The Effects of Anthocyanins Added to Semen Diluent on Semen Quality, Semen Antioxidant Capacity, and Sperm Apoptosis in Zi Geese
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Anthocyanins and Anthocyanins Solution Preparation
2.3. Experimental Animals
2.4. Semen Collection
2.5. Semen Processing
2.6. Evaluation of Sperm Quality
2.6.1. Single Semen Volume
2.6.2. Semen pH Value
2.6.3. Sperm Survival Rate
2.6.4. Sperm Parameters
2.7. Mitochondrial Activity Assay
2.8. Semen Antioxidant Capacity Detection
2.9. Sperm Apoptosis Detection
2.10. Relative mRNA Expression Detection
2.11. Western Blot Detection
2.12. DNA Fragment Index Detection
2.13. Data Analysis
3. Experimental Results
3.1. Semen Collection Situation
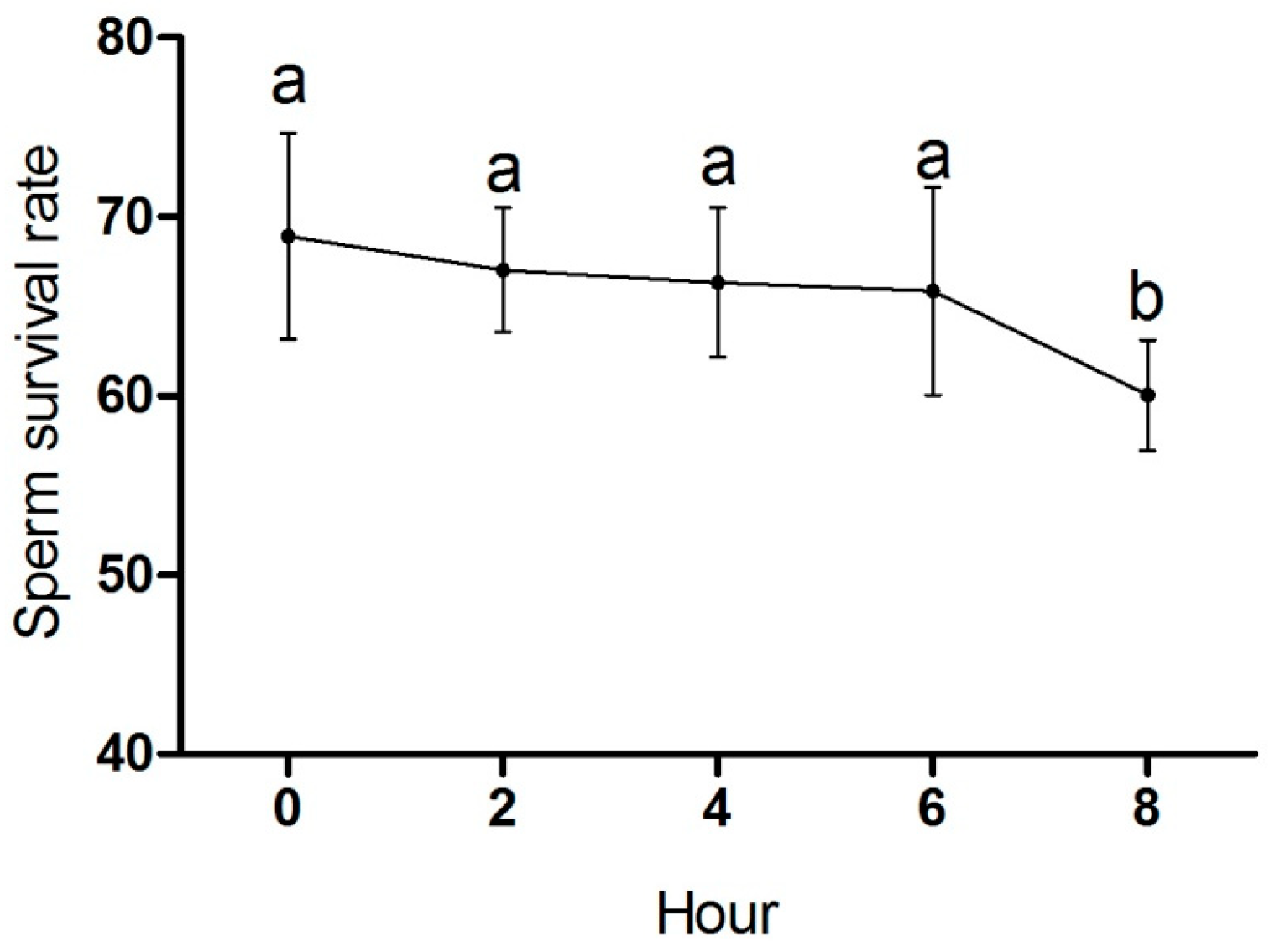
3.2. Effect of Semen Storage Time on Sperm Survival Rate of Zi Geese
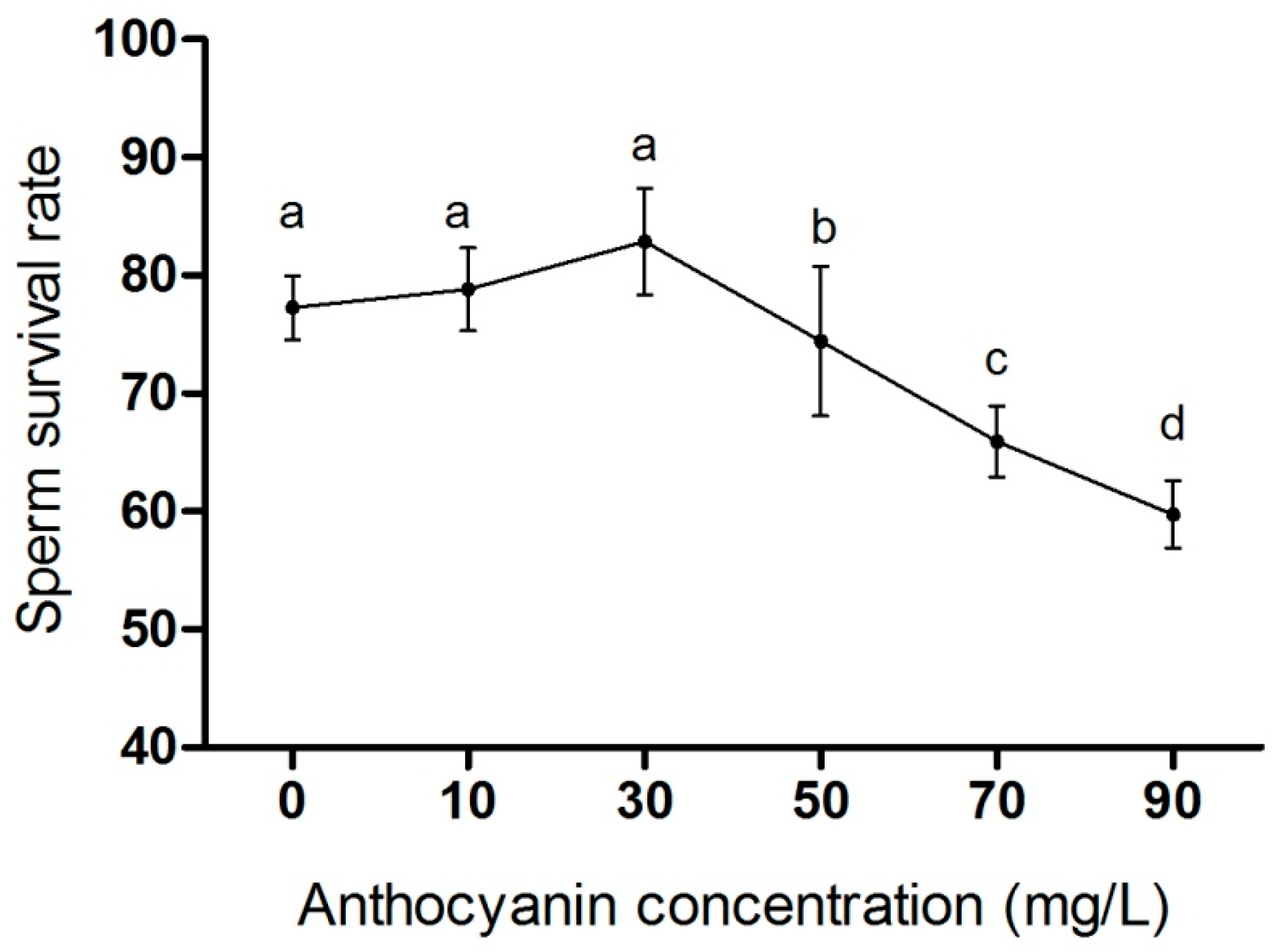
3.3. Effects of Different Anthocyanin Concentrations on Sperm Survival Rate of Zi Geese
3.4. Effects of Anthocyanins on Semen Quality of Zi Geese
3.5. Effects of Anthocyanins on Sperm Dynamics Parameters
3.6. The Effect of Anthocyanins on the Antioxidant Capacity of Goose Semen
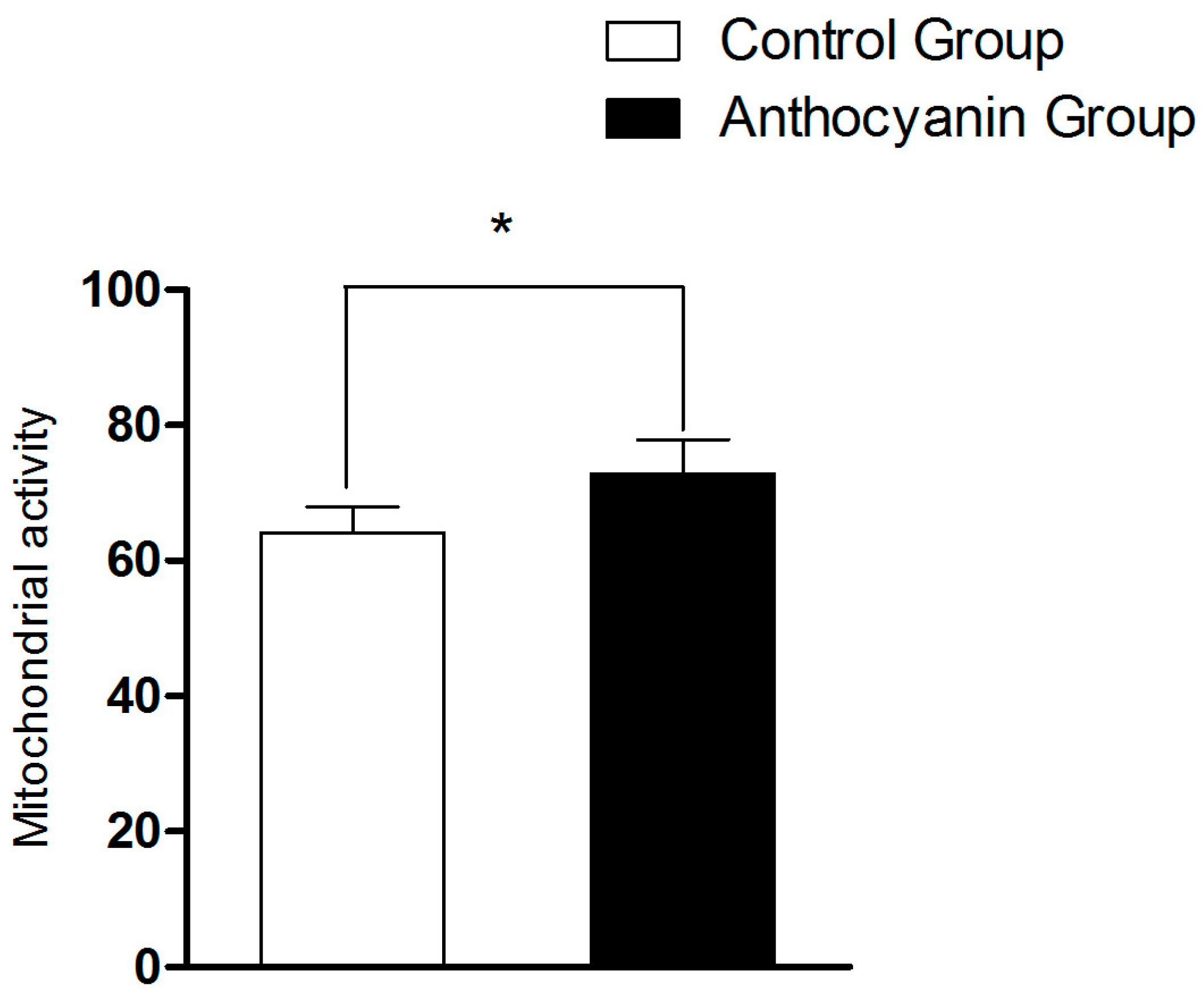
3.7. The Effect of Anthocyanins on Mitochondrial Activity of Goose Sperm
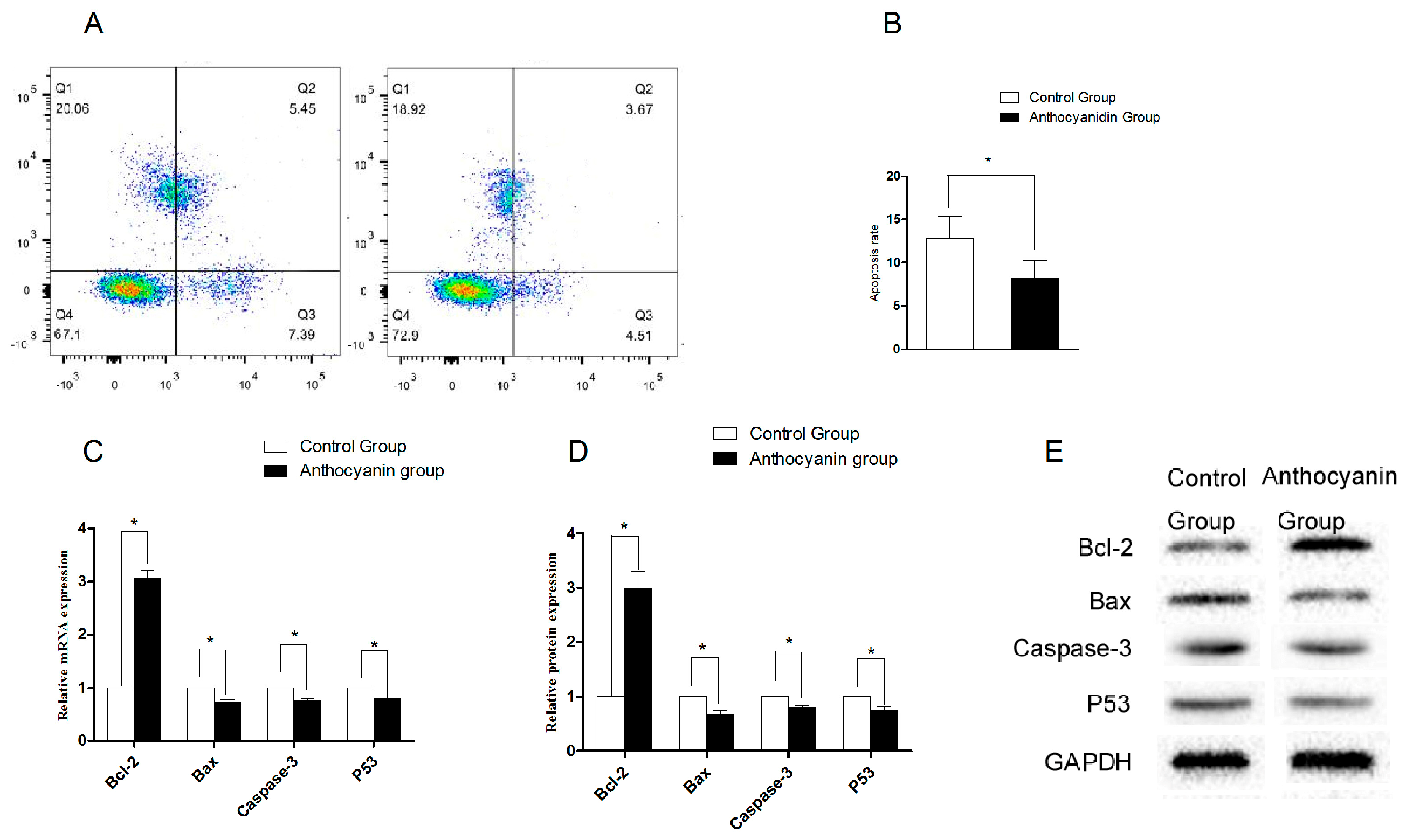
3.8. Effects of Anthocyanins on Sperm Apoptosis in Zi Geese
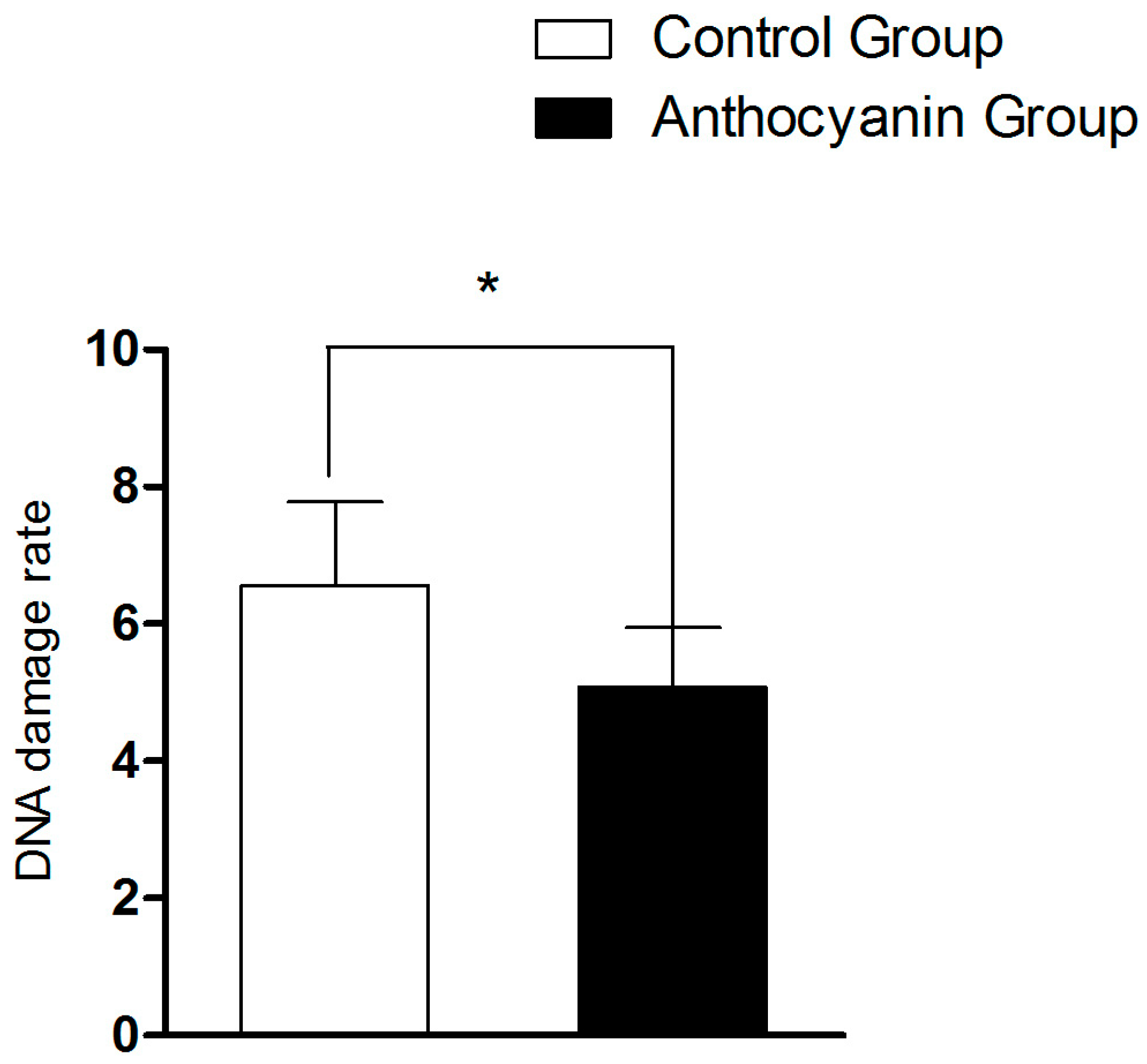
3.9. The Effect of Anthocyanins on DNA Fragmentation Rate of Goose Sperm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, W.Q.; Zhao, D.H.; Cheng, H.Z.; Wang, S.B.; Yang, J.; Cui, H.X.; Lu, M.Y.; Wu, H.Z.; Xu, L.; Liu, G.J. Effects of dietary enteromorpha powder on reproduction-related hormones and genes during the late laying period of zi geese. Anim. Biosci. 2021, 34, 457–462. [Google Scholar] [CrossRef]
