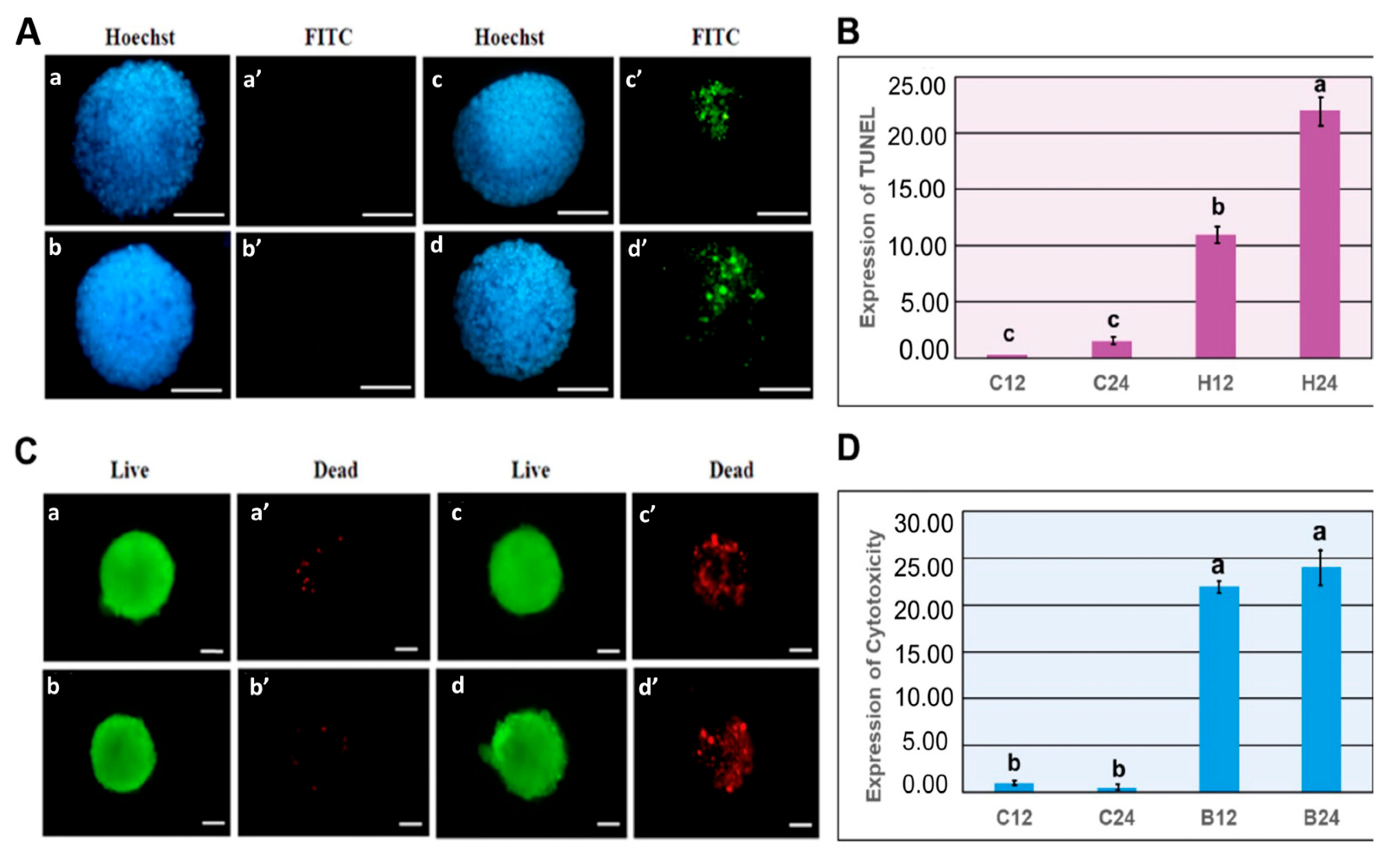
3.3. Experiment 3: Effects of Mild and Severe Heat Shock on HSP70, HSP72 and Caspase-9 Expression in mEBs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oke, O.; Uyanga, V.; Iyasere, O.; Oke, F.; Majekodunmi, B.C.; Logunleko, M.; Abiona, J.A.; Nwosu, E.U.; Abioja, M.O.; Daramola, J.O.; et al. Environmental stress and livestock productivity in hot-humid tropics: Alleviation and future perspectives. J. Therm. Biol. 2021, 100, 103077. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.R.; Yasoob, T.B.; Zhang, Z.; Yu, D.; Feng, J.; Zhu, X.; Hang, S. Supplementation of Moringa oleifera leaf powder orally improved productive performance by enhancing the intestinal health in rabbits under chronic heat stress. J. Therm. Biol. 2020, 93, 102680. [Google Scholar] [CrossRef]
- Ross, J.W.; Hale, B.J.; Seibert, J.T.; Romoser, M.R.; Adur, M.K.; Keating, A.F.; Baumgard, L.H. Physiological mechanisms through which heat stress compromises reproduction in pigs. Mol. Reprod. Dev. 2017, 84, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional physiology and biochemistry of dairy cattle under the influence of heat stress: Consequences and opportunities. Animals 2020, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Sailo, L.; Verma, N.; Bharti, P.; Saikia, J.; Kumar, R. Impact of heat stress on health and performance of dairy animals: A review. Vet. World 2016, 9, 260–268. [Google Scholar] [CrossRef]
- Peña, S.T., Jr.; Stone, F.; Gummow, B.; Parker, A.J.; Paris, D.B. Susceptibility of boar spermatozoa to heat stress using in vivo and in vitro experimental models. Trop. Anim. Health Prod. 2021, 53, 97. [Google Scholar] [CrossRef]
- Morrell, J.M. Heat stress and bull fertility. Theriogenology 2020, 153, 62–67. [Google Scholar] [CrossRef]
- Correa-Calderón, A.; Angulo-Valenzuela, I.; Betancourth, F.; Oroz-Rojo, F.; Fierros-Castro, K.; Macías-Cruz, U.; Díaz-Molina, R.; Avendaño-Reyes, L. Conception rate following artificial insemination with sexed semen in Holstein heifers under artificial cooling during summer compared with winter season. Trop. Anim. Health Prod. 2020, 52, 203–209. [Google Scholar] [CrossRef]
- Khan, A.; Dou, J.; Wang, Y.; Jiang, X.; Khan, M.Z.; Luo, H.; Usman, T.; Zhu, H. Evaluation of heat stress effects on cellular and transcriptional adaptation of bovine granulosa cells. J. Anim. Sci. Biotechnol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Lang, L.; Wang, Z.Z.; Liu, B.; Shen, C.Q.; Tu, J.Y.; Wang, S.C.; Lei, R.L.; Peng, S.Q.; Xiao, X.; Zhao, Y.J.; et al. The effects and mechanisms of heat stress on mammalian oocyte and embryo development. J. Therm. Biol. 2024, 124, 103927. [Google Scholar] [CrossRef]
- Ju, J. Cellular responses of oocytes and embryos under thermal stress: Hints to molecular signaling. Anim. Reprod. 2018, 2, 79–90. Available online: https://www.animal-reproduction.org/article/5b5a6085f7783717068b47ec (accessed on 10 November 2025).
