1. Introduction
Cell injury or death caused by infectious and non-infectious agents, physical trauma, extreme temperatures (heat or cold), radiation, or malignant cells can trigger a highly coordinated sequence of fluid and cellular responses within vascularized tissues, a process known as inflammation. This response leads to the buildup of fluid, electrolytes, plasma proteins, and leukocytes in extravascular tissue. Clinically, inflammation is identified by redness, heat, swelling, pain, and loss of function in the affected tissue. It typically acts as a defense mechanism, aiming to dilute, contain, and remove the cause of injury, while also initiating tissue repair. Leukocytes play an essential role in this process, forming a key part of the immune system and defending the body against harmful pathogens, including bacteria, viruses, fungi, and parasites [
1].
In human medicine, the intensive care infection score (ICIS) has been investigated for critically ill patients to differentiate between infectious and non-infectious processes [
2]. ICIS comprises five blood-cell-derived parameters: the mean fluorescence intensity of mature (segmented) neutrophils (NE-SFL), the difference in hemoglobin concentration between new (RET-H
e) and mature (RBC-H
e) red blood cells (Delta-H
e), total segmented neutrophil count, antibody-secreting lymphocytes, and immature granulocyte count [
3]. In clinical settings, physicians may encounter challenges in promptly establishing a diagnosis, often due to inconclusive clinical findings, insufficient diagnostic markers, or delays in obtaining microbiological results [
2,
4]. Measurement of acute-phase markers, like C-reactive protein (CRP), but especially procalcitonin (PCT), are time-consuming and costly [
5]. There is a need for new and reliable approaches for the rapid diagnosis of sepsis [
2]. Studies in humans have shown that the ICIS is potentially useful for the prediction of infection and its severity in critically ill patients [
3], and that it serves as a reliable marker with comparable or higher sensitivity and specificity than CRP and PCT [
2]. Furthermore, ICIS offers several advantages over conventional inflammatory markers such as CRP or PCT. One benefit is that no additional blood sample is needed, provided that a white blood cell (WBC) analysis has already been performed using a K
3EDTA sample. In this case, ICIS can be measured from the same tube. Another advantage lies in the low associated costs, as ICIS is measured on the same analyzer used for WBC assessment [
3].
In veterinary medicine, dogs are often presented to veterinary clinics due to a wide range of health issues. Inflammation is often the underlying cause of the condition. Canine WBC counts and their specific subpopulations can vary significantly during inflammation, primarily due to interactions between the immune system and the pathogen, as well as the host’s inflammatory response. Therefore, information about the leukogram, including WBC count, leukocyte differential, and morphological changes in leukocytes, provides valuable insights into the type and stage of inflammation in dogs, and has been used for decades to diagnose inflammation in the canine patient. The leukogram is moderately sensitive in detecting inflammation; therefore, a normal leukogram does not rule out inflammatory disease [
6]. Measurement of acute phase proteins, such as CRP in dogs, is a more sensitive test. CRP increases rapidly as part of a number of inflammatory diseases [
7,
8] and plays important roles by protecting against infection, clearance of damaged tissue, prevention of autoimmunization, and regulation of the inflammatory response [
9].
In contrast to human medicine, the evaluation of morphological WBC changes in dogs relies on the examination of a stained blood smear, which is time-consuming and depending on additional laboratory equipment (such as staining capabilities, a microscope) and trained staff. A more rapidly responding parameter for predicting inflammation in dogs, that could be analyzed faster and cheaper, would therefore be beneficial.
ICIS is currently not available for animal patients. However, individual parameters, such as mean fluorescence intensity of neutrophils (NE-SFL) and difference in hemoglobin concentration between immature and mature red blood cells (Delta-H
e), are available. Modern hematological analyzers, such as the Sysmex XN-V Series, are capable of measuring more parameters than the traditional complete blood cell count [
10]. In the white blood cell differential (WDF) channel of the Sysmex XN-1000V hematology device (Sysmex Corporation, Kobe, Japan), fluorescence flow cytometry is applied to measure neutrophilic granulocytes among other white blood cells. In this channel, cells are differentiated based on side fluorescent light (SFL), side scattered light (SSC), and forward scattered light (FSC) signals [
11]. Depending on individual cell characteristics, differences in these light and fluorescence signals might be detected and reported as NE-FSC, NE-SSC, and NE-SFL values. Information about the performance of these novel parameters in inflammatory or infectious and non-inflammatory diseases in dogs, and whether or not they can be an early and effective marker of inflammation, is lacking. A first study by O’Toole et al. suggests that NE-SSC, and particularly NE-SFL, may serve as promising markers of systemic inflammation in dogs and cats [
12].
The aim of this study was to evaluate retrospectively three novel neutrophilic granulocyte parameters NE-SFL, NE-SSC, and NE-FSC in healthy dogs and dogs with and without inflammatory diseases, and to compare these parameters with established inflammatory markers such as CRP, WBC count, the presence of bands, and toxic changes.
For this, a control group of healthy dogs was determined (1) and different disease groups were established (septic dogs, dogs with pyometra, steroid-responsive meningitis-arteritis (SRMA) and idiopathic epilepsy) to compare NE-SFL, NE-SSC and NE-FSC values with traditional inflammatory markers (CRP concentration, total WBC count, presence of bands and neutrophilic toxic changes) (2).
2. Materials and Methods
2.1. Novel Neutrophilic Granulocyte Parameters NE-SFL, NE-SSC, and NE-FSC from Sysmex XN-1000V
The Sysmex XN-1000V veterinary hematology analyzer (Sysmex Corporation, Kobe, Japan) is the latest hematological high-throughput device on the veterinary market and is derived from the Sysmex XN Series technological platform. It is the successor of the Sysmex XT-2000iV and is characterized by important new features, including an optic-fluorescent analysis for platelets (platelet fluorescence channel, using an oxazine-based fluorescent dye), the counting of nucleated red blood cells in the WNR-channel [
13] with every complete blood count and a body fluid application to measure fluids such as cerebrospinal and bronchoalveolar lavage fluids [
14,
15,
16]. Generally, the WDF channel differentiates leukocytes and therefore counts neutrophils, lymphocytes, monocytes, and eosinophils through fluorescence flow cytometry, utilizing a red semiconductor laser with a wavelength of 633 nm [
11]. In this process, leukocytes are first permeabilized using a surfactant and then stained with a fluorescent polymethine dye, which binds to cytoplasmatic organelles and nucleic acids (DNA and RNA) [
17]. By doing this, three signals are recorded in the white blood cell differential scattergram: side fluorescent light (SFL, DNA/RNA content), side scattered light (SSC, granularity), and forward scattered light (FSC, cell size) [
18]. In the same process, novel parameters, such as the NE-FSC, NE-SSC, and NE-SFL, are determined as research parameters that could be displayed on customizable User Screens within the instrument’s Browser section of the IPU.