- Kovacs-Koplikne, E. Problems in using artificial insemination in breeding geese. L’Alimentation Et La Vie 1969, 57, 3. [Google Scholar]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. 2017, 57, 3072–3083. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Saini, R.K.; Khan, M.I.; Shang, X.M.; Kumar, V.; Kumari, V.; Kesarwani, A.; Ko, E.Y. Dietary sources, stabilization, health benefits, and industrial application of anthocyanins-a review. Foods 2024, 13, 1227. [Google Scholar] [CrossRef]
- Li, A.R.; Xiao, R.S.; He, S.J.; An, X.Y.; He, Y.; Wang, C.T.; Yin, S.; Wang, B.; Shi, X.W.; He, J.R. Research advances of purple sweet potato anthocyanins: Extraction, identification, stability, bioactivity, application, and biotransformation. Molecules 2019, 24, 3816. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Lu, P.; Li, Z.Q.; Yuan, C.S.; Liu, H.Y.; Zhao, J.; Lu, W.F.; Wang, J. Oligomeric proanthocyanidins and bamboo leaf flavonoids improve the quality of bull semen cryopreservation. Molecules 2022, 27, 1144. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Weike, S.Y.; Li, Y.; Chen, M.J.; Hu, Y.M.; Liu, B.; Yang, G.S.; Hu, J.H. Effects of oligomeric proanthocyanidins on quality of boar semen during liquid preservation at 17 °C. Anim. Reprod. Sci. 2018, 198, 47–56. [Google Scholar] [CrossRef]
- AlGhannam, S.M.; El-Rahman, S.N.A. Antioxidant evaluation study of black rice anthocyanins nano-composite as prospective against infertility induced by alcl3 in rats. Braz. J. Biol. 2024, 84, e280570. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Luo, Y.H.; Ma, C.; Dong, L.; Chen, F. Blueberry anthocyanins extract attenuates acrylamide-induced oxidative stress and neuroinflammation in rats. Oxid. Med. Cell Longev. 2022, 2022, 7340881. [Google Scholar] [CrossRef] [PubMed]