- Ju, J.C.; Tseng, J.K. Nuclear and cytoskeletal alterations of in vitro matured porcine oocytes under hyperthermia. Mol. Reprod. Dev. 2004, 68, 125–133. [Google Scholar] [CrossRef]
- Ju, J.C.; Parks, J.E.; Yang, X. Thermotolerance of IVM-derived bovine oocytes and embryos after short-term heat shock. Mol. Reprod. Dev. 1999, 53, 336–340. [Google Scholar] [CrossRef]
- Wrzecińska, M.; Kowalczyk, A.; Kordan, W.; Cwynar, P.; Czerniawska-Piątkowska, E. Disorder of biological quality and autophagy process in bovine oocytes exposed to heat stress and the effectiveness of in vitro fertilization. Int. J. Mol. Sci. 2023, 24, 11164. [Google Scholar] [CrossRef]
- Houston, B.J.; Nixon, B.; Martin, J.H.; De Iuliis, G.N.; Trigg, N.A.; Bromfield, E.G.; McEwan, K.E.; Aitken, R.J. Heat exposure induces oxidative stress and DNA damage in the male germ line. Biol. Reprod. 2018, 98, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Miętkiewska, K.; Kordowitzki, P.; Pareek, C.S. Effects of heat stress on bovine oocytes and early embryonic development—An update. Cells 2022, 11, 4073. [Google Scholar] [CrossRef] [PubMed]
- Bardot, E.S.; Hadjantonakis, A.K. Mouse gastrulation: Coordination of tissue patterning, specification and diversification of cell fate. Mech. Dev. 2020, 163, 103617. [Google Scholar] [CrossRef]
- Habibi, I.P.; Ostad, S.N.; Heydari, A.; Aliebrahimi, S.; Montazeri, V.; Foroushani, A.R.; Monazzam, M.R.; Ghazi-Khansari, M.; Golbabaei, F. Effect of heat stress on DNA damage: A systematic literature review. Int. J. Biometeorol. 2022, 66, 2147–2158. [Google Scholar] [CrossRef]
- Cowell, W.; Ard, N.; Herrera, T.; Medley, E.A.; Trasande, L. Ambient temperature, heat stress and fetal growth: A review of placenta-mediated mechanisms. Mol. Cell. Endocrinol. 2023, 576, 112000. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Kasimanickam, V. Impact of heat stress on embryonic development during first 16 days of gestation in dairy cows. Sci. Rep. 2021, 11, 14839. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, C.Y.; Zhang, H.; Zhang, P.; Shahzad, M.; Du, W.; Zhao, X. Heat-Stress Impacts on Developing Bovine Oocytes: Unraveling Epigenetic Changes, Oxidative Stress, and Developmental Resilience. Int. J. Mol. Sci. 2024, 25, 4808. [Google Scholar] [CrossRef]
- Amaz, S.A.; Mishra, B. Embryonic thermal manipulation: A potential strategy to mitigate heat stress in broiler chickens for sustainable poultry production. J. Anim. Sci. Biotechnol. 2024, 15, 75. [Google Scholar] [CrossRef]
- Cheng, M.B.; Wang, X.; Huang, Y.; Zhang, Y. Hyperthermia depletes Oct4 in mouse blastocysts and stem cells. Stem Cell Res. Ther. 2020, 11, 195. [Google Scholar] [CrossRef]