NE-SFL is considered an indicator of neutrophil activation, as the fluorescent dye binds to nucleic acids within both cytoplasmic organelles and the nucleus in activated cells [
17,
19]. Its intensity corresponds to the composition and quantity of nucleic acids and cellular organelles [
20].
NE-SSC measures the nucleus and internal granularity of neutrophils [
21]. When neutrophil complexity increases due to functional changes, such as toxic granulation or vacuolization, their position in the scattergram shifts accordingly [
22].
The NE-FSC signal detects cell size [
21]. Activated neutrophils may show an abnormal cell size, leading to an altered FSC signal [
23].
The positioning of the neutrophil granulocyte population in the scattergram allows an evaluation of the activity of the neutrophils [
22] and generates the parameters NE-SFL, NE-SSC, and NE-FSC.
Prior to the measurement of study samples, internal quality control was performed once daily by measuring control material provided by the manufacturer (XN-Check L1-L3). The controls were processed like patient samples and measured on a properly calibrated and fully functional device in quality control mode. The measurement results must fall within the assay ranges specified on the assay sheet. Control materials were stored at a temperature of 2–8 °C and warmed up to room temperature for analysis.
2.2. Blood Smear Evaluation
Blood smear preparation was performed within one hour after sample collection by trained laboratory technicians. The smears were air-dried for 20 min before staining. Staining was performed on a HEMA-TEK 2000 slide stainer (Siemens Healthcare GmbH, Erlangen, Germany) with modified Wright’s stain. Two experienced laboratory technicians performed a 200 WBC differential by microscopic blood smear evaluation, using a Leica DM LB2 microscope (Leica Microsystems AG, Heerbrugg, Switzerland) with 50× and 100× oil immersion objectives. Relative and absolute numbers of bands were determined. Bands were defined with unsegmented and smooth nuclear walls and immature chromatin structure. The laboratory’s reference interval ranged from 0 to 0.084 × 103/µL.
Additionally, the quantity and degree of toxic changes in neutrophils were assessed semiquantitatively. The presence of Döhle bodies, foamy cytoplasm, basophilic cytoplasm and toxic granulation indicated toxicity. The quantity and the degree were judged according to the grading scheme of Harvey [
24]. Toxic changes in neutrophils were not observed in the healthy control group.
2.3. Measurement of Canine C-Reactive Protein (CRP)
CRP concentrations were determined on a fully automated chemistry analyzer (Cobas C 501, Roche Diagnostics, Rotkreuz, Switzerland) using two commercially available test kits validated for the canine species (Randox Laboratories Ltd., Crumlin, UK and Gentian Canine CRP Immunoassay, Orlando, USA), which have been previously validated [
25,
26]. The cut-off value for healthy control dogs was ≤10.7 mg/L (Randox Laboratories Ltd., UK).
The study population was retrospectively assembled from cases between 2018 and 2024. Until December 2022, CRP concentrations were measured using the Randox assay; from January 2023 onward, the Gentian assay was used. The cut-off values applied were >10.7 mg/L for Randox and >10.2 mg/L for Gentian.
2.4. Establishment of a Control Group for NE-SFL, NE-SSC, and NE-FSC
In the first part of this study, 21 healthy dogs were prospectively selected to establish a control group for the novel neutrophilic granulocyte parameters. The following criteria had to be met: the dogs had to be anamnestically and clinically healthy, free of medication for at least one month, with CRP concentration, WBC count, and neutrophil cell count within the reference range and absence of left shift and toxic changes. To ensure this, dog owners were interviewed about their pets’ health status, medication history, and additional details, such as breed, age, gender, and neuter status, were recorded. Dogs that did not meet these criteria or had values outside the reference ranges were excluded from the healthy control group. Neither gender nor neuter status served as exclusion criteria for the healthy dogs; however, they had to be at least one year old to eliminate the influence of age-related blood changes observed in younger dogs [
27]. Greyhounds and Greyhound mix breeds were excluded from the study due to their well-documented clinicopathologic differences, particularly in hematologic profiles, which distinguish them from other dog breeds [
28].
All healthy dogs of the control group were privately owned, and their owners provided written consent for study participation. Approval from the Cantonal Veterinary Office (ZH 057/19, Zurich, Switzerland) was obtained prior to conducting the study. Before blood sampling, each dog underwent a brief physical examination. Venous blood was then collected using a 20-gauge (0.9 × 25 mm) needle from either the cephalic vein (Vena cephalica antebrachii) of the front leg or the saphenous vein (V. saphena) of the hind leg, without sedation. If necessary, the puncture site was shaved and disinfected beforehand. Blood was drawn into sterile K3EDTA tubes (1.3 mL) and serum tubes (4 mL). After 30 min, whole blood samples were centrifuged at 2000× g (Rotina 380 R, Hettich, Bäch, Switzerland), and the serum was subsequently collected. All blood samples were analyzed within four hours at the Clinical Laboratory of the Vetsuisse Faculty, University of Zurich, Switzerland. A complete blood count, including WBC differentiation, was performed using the Sysmex XN-1000V hematology analyzer (Sysmex Corporation, Kobe, Japan), and blood smears were evaluated for the presence of platelet clumps and morphological abnormalities. CRP concentrations were determined using a canine CRP assay (Randox Laboratories Ltd., UK).
2.5. Data Collection for Study Group
In the second part of this study, the performance of the novel neutrophilic granulocyte parameters in inflammatory and non-inflammatory diseases was retrospectively evaluated in a total of 84 diseased dogs, comprising the study population. The study population was organized into two main groups. Group 1 covered dogs with non-inflammatory diseases and contained 20 dogs with idiopathic epilepsy. Group 2 enclosed dogs with inflammatory diseases, and the corresponding subgroups contained 23 dogs with SRMA, 20 dogs with pyometra, and 21 dogs with sepsis or septic abdomen.