- Isaak, C.K.; Petkau, J.C.; Blewett, H.; Karmin, O.; Siow, Y.L. Lingonberry anthocyanins protect cardiac cells from oxidative-stress-induced apoptosis. Can. J. Physiol. Pharmacol. 2017, 95, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Pawłowicz, P.; Stachowiak, G.; Bielak, A.; Wilczyński, J. Administration of natural anthocyanins derived from chokeberry (aronia melanocarpa) extract in the treatment of oligospermia in males with enhanced autoantibodies to oxidized low density lipoproteins (olab). Clin. Trial 2001, 72, 6. [Google Scholar]
- Zhou, L.; Zhang, C.Q.; Qiang, Y.; Huang, M.; Ren, X.M.; Li, Y.H.; Shao, J.H.; Xu, L.C. Anthocyanin from purple sweet potato attenuates lead-induced reproductive toxicity mediated by jnk signaling pathway in male mice. Ecotox Environ. Safe 2021, 224, 112683. [Google Scholar] [CrossRef]
- Harraway, C.; Berger, N.G.; Dubin, N.H. Semen ph in patients with normal versus abnormal sperm characteristics. Am. J. Obstet. Gynecol. 2000, 182, 3. [Google Scholar] [CrossRef]
- Jing, J.; Peng, Y.H.; Fan, W.M.; Han, S.Y.; Peng, Q.H.; Xue, C.R.; Qin, X.R.; Liu, Y.; Ding, Z.D. Obesity-induced oxidative stress and mitochondrial dysfunction negatively affect sperm quality. FEBS Open Bio 2023, 13, 763–778. [Google Scholar] [CrossRef]
- Hu, R.Z.; Yang, X.Z.; Gong, J.T.; Lv, J.; Yuan, X.P.; Shi, M.K.; Fu, C.X.; Tan, B.; Fan, Z.Y.; Chen, L.; et al. Patterns of alteration in boar semen quality from 9 to 37 months old and improvement by protocatechuic acid. J. Anim. Sci. Biotechnol. 2024, 15, 78. [Google Scholar] [CrossRef]