- Shiota, K. Induction of Neural Tube Defects and Skeletal Malformations in Mice following Brief Hyperthermia in utero. Neonatology 1988, 53, 86. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Bao, E.; Tang, S. The protective role of heat shock proteins against stresses in animal breeding. Int. J. Mol. Sci. 2024, 25, 8208. [Google Scholar] [CrossRef]
- Javid, B.; MacAry, P.A.; Lehner, P.J. Structure and function: Heat shock proteins and adaptive immunity. J. Immunol. 2007, 179, 2035–2040. [Google Scholar] [CrossRef]
- Hassan, F.U.; Nawaz, A.; Rehman, M.S.; Ali, M.A.; Dilshad, S.M.; Yang, C. Prospects of HSP70 as a genetic marker for thermo-tolerance and immuno-modulation in animals under climate change scenario. Anim. Nutr. 2019, 5, 340–350. [Google Scholar] [CrossRef]
- Simao, J.d.J.; Bispo, A.F.d.S.; Plata, V.T.G.; Abel, A.B.M.; Saran, R.J.; Barcella, J.F.; Alonso, J.C.C.; Santana, A.V.; Armelin-Correa, L.M.; Alonso-Vale, M.I.C. The activation of the NF-κB pathway in human adipose-derived stem cells alters the deposition of epigenetic marks on H3K27 and is modulated by fish oil. Life 2024, 14, 1653. [Google Scholar] [CrossRef]
- Leahy, A.; Xiong, J.; Kuhnert, F.; Stuhlmann, H. Use of Developmental Marker Genes to Define Temporal and Spatial Patterns of Differentiation during Embryoid Body Formation. J. Exp. Zool. 1999, 284, 67–81. [Google Scholar] [CrossRef]
- Girgin, M.U.; Broguiere, N.; Hoehnel, S.; Brandenberg, N.; Mercier, B.; Arias, A.M.; Lutolf, M.P. Bioengineered embryoids mimic post-implantation development in vitro. Nat. Commun. 2021, 12, 5140. [Google Scholar] [CrossRef]
- Intawicha, P.; Ou, Y.W.; Lo, N.W.; Zhang, S.C.; Chen, Y.Z.; Lin, T.A.; Su, H.L.; Guu, H.F.; Chen, M.J.; Lee, K.H.; et al. Characterization of embryonic stem cell lines derived from New Zealand white rabbit embryos. Cloning Stem Cells 2009, 11, 27–38. [Google Scholar] [CrossRef]
- SAS Institute. SAS User’s Guide Statistics, version 6.03; SAS Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Choi, D.; Lee, H.J.; Jee, S.; Jin, S.; Koo, S.K.; Paik, S.S.; Jung, S.C.; Hwang, S.Y.; Lee, K.S.; Oh, B. In vitro differentiation of mouse embryonic stem cells: Enrichment of endodermal cells in the embryoid body. Stem Cells 2005, 23, 817–827. [Google Scholar] [CrossRef]
- Boni, R. Heat stress, a serious threat to reproductive function in animals and humans. Mol. Reprod. Dev. 2019, 86, 1307–1323. [Google Scholar] [CrossRef]
- Omori, H.; Otsu, M.; Suzuki, A.; Nakayama, T.; Akama, K.; Watanabe, M.; Inoue, N. Effects of heat shock on survival, proliferation and differentiation of mouse neural stem cells. Neurosci. Res. 2014, 79, 13–21. [Google Scholar] [CrossRef]
- Li, J.; Labbadia, J.; Morimoto, R.I. Rethinking HSF1 in stress, development, and organismal health. Trends Cell Biol. 2017, 27, 895–905. [Google Scholar] [CrossRef]