The selection of animals in the study group was performed retrospectively between January 2018 and May 2024, based on the diagnosis of board-certified specialists. Inclusion criteria for all subgroups were as follows: (1) CRP concentration were measured, (2) WBC count and the novel neutrophilic granulocyte parameters (NE-SFL, NE-SSC, and NE-FSC) were measured on the Sysmex XN-V hematology analyzer, and (3) WBC differentiation (including count of bands) and toxic changes (quantity and degree) were evaluated manually on stained blood smears. All parameters were analyzed on the day of admission to the clinic for small animals, Vetsuisse Faculty, University of Zurich, Switzerland. Dogs were excluded if any data was lacking.
The diagnosis of idiopathic epilepsy was based on the anamnesis (and ruling out other possible causes of seizures), neurological exam, and further diagnostics, like analysis of the cerebrospinal fluid or results from MRI/CT. The diagnosis of SRMA was based on clinical signs (neck stiffness, neck pain and fever without neurological deficits), cerebrospinal fluid analysis and a positive response to corticosteroid therapy. The diagnosis of pyometra was based on bacteriological culture of uterine contents, with additional support from hematological and biochemical findings, as well as abdominal imaging (ultrasound and/or radiographs). Diagnosis of sepsis was based on the following two criteria: (1) Having two or more systemic inflammatory response syndrome (SIRS) criteria. The SIRS-criteria utilized were: temperature <38.1 °C/>39.2 °C, heart rate >120/min., respiratory rate >20/min., WBC count <6 × 10
3/µL or >16 × 10
3/µL or bands >3% [
29]. (2) Testing positive for bacterial culture in an abdominal organ (excluding the urogenital tract) or the peritoneal cavity.
Medical records of all subgroups were extracted from the clinical information system (Vetera GmbH, Eltville am Rhein, Germany). Blood analysis results were extracted from the laboratory information systems (Vianova, Clinisys, Gent, Belgium), along with hematology data from the Sysmex XN-1000V instrument of the Clinical Laboratory of the Vetsuisse Faculty of Zurich, Switzerland.
2.6. Manual Gating
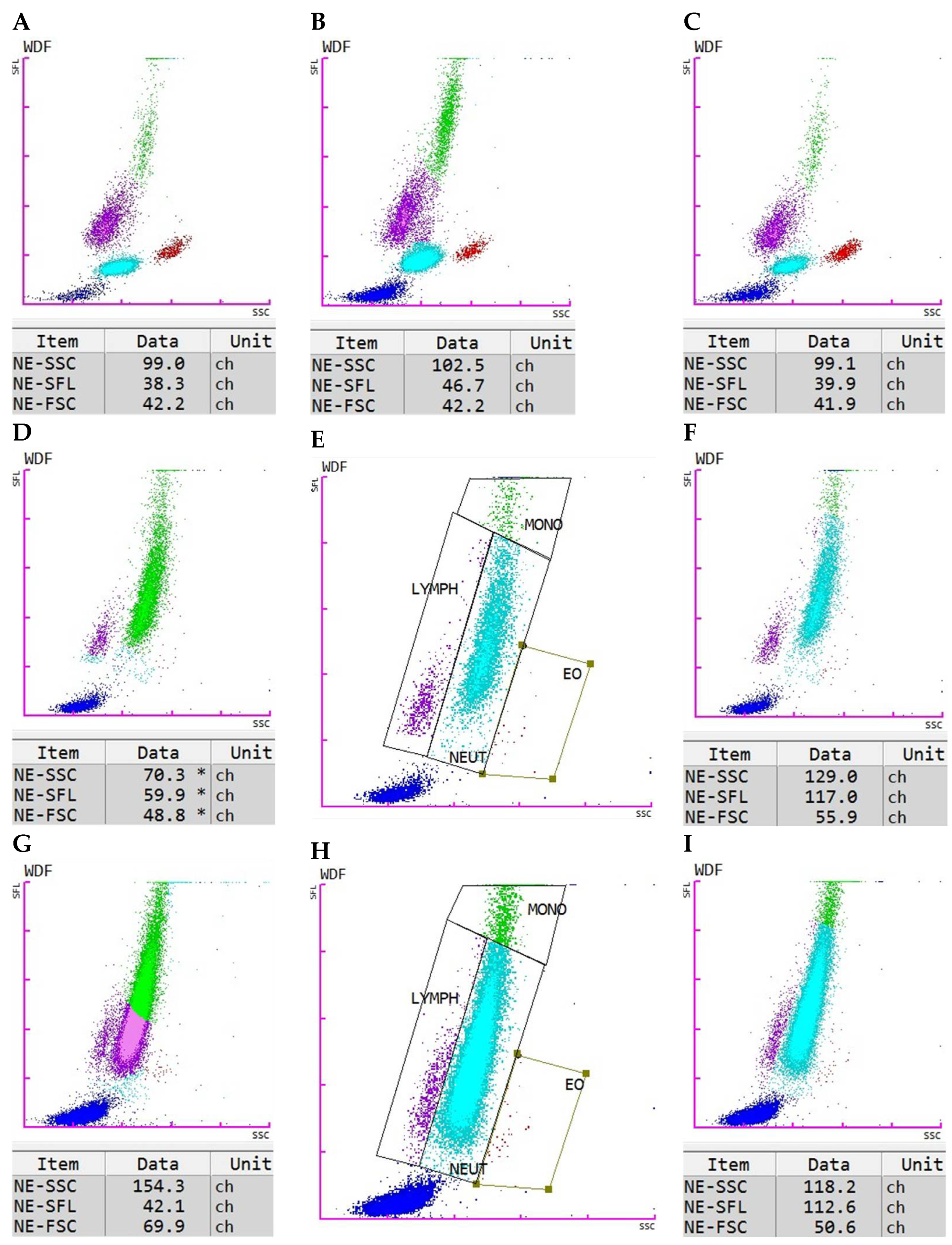
When the notification “WBC Abn Scattergram” is given by the Sysmex XN-V with the result report, it points towards an abnormal pattern of the cell clusters displayed in the scattergram. General reasons could be alterations in cell morphology, presence of immature, reactive, atypical, or neoplastic cells, as well as the occurrence of interfering conditions (e.g., marked platelet clumping). In our case, most abnormal scattergram flags occurred due to the presence of immature neutrophilic granulocytes in the sample or because the neutrophils showed cytoplasmic toxicity. In some cases, these neutrophils were misclassified as monocytes or monocytes and lymphocytes. For these cases, manual gating was performed using the instrument’s Manual Analysis software tool (XN-1000V, software version 3.07). Applying the new gates corrected the cell count, as well as the respective neutrophil values NE-SFL, NE-SSC, and NE-FSC. The newly created gates were saved as a new analysis profile to reapply them to similar cases. This approach eliminated the need to perform gating from scratch for each sample; instead, only a cross-check was required to ensure that the cell clusters were properly positioned within the applied gates. If not, the respective gates could be adjusted as needed.