- Sheng, W.; Zhang, Y.-S.; Li, Y.-Q.; Wu, X.-N.; Chai, L.-M.; Yue, L.-F.; Liu, J.; Ding, J.; Li, X.-R.; Chen, M.; et al. Effect of yishenjianpi recipe on semen quality and sperm mitochondria in mice with oligoasthenozoospermia induced by tripterygium glycosides. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 87–95. [Google Scholar] [CrossRef]
- Zhu, F. Anthocyanins in cereals: Composition and health effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef]
- Hu, R.; Yang, X.; Wang, L.; Su, D.; He, Z.; Li, J.; Gong, J.; Zhang, W.; Ma, S.; Shi, M.; et al. Gut microbiota dysbiosis and oxidative damage in high-fat diet-induced impairment of spermatogenesis: Role of protocatechuic acid intervention. Food Front. 2024, 5, 2566–2578. [Google Scholar] [CrossRef]
- Bonarska-Kujawa, D.; Pruchnik, H.; Kleszczynska, H. Interaction of selected anthocyanins with erythrocytes and liposome membranes. Cell Mol. Biol. Lett. 2012, 17, 289–308. [Google Scholar] [CrossRef]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef] [PubMed]
- John, J.C.S.; Bowles, E.J.; Amaral, A. Sperm mitochondria and fertilisation. Soc. Reprod. Fertil. Suppl. 2007, 65, 18. [Google Scholar]
- Yuan, Z.Q.; Zhang, J.Y.; Tu, C.C.; Wang, Z.J.; Xin, W.P. The protective effect of blueberry anthocyanins against perfluorooctanoic acid-induced disturbance in planarian. Ecotox. Environ. Safe 2016, 127, 170–174. