Physiological Responses to Thermal Stress in the Liver of Gymnocypris eckloni Revealed by Multi-Omics
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Heat Acclimation
2.2. Histological Analysis
2.3. mRNA and Small RNA Sequencing
2.4. Identification and Functional Enrichment Analysis of Differentially Expressed Genes, miRNAs, and miRNA Targets
2.5. Quantification of Genes and Statistical Analysis
2.6. UHPLC-MS/MS Analysis, Metabolite Identification, and Data Analysis
2.7. Interaction Analysis of mRNA, miRNA, and Metabolite
2.8. Statistical Analysis and Pathway Analysis
3. Results
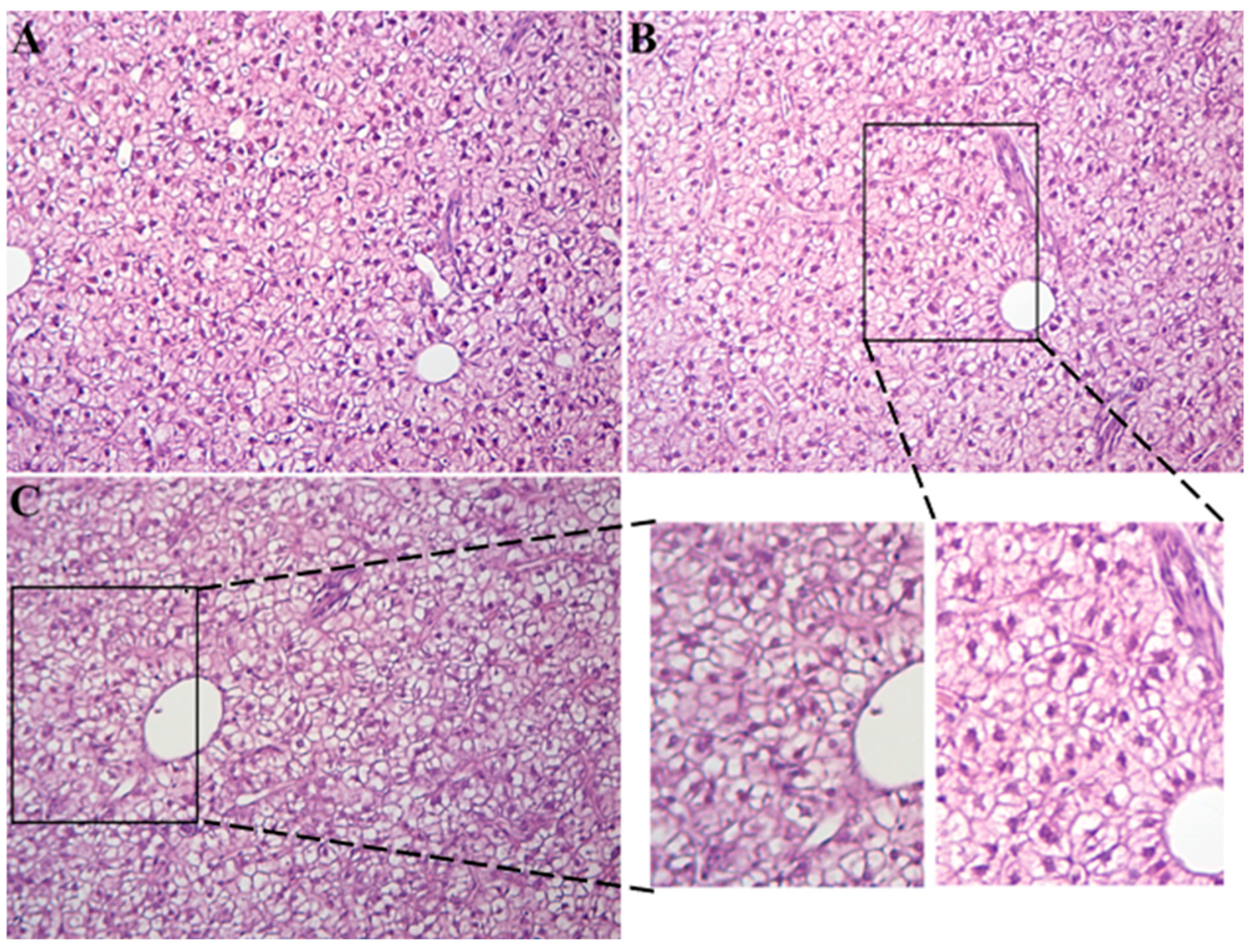
3.1. Fish Growth Parameters and Histopathological Change
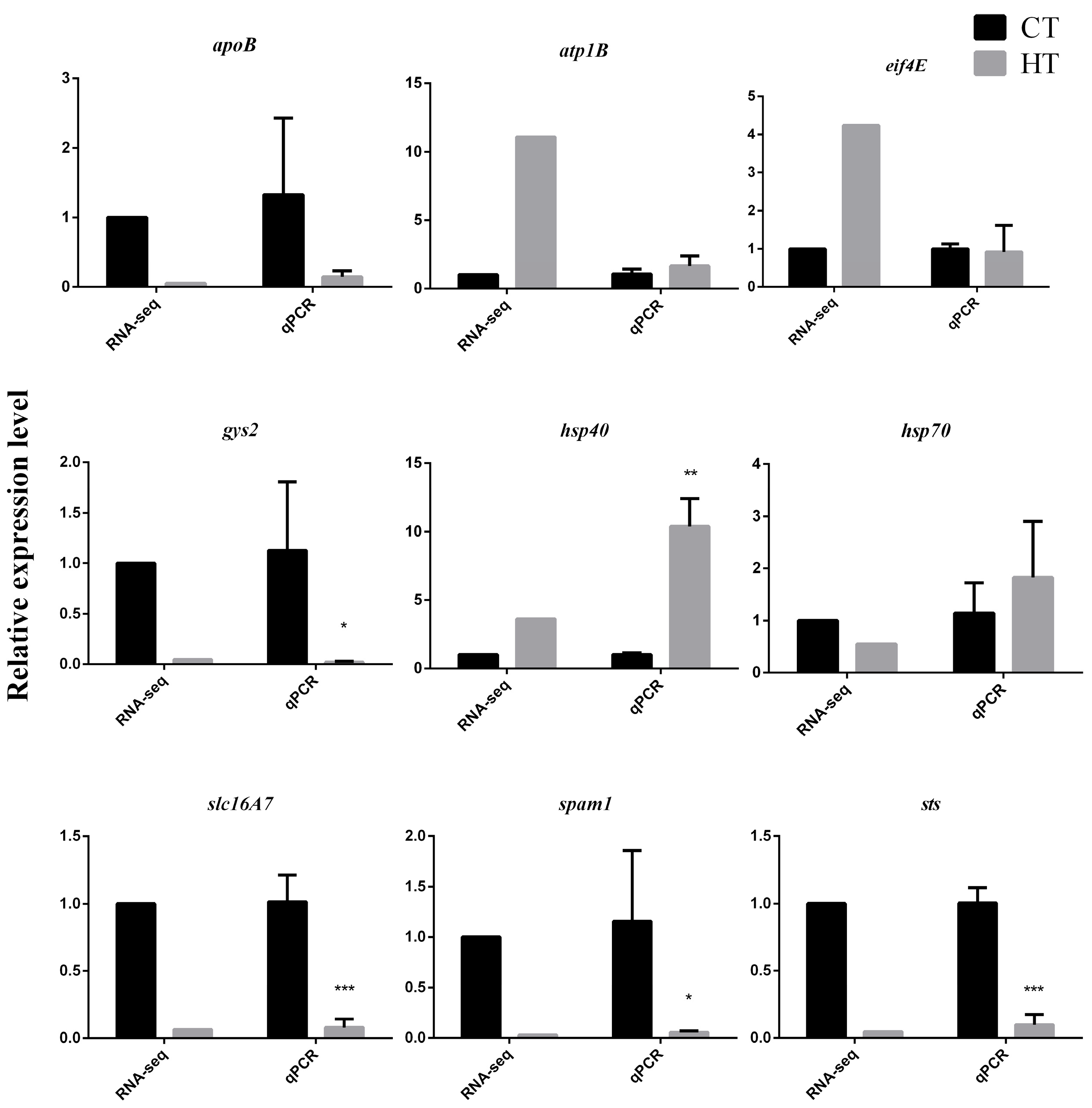
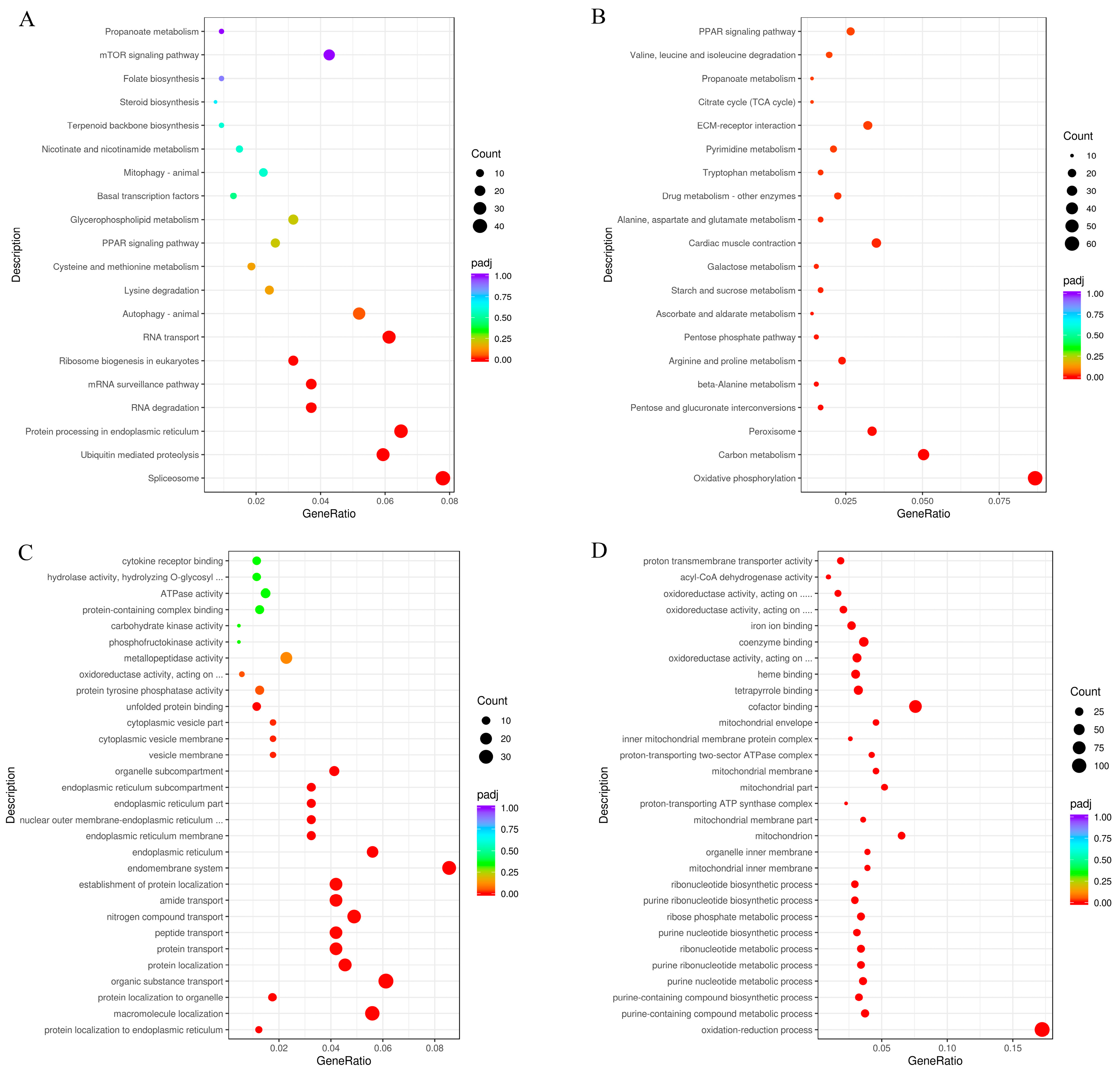
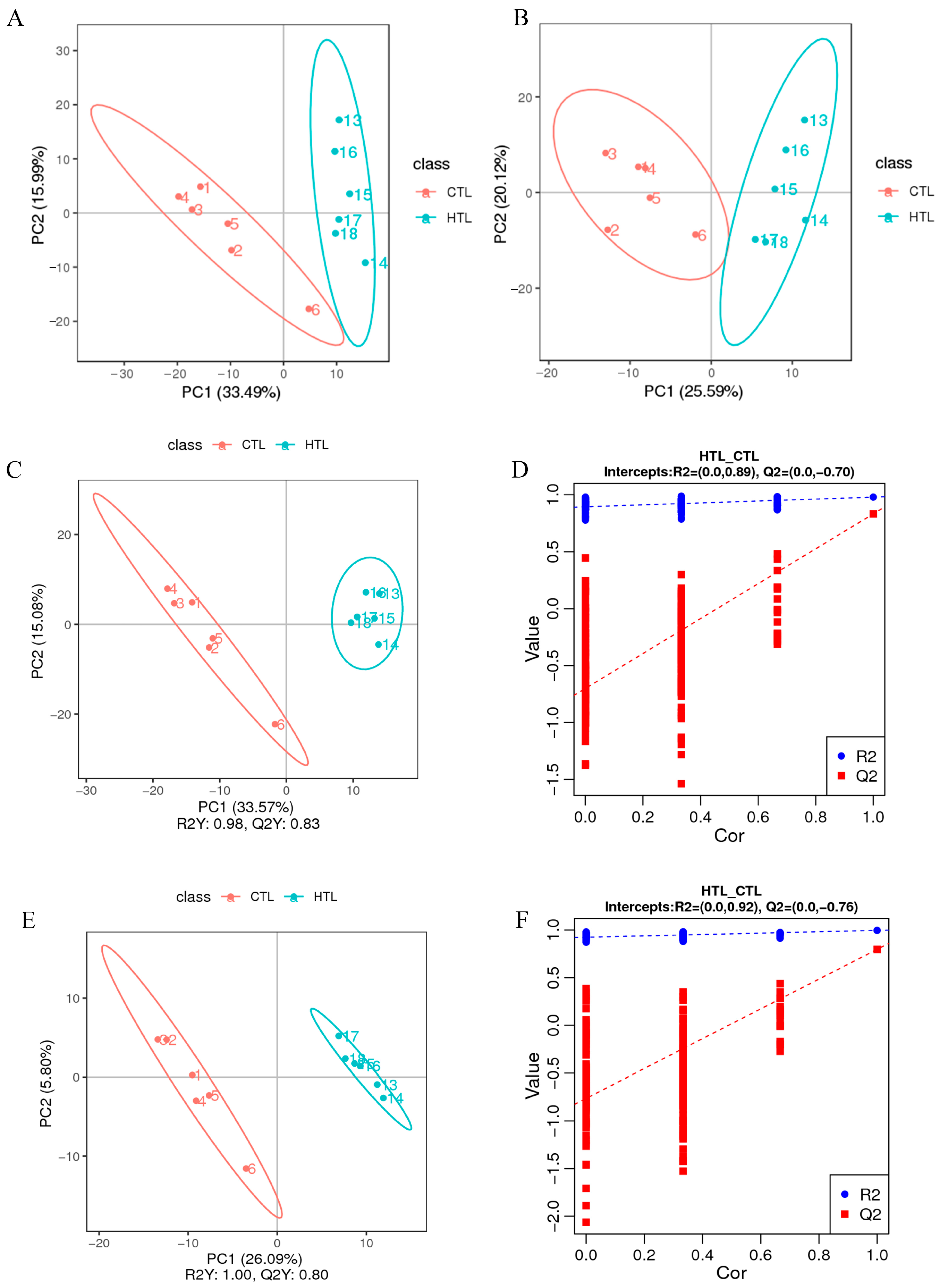
3.2. RNA-Seq, Small RNA-Seq, and Analysis
Pathways Involving DEGs and miRNA-Targets
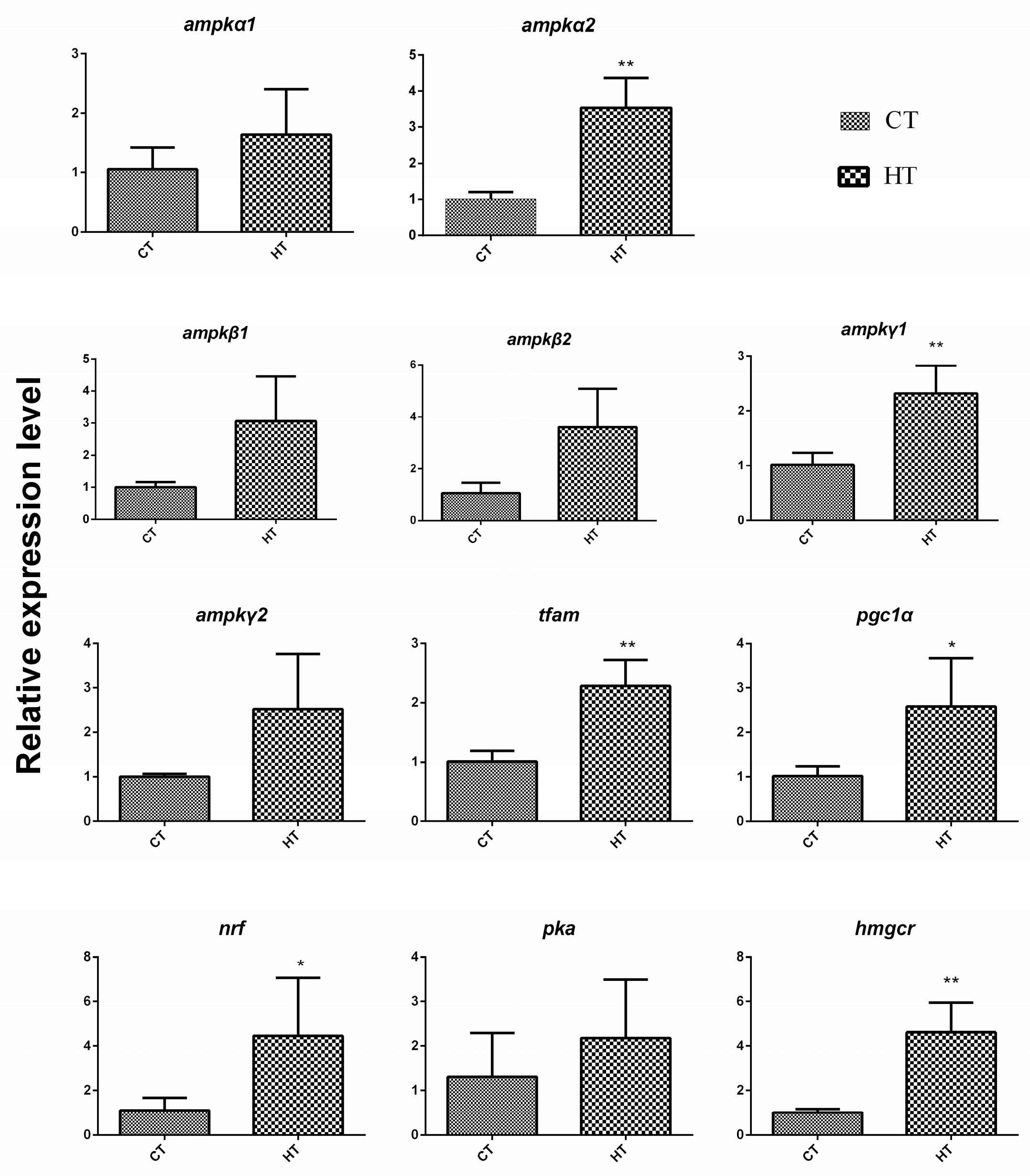