2.7. Statistical Analysis
The statistics of the control group were generated using the Analyse-it (version 6.15, Analyse-it Software Ltd., Leeds, UK), an add-in for Microsoft Excel (version 2021, Microsoft Corporation, Redmond, WA, USA). For the control group, minimum, maximum, and median values were calculated for Ne-SFL, NE-SSC, and NE-FSC.
Statistical analysis for the study groups and comparisons between the study groups and the healthy control group were performed using GraphPad Prism 10 (version 10, GraphPad Software, San Diego, CA, USA). To test small sample sizes (<50) for normal distributions, the Shapiro–Wilk test is recommended [
30] and was used. It demonstrated that the data of all groups were mostly not normally distributed; therefore, nonparametric tests were used [
31]. Group comparisons between all five groups were performed using the Kruskal–Wallis test (p
KW), followed by Dunn’s post hoc test (p
Dunn) [
31]. Spearman’s correlation coefficient (p
S) was used [
32] to assess the correlations between NE-SFL, NE-SSC, NE-FSC, and other hematological parameters, namely the WBC count, CRP concentration, count of bands, and toxic changes. The strength of the correlation was interpreted as follows: coefficients between 0.0 and 0.1 were considered to indicate no correlation, 0.1 to 0.3 a low correlation, 0.3 to 0.5 a moderate correlation, 0.5 to 0.7 a strong correlation, and values above 0.7 a very strong correlation [
33]. For all calculated
p-values (p
KW, p
Dunn, p
S,), a significance threshold of
p < 0.05 was applied.
Descriptive statistics for the study groups (median and interquartile range) were calculated using Microsoft Excel (version 2021, Microsoft Corporation, Redmond, WA, USA) with built-in formulas.
4. Discussion
This study aimed to evaluate the diagnostic potential of the novel neutrophilic granulocyte parameters—NE-SFL, NE-SSC, and NE-FSC—measured by the Sysmex XN-1000V hematology analyzer, in distinguishing between inflammatory and non-inflammatory conditions in dogs. Our results demonstrate that these parameters, especially NE-SFL and NE-SSC, have promising value as indicators of inflammation in dogs. The establishment of a control group of healthy dogs for NE-SFL, NE-SSC, and NE-FSC represents a crucial first step toward the introduction of these parameters into clinical