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Yu, Q.W.; Zhang, L.Y.; Jiang, Z.Z. Cyclophilin d-mediated mitochondrial permeability transition regulates mitochondrial function. Curr. Pharm. Design 2023, 29, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.Y.; Zheng, J.W.; Herrera-Balandrano, D.D.; Zhang, X.X.; Huang, W.Y.; Sui, Z.Q. In vivo antioxidant activity of rabbiteye blueberry (cv. ‘Brightwell’) anthocyanin extracts. J. Zhejiang Univ.-Sci. B 2023, 24, 602–616. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, F.; Cao, X.; Chen, G.; Guo, Q.; Ying, X.; Guo, W.; Lu, L.; Ding, Z. Con a-binding protein zn-a2-glycoprotein on human sperm membrane is related to acrosome reaction and sperm fertility. Int. J. Androl. 2012, 35, 145–157. [Google Scholar] [CrossRef]
- Mao, H.T.; Yang, W.X. Modes of acrosin functioning during fertilization. Gene 2013, 526, 75–79. [Google Scholar] [CrossRef]
- Torabi, S.S.S.; Mohammadi, T.; Rezaei, M. The impact of procyanidin supplementation on turkey semen quality, oxidative stress parameters, and fertilization during preservation at −196 °C. Cryobiology 2025, 120, 105286. [Google Scholar] [CrossRef]
- Xie, D.E.; Lu, C.; Zhu, Y.; Zhu, S.L.; Yang, E.J.; Jin, X. Analysis on the association between sperm DNA fragmentation index and conventional semen parameters, blood microelements and seminal plasma ros in male patients with infertility. Exp. Ther. Med. 2018, 15, 5173–5176. [Google Scholar] [CrossRef]
- Yamuangmorn, S.; Prom-u-Thai, C. The potential of high-anthocyanin purple rice as a functional ingredient in human health. Antioxidants 2021, 10, 833. [Google Scholar] [CrossRef]
- Verma, N.; Pink, M.; Schmitz-Spanke, S. A new perspective on calmodulin-regulated calcium and ros homeostasis upon carbon black nanoparticle exposure. Arch. Toxicol. 2021, 95, 2007–2018. [Google Scholar] [CrossRef]