3.3. Integrated Analysis of DEGs and DEMiRs, and Potential miRNA-mRNA Regulatory Networks
3.4. Metabolite Profiling in Response to Thermal Stress
Differential Metabolites and Metabolic Pathways in Response to Thermal Stress
3.5. Integrated Analysis of DEMs and Transcriptome
4. Discussion
4.1. Growth Parameters Comparison and Histopathological Change
4.2. Transcriptional Response to Thermal Stress
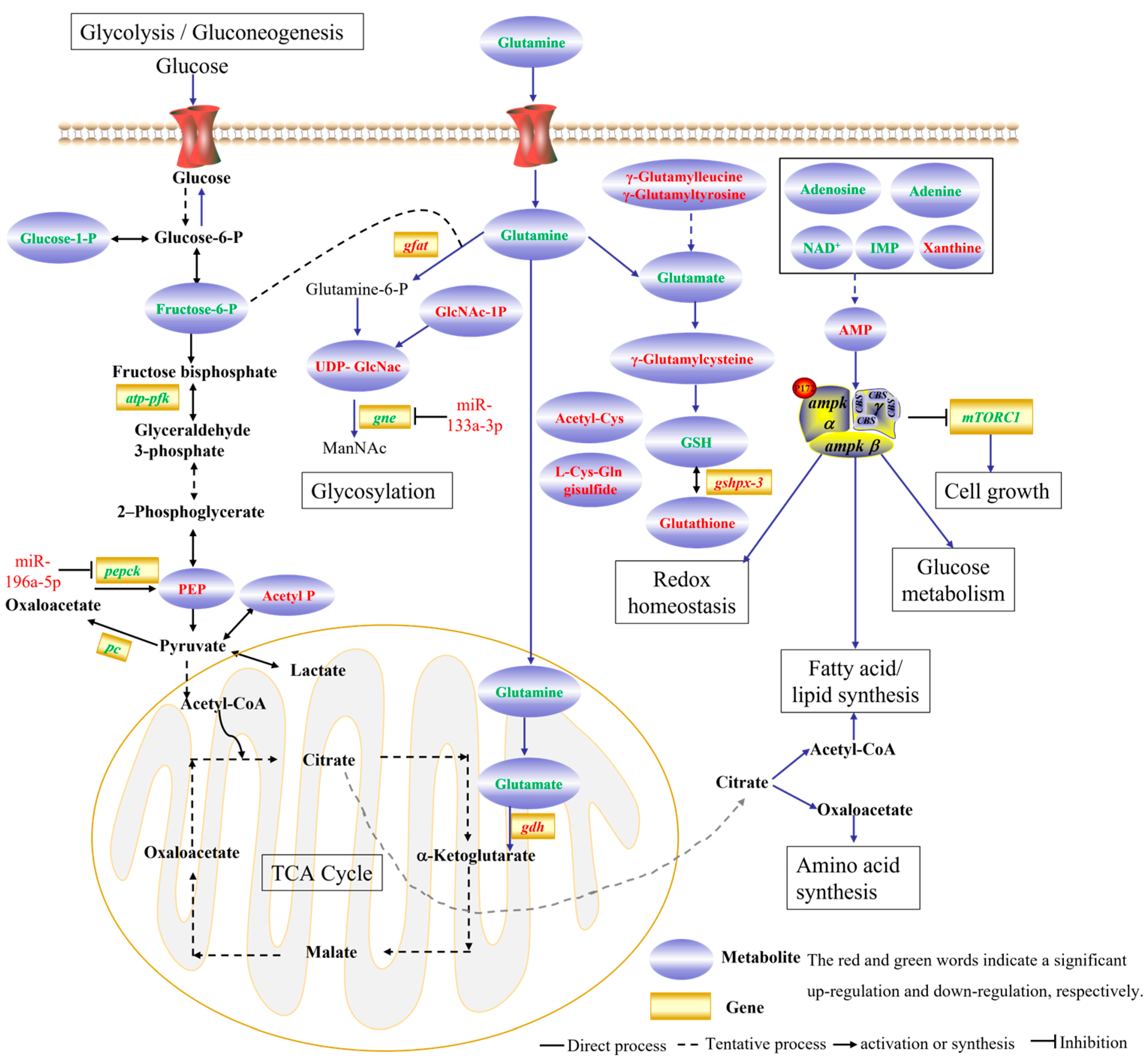