veterinary diagnostics. The defined value ranges provide a baseline for comparison and serve as an initial orientation to guide future studies in assessing their clinical significance. Notably, control samples did not require manual gating, supporting the robustness of these measurements in healthy dogs.
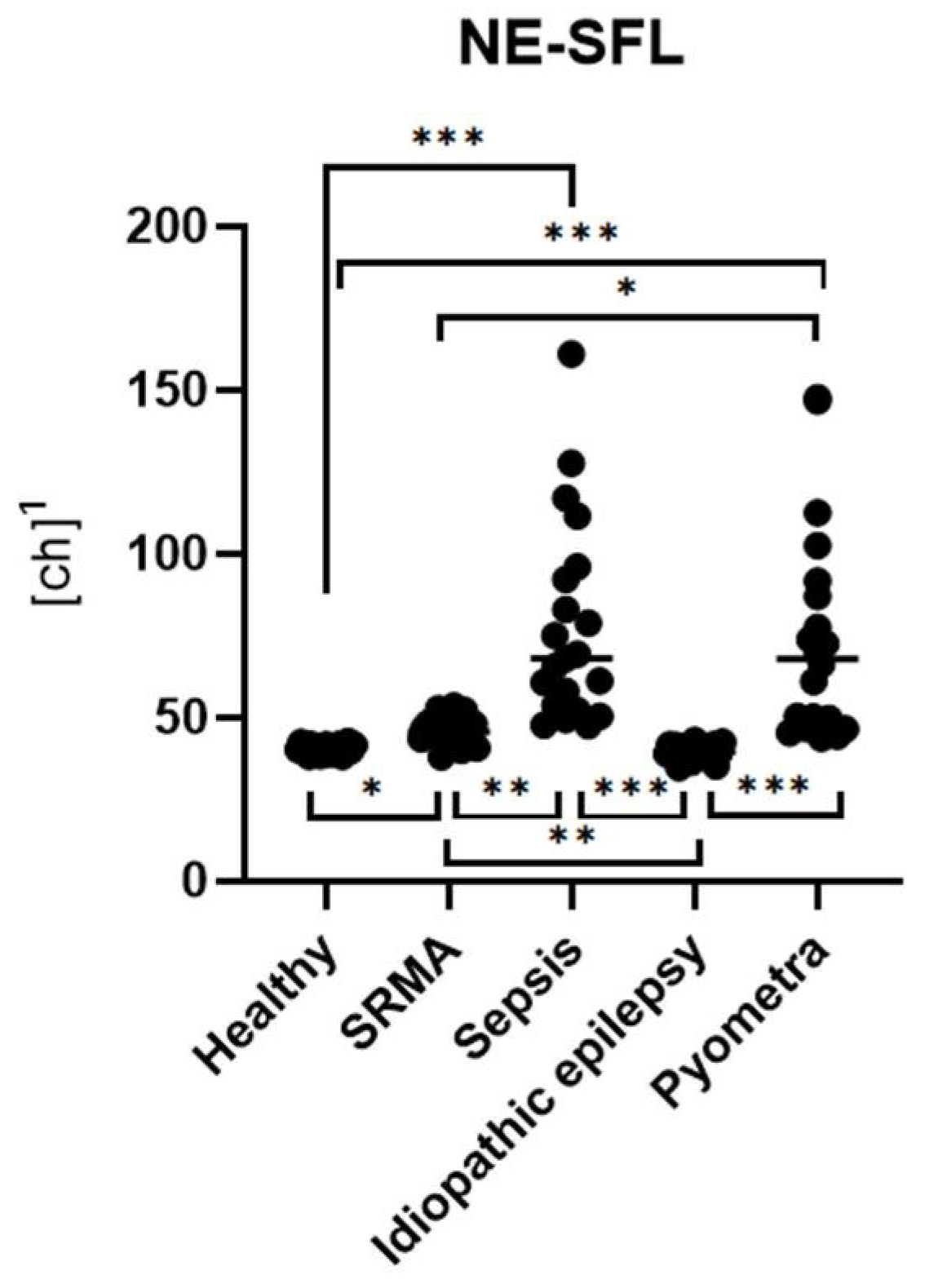
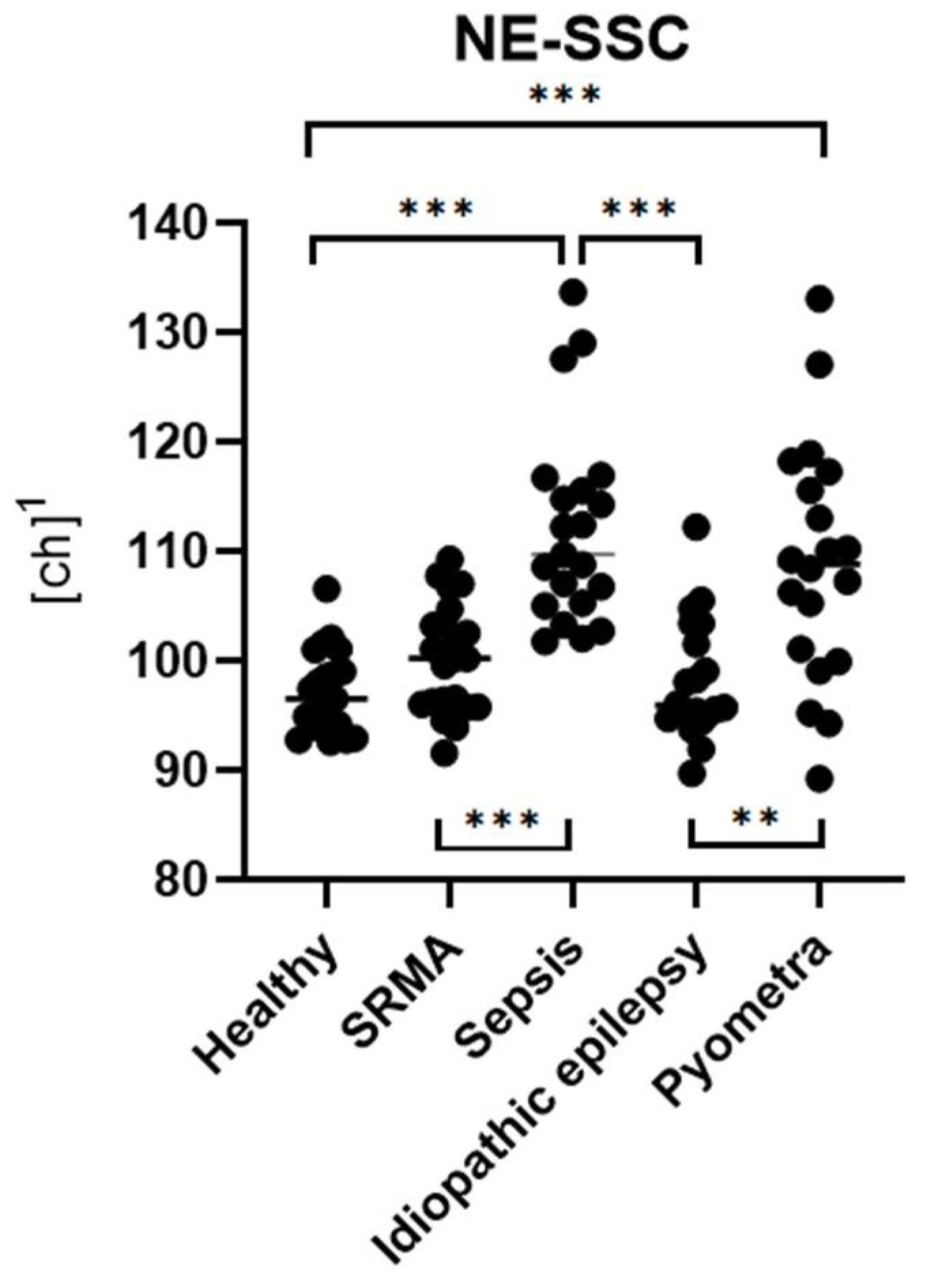
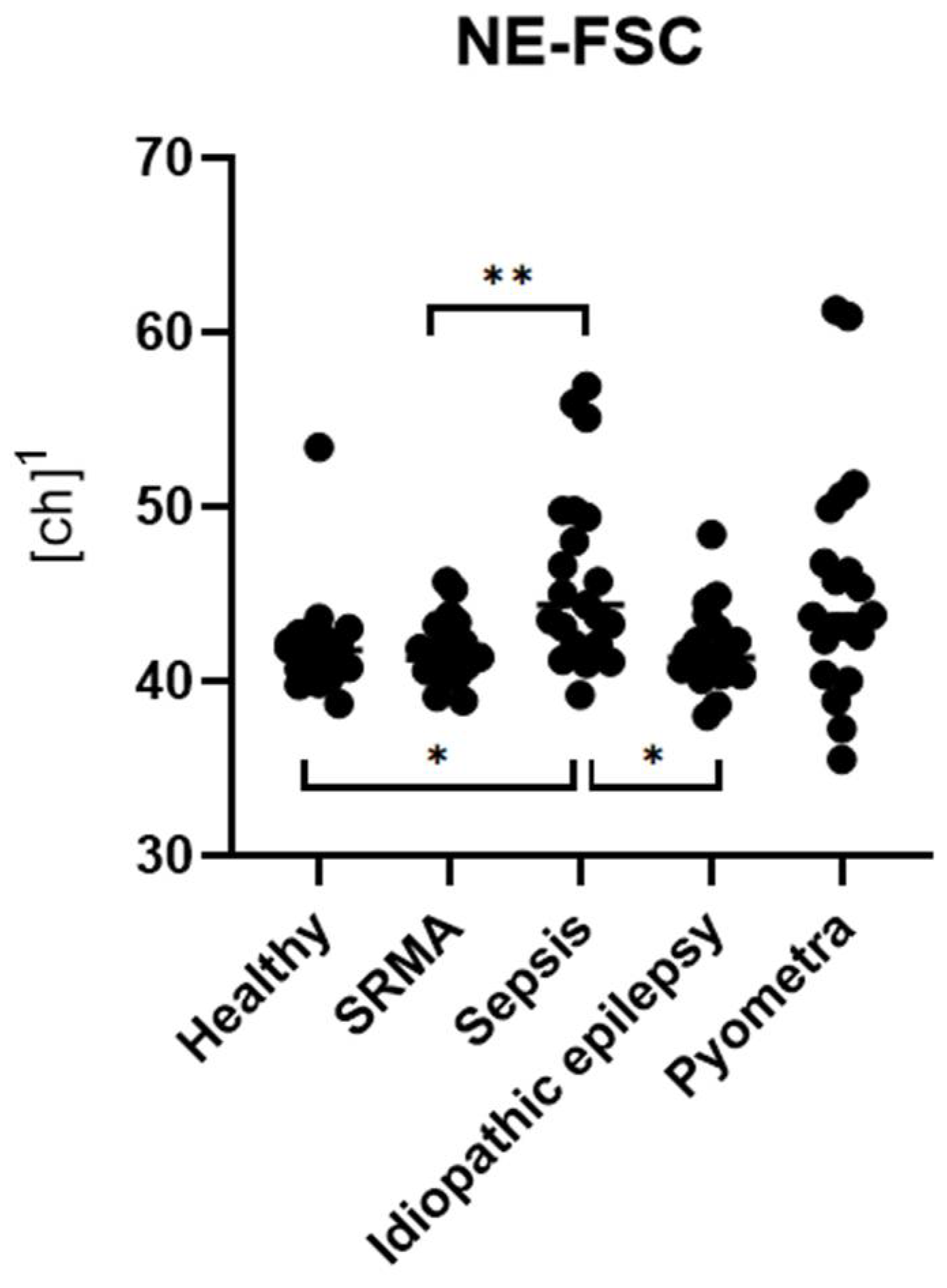
In the clinical part of the study, dogs diagnosed with inflammatory diseases, such as sepsis and pyometra, showed significantly higher levels of NE-SFL and NE-SSC compared to healthy controls. Furthermore, these parameters were also significantly increased relative to non-inflammatory cases (idiopathic epilepsy). NE-FSC was only significantly increased in the sepsis group, suggesting its utility might not be very specific to certain types or severities of inflammation.
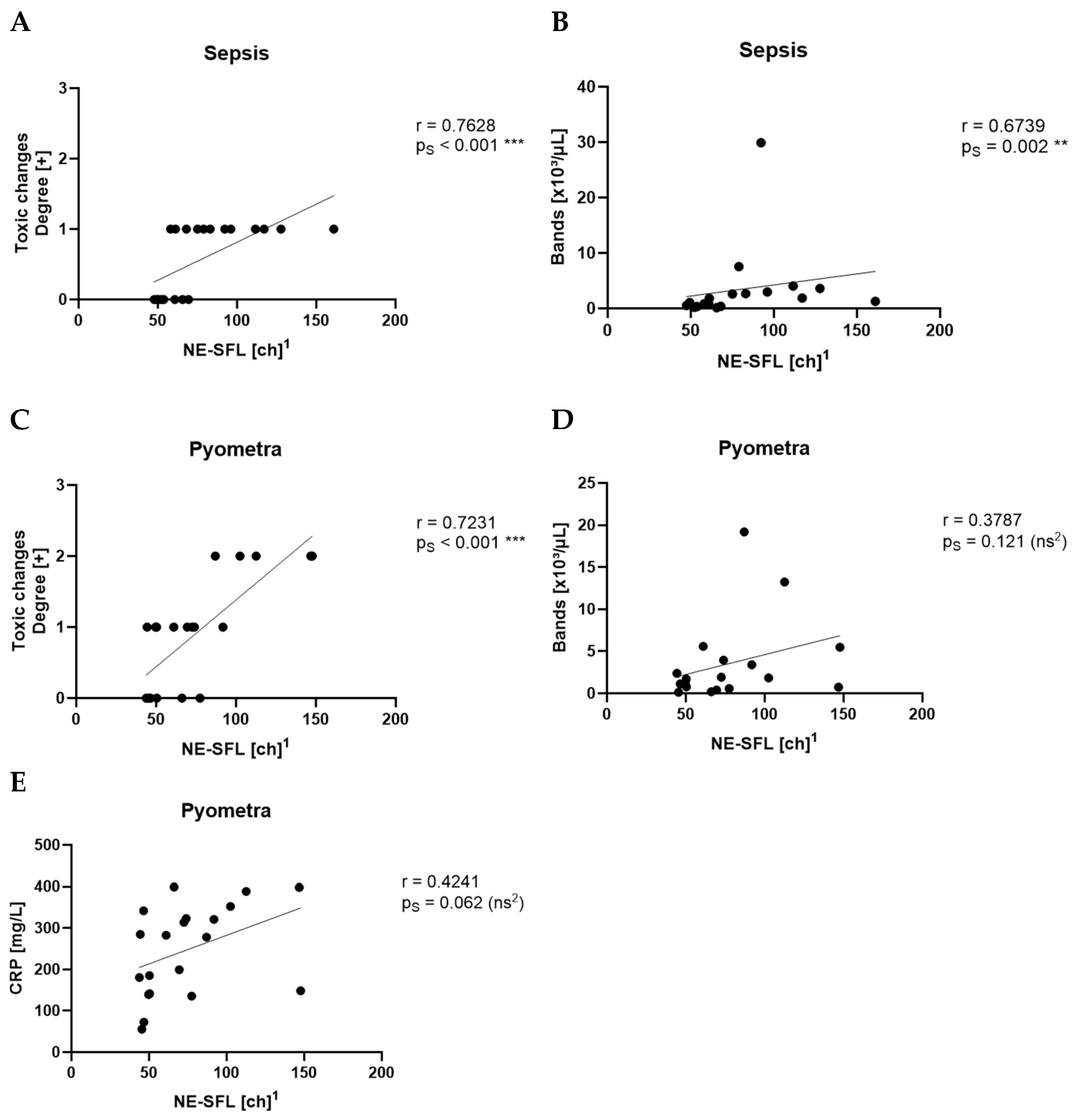
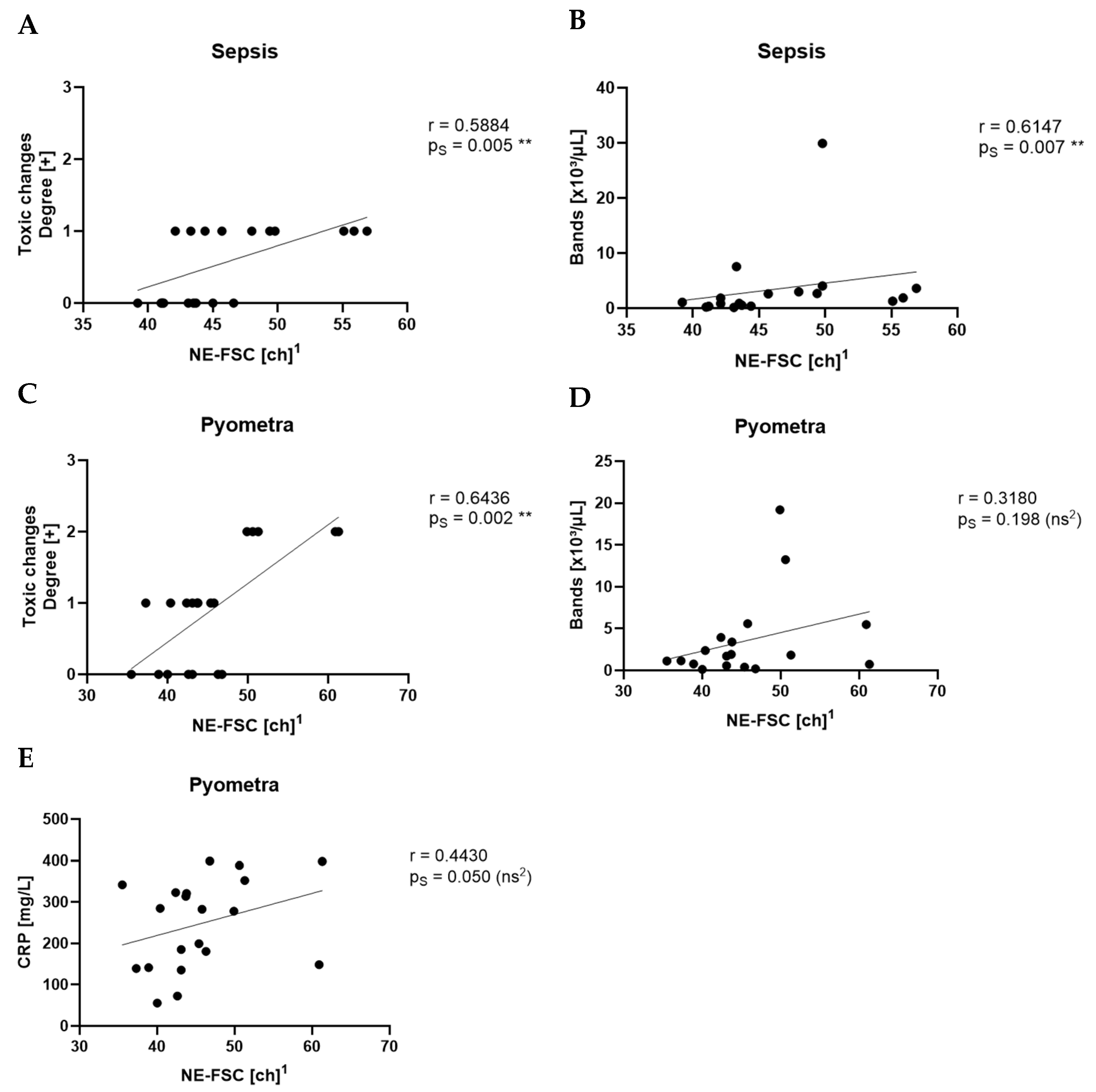
Among the three parameters, NE-SFL showed the most consistent and significant differentiation across the tested disease groups. It was elevated in all inflammatory conditions and showed clear separation from both healthy controls and dogs with idiopathic epilepsy. This supports previous findings in human medicine, where NE-SFL, as part of the ICIS, has been shown to significantly discriminate between patients with infection and patients without infection [
2,
3]. The fluorescence signal reflects increased nucleic acid content due to enhanced cellular activity and correlates well with the presence of toxic changes in neutrophils, which are commonly seen during inflammation [
34], and increased numbers of bands. It also aligns with previous findings, which showed that NE-SFL was significantly increased in dogs with systemic inflammation and positively correlated with CRP concentrations [
12]. In contrast to the study by O’Toole et al. the present study showed that Ne-SFL and CRP concentrations did not correlate. This might be due to the low number of animals within each disease group, whereas O’Toole et al. included various diseases in one large group. This study further distinguishes itself by incorporating manual gating of samples, focusing on smaller yet clearly defined disease groups, and including a healthy control group for comparison.