- Abdurrahim, A.E.; Mazurak, V.C.; Chen, L.Y. Gingerols synergize with anthocyanins to induce antioxidant activity. Front. Nutr. 2023, 10, 1229015. [Google Scholar] [CrossRef]
- Czerski, L.; Nuñez, G. Apoptosome formation and caspase activation: Is it different in the heart? J. Mol. Cell Cardiol. 2004, 37, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Feinstein-Rotkopf, Y.; Arama, E. Can’t live without them, can live with them: Roles of caspases during vital cellular processes. Apoptosis 2009, 14, 980–995. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.B.; Liu, Y.; Ding, B.; Lu, S.Q.; Ni, B.Y.; Chen, Y.T.; Yang, L.; Liu, Y.N.; Zhang, Y.C.; Wang, Y.X.; et al. Anthocyanins from blueberry ameliorated arsenic-induced memory impairment, oxidative stress, and mitochondrial-biosynthesis imbalance in rat hippocampal neurons. Cell Signal 2024, 119, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Park, C.; Hwangbo, H.; Kim, S.O.; Noh, J.S.; Park, S.H.; Hong, S.H.; Hong, S.H.; Kim, G.Y.; Choi, Y.H. Anthocyanins inhibits oxidative injury in human retinal pigment epithelial arpe-19 cells activating heme oxygenase-1. J. Microbiol. Biotechnol. 2024, 34, 596–605. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Wen, Q.L.; Cai, Y.F.; Liu, Y.Y.; Ma, W.L.; Li, Q.L.; Song, F.H.; Guo, Y.W.; Zhu, L.; Ge, J.Y.; et al. Alantolactone induces concurrent apoptosis and gsdme-dependent pyroptosis of anaplastic thyroid cancer through ros mitochondria-dependent caspase pathway. Phytomedicine 2023, 108, 154528. [Google Scholar] [CrossRef]
- Huang, Z.B.; Shi, X.Y.; Li, M.; Huang, Q.M.; Xie, L.M. Monacolin k induces apoptosis of human glioma u251 cells by triggering ros-mediated oxidative damage and regulating mapks and nf-?B pathways. ACS Chem. Neurosci. 2023, 14, 1331–1341. [Google Scholar]