4.3. Metabolic Response to Thermal Stress
4.4. Regulation Network in Response to Chronic Thermal Stress in G. eckloni
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soomro, S.-E.-H.; Shi, X.; Guo, J.; Ke, S.; Hu, C.; Asad, M.; Jalbani, S.; Zwain, H.M.; Khan, P.; Boota, M.W. Are global influences of cascade dams affecting river water temperature and fish ecology? Appl. Water Sci. 2023, 13, 106. [Google Scholar] [CrossRef]
- Dadras, H.; Dzyuba, B.; Cosson, J.; Golpour, A.; Siddique, M.A.M.; Linhart, O. Effect of water temperature on the physiology of fish spermatozoon function: A brief review. Aquac. Res. 2017, 48, 729–740. [Google Scholar] [CrossRef]
- Menon, S.V.; Kumar, A.; Middha, S.K.; Paital, B.; Mathur, S.; Johnson, R.; Kademan, A.; Usha, T.; Hemavathi, K.N.; Dayal, S.; et al. Water physicochemical factors and oxidative stress physiology in fish, a review. Front. Environ. Sci. 2023, 11, 1240813. [Google Scholar] [CrossRef]
- Liu, T.Y.; Li, L.; Yang, Y.C.; Li, J.R.; Yang, X.T.; Li, L.; Zheng, Z.Y.; Yang, B.Y.; Zhang, P.Y.; Liu, H.Y. Effects of chronic cold stress and thermal stress on growth performance, hepatic apoptosis, oxidative stress, immune response and gut microbiota of juvenile hybrid sturgeon (Acipenser baerii Q x A. schrenkii d). Fish Shellfish Immunol. 2025, 157, 110078. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.A.; Dunn, J. Temperature acclimation