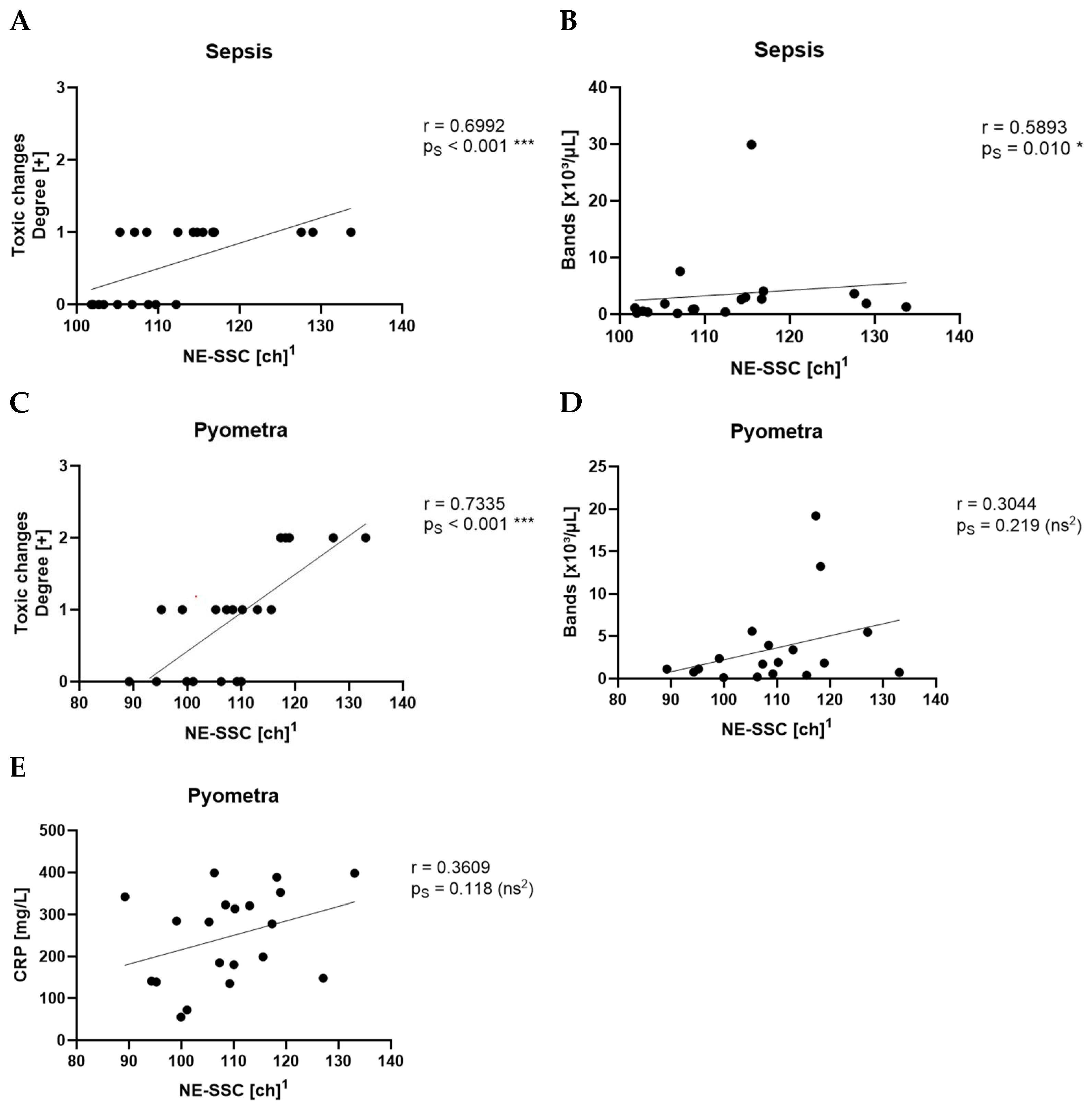
NE-SSC, reflecting the granularity and internal complexity of neutrophils, was also significantly elevated in inflammatory conditions. This is supported by the fact that NE-SSC has shown a strong correlation with toxic changes in the pyometra and sepsis groups, suggesting that structural and morphological changes in neutrophils accompany activation and toxic transformation.
NE-FSC, which correlates with neutrophil size, was only elevated in the sepsis group but not in pyometra, SRMA or idiopathic epilepsy cases. This could imply that NE-FSC is more reflective of neutrophil swelling or cellular enlargement, a change possibly more characteristic of bacterial infections. This would be consistent with the fact that NE-FSC correlated with toxic changes and the count of bands in the sepsis and pyometra groups. Its lack of elevation in SRMA cases suggests that NE-FSC may not be as sensitive in detecting sterile, immune-mediated inflammation. Dogs with idiopathic epilepsy, representing the non-inflammatory group, exhibited NE-SFL, NE-SSC, and NE-FSC values within the value ranges of the control group. This confirms that the novel neutrophil parameters are not elevated in this non-inflammatory disease, supporting their specificity for inflammatory processes.
The necessity for manual gating in cases of pyometra and sepsis due to abnormal scattergram flags highlights a practical challenge. In severe inflammatory states, increased cytoplasmic toxicity and the presence of bands may disrupt automated classification, requiring manual correction for accurate analysis. This requirement may limit practical application of these parameters in emergency settings since trained staff with the right expertise would be needed to perform the manual gating. Further refinement of automated analysis algorithms or the careful application of the software’s manual gating options are crucial to improve the accuracy and efficiency of these measurements and, therefore, reduce this issue in the future.
The results also align with the current trend in both human and veterinary medicine toward rapid diagnostics that can reduce dependence on time-consuming, costly tests like CRP and PCT. Unlike CRP or PCT, these novel parameters are obtained from the routinely performed WBC analysis using the Sysmex XN-1000V analyzer, thereby eliminating the need for additional blood sampling and providing a practical advantage in routine diagnostics, which represents a step forward in veterinary hematological diagnostics.
A key limitation of this study is the relatively small sample size for each disease category and the healthy control group, which may affect the statistical power and generalizability of the results. Future studies with larger cohorts and a broader range of inflammatory conditions are needed to further validate the diagnostic performance and to establish reference values for these novel parameters. Additionally, longitudinal studies assessing changes in NE-SFL, NE-SSC, and NE-FSC over time in response to treatment could provide insights into their potential role in monitoring disease progression and therapeutic efficiency.
Another potential area of research is the comparison of these novel parameters with existing inflammatory markers in a prospective clinical setting. Evaluating their sensitivity and specificity in combination with traditional diagnostics could help establish optimal diagnostic algorithms for inflammatory diseases in veterinary medicine. Furthermore, investigating their utility in distinguishing bacterial from non-bacterial inflammations could contribute to more targeted antibiotic usage, addressing concerns related to antimicrobial resistance. Future research should also focus on evaluating these novel parameters in juvenile dogs (<1 year) and in Greyhounds.
Additionally, in the future, Delta-H
e should also be the target of a similar study. Since it is part of the ICIS in human diagnostics, there might be some potential use in veterinary medicine too. Delta-H
e is the difference between reticulocyte (Ret-H
e) and erythrocyte hemoglobin content (RBC-H
e) [
35]. In human medicine, a negative value for Delta-H
e indicates an impaired hemoglobinization of newly formed reticulocytes compared with the hemoglobin content of mature erythrocytes. This suggests that an insufficient iron supply for hemoglobin synthesis could be due to a high-grade inflammation [
36]. In veterinary medicine, Delta-H
e is especially investigated for its role in differentiating between chronic hemorrhagic and chronic inflammatory anemia, confirming its value as an inflammatory marker similar to humans [
37].