- Gao, S.; Chen, T.F.; Choi, M.Y.; Liang, Y.W.; Xue, J.Y.; Wong, Y.S. Cyanidin reverses cisplatin-induced apoptosis in hk-2 proximal tubular cells through inhibition of ros-mediated DNA damage and modulation of the erk and akt pathways. Cancer Lett. 2013, 333, 36–46. [Google Scholar] [CrossRef]
| Ingredient | Content |
|---|---|
| Fructose (g) | 1.000 |
| Magnesium chloride (g) | 0.068 |
| Sodium acetate (g) | 0.857 |
| Potassium citrate (g) | 0.128 |
| Sodium glutamate (g) | 1.920 |
| Penicillin (IU) | 100,000 |
| Streptomycin (IU) | 100,000 |
| Distilled water (mL) | 100 |
| Gene | Accession Number | Primer Sequence | Product Size (bp) |
|---|---|---|---|
| Bcl-2 | KM040761 | F: 5′-GAGTACCTGAACCGGCATCT-3′ R: 5′-CAGCCTCCGTTATCCTGGTA-3′ | 151 |
| Bax | XM_066985593 | F: 5′-GGAATTCGCCGAGATGTCCG-3′ R:5′-CGGGATCCGGTCTGATCGTCG-3′ | 129 |
| Caspase-3 | XM_066996225 | F: 5′-ATGGCCCTGGACATTGAC-3′ R: 5′-TTGCTGTCGCTGCTTGT-3′ | 135 |
| P53 | XM_013175338 | F: 5′-GAGTCTGCCCGGACGAT-3′ R: 5′-TCCTCGGTGCTCGATGT-3′ | 145 |
| Control Group | Anthocyanin Group | ||
|---|---|---|---|
| pH value | 7.32 ± 0.54 | 7.20 ± 0.63 | |
| Sperm concentration (pcs/mL) | 3.85 ± 0.29 | 3.77 ± 0.16 | |
| Sperm motility | 0.70 ± 0.05 b | 0.76 ± 0.03 a | |
| Sperm survival rate (%) | 76.98 ± 5.67 b | 81.08 ± 3.85 a | |
| Sperm abnormality rate (%) | 8.71 ± 0.64 | 8.62 ± 0.71 | |
| Sperm mortality rate (%) | 23.02 ± 1.68 a | 18.92 ± 2.34 b | |
| Membrane integrity rate (%) | 67.56 ± 2.60 b | 72.63 ± 3.09 a | |
| Acrosome integrity rate (%) | 78.80 ± 4.52 b | 92.94 ± 4.67 a | |
| Control Group | Anthocyanin Group | |
|---|---|---|
| VCL (μm/s) | 103.87 ± 6.22 b | 118.84 ± 7.32 a |