- Kaitsuka, T.; Hakim, F. Response of pluripotent stem cells to environmental stress and its application for directed differentiation. Biology 2021, 10, 84. [Google Scholar] [CrossRef]
- Anckar, J.; Sistonen, L. Regulation of HSF1 function in the heat stress response: Implications in aging and disease. Annu. Rev. Biochem. 2011, 80, 1089–1115. [Google Scholar] [CrossRef]
- Zhang, B.; Fan, Y.; Cao, P.; Tan, K. Multifaceted roles of HSF1 in cell death: A state-of-the-art review. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188591. [Google Scholar] [CrossRef]

| Gene | Primer Sequences (Forward/Reverse) | Annealing Temperature | Products Size | Accession Numbers |
|---|---|---|---|---|
| Nestin | F-AGCAACTGGCACACCTCAA R-ACATCCTGGGCTCTGACCT | 59 °C | 370 bp | NM_016701.3 |
| Pax6 | F-AGACTTTAACCAAGGGCGGT R-TAGCCAGGTTGCGAAGAACT | 58 °C | 562 bp | NM_013627.6 |
| Flk-1 | F-AGGTGCCTCCCCATACCCTGG R-GGCCGGCTCTTTCGCTTACTG | 65 °C | 396 bp | NM_010612.3 |
| Brachyury | F-ATAACGCCAGCCCACCTAC R-CGGAGAACCAGAGACGAG | 58 °C | 196 bp | NM_009309.2 |
| TTR | F-AGTCCTGGATGCTGTCCGAG R-TTCCTGAGCTGCTAACACGG | 59 °C | 441 bp | NM_013697.5 |
| AFP | F-GCTCACACCAAAGCGTCAAC R-CCTGTGAACTCTGGTATCAG | 54 °C | 411 bp | NM_007423.4 |
| GAPDH | F-GAGCCAAACGGGTCATCATCT R-AGGGGCCATCCACAGTCTTCT | 65 °C | 231 bp | NM_008084.4 |
| Gene | Primer Sequences (Forward/Reverse) | Products Size | Accession Numbers |
|---|---|---|---|
| HSP72 | F-ACGGCATCTTCGAGGTGAA R-TGTTCTGGCTGATGTCCTTCT | 129 bp | NM_010479.2 |
| HSP70 | F-AAGAACGCGCTCGAGTCCTAT R-GAGATGACCTCCTGGCACTTGT | 122 bp | NM_010478.3 |
| Caspase 9 | F-TTGTCTCCTGGAGGGACAAGAA R-AAGGAGGGACTGCAGGTCTTC | 101 bp | NM_015733.6 |
| GAPDH | F-CTGCACCACCAACTGCTTAGC R-CAGTCTTCTGGGTGGCAGTGA | 110 bp | NM_008084.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Intawicha, P.; Sonsiri, K.; Yang, C.-R.; Lo, N.-W.; Tang, P.-C.; Ju, J.-C. Heat Shock Differentially Compromises Embryonic Development and Gene Expression in a Mouse Embryoid Body Model System. Animals 2025, 15, 3293. https://doi.org/10.3390/ani15223293
Intawicha P, Sonsiri K, Yang C-R, Lo N-W, Tang P-C, Ju J-C. Heat Shock Differentially Compromises Embryonic Development and Gene Expression in a Mouse Embryoid Body Model System. Animals. 2025; 15(22):3293. https://doi.org/10.3390/ani15223293
Chicago/Turabian StyleIntawicha, Payungsuk, Kamonthip Sonsiri, Chun-Ru Yang, Neng-Wen Lo, Pin-Chi Tang, and Jyh-Cherng Ju. 2025. "Heat Shock Differentially Compromises Embryonic Development and Gene Expression in a Mouse Embryoid Body Model System" Animals 15, no. 22: 3293. https://doi.org/10.3390/ani15223293
APA StyleIntawicha, P., Sonsiri, K., Yang, C.-R., Lo, N.-W., Tang, P.-C., & Ju, J.-C. (2025). Heat Shock Differentially Compromises Embryonic Development and Gene Expression in a Mouse Embryoid Body Model System. Animals, 15(22), 3293. https://doi.org/10.3390/ani15223293