| VSL (μm/s) | 68.46 ± 8.13 b | 72.83 ± 7.20 a |
| VAP (μm/s) | 80.63 ± 6.38 b | 89.91 ± 5.48 a |
| LIN (%) | 63.44 ± 5.47 | 62.98 ± 8.01 |
| Control Group | Anthocyanin Group | |
|---|---|---|
| ROS (OD Value) | 0.63 ± 0.04 a | 0.36 ± 0.03 b |
| MDA (nmol/L) | 7.64 ± 0.81 a | 5.18 ± 0.72 b |
| SOD (U/mL) | 166.07 ± 12.38 b | 220.39 ± 11.84 a |
| GPx (U/L) | 118.55 ± 8.61 b | 152.02 ± 13.46 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zeng, C.; Zhao, P.; Pan, Y.; Zang, Z.; Zhang, Y.; Yue, S.; Liu, S.; Zheng, P.; Huang, H.; et al. The Effects of Anthocyanins Added to Semen Diluent on Semen Quality, Semen Antioxidant Capacity, and Sperm Apoptosis in Zi Geese. Animals 2025, 15, 3281. https://doi.org/10.3390/ani15223281
Wang S, Zeng C, Zhao P, Pan Y, Zang Z, Zhang Y, Yue S, Liu S, Zheng P, Huang H, et al. The Effects of Anthocyanins Added to Semen Diluent on Semen Quality, Semen Antioxidant Capacity, and Sperm Apoptosis in Zi Geese. Animals. 2025; 15(22):3281. https://doi.org/10.3390/ani15223281
Chicago/Turabian StyleWang, Size, Chuicheng Zeng, Puxuan Zhao, Yue Pan, Zhengyu Zang, Yuanliang Zhang, Shan Yue, Shengjun Liu, Peng Zheng, He Huang, and et al. 2025. "The Effects of Anthocyanins Added to Semen Diluent on Semen Quality, Semen Antioxidant Capacity, and Sperm Apoptosis in Zi Geese" Animals 15, no. 22: 3281. https://doi.org/10.3390/ani15223281
APA StyleWang, S., Zeng, C., Zhao, P., Pan, Y., Zang, Z., Zhang, Y., Yue, S., Liu, S., Zheng, P., Huang, H., & Zhao, X. (2025). The Effects of Anthocyanins Added to Semen Diluent on Semen Quality, Semen Antioxidant Capacity, and Sperm Apoptosis in Zi Geese. Animals, 15(22), 3281. https://doi.org/10.3390/ani15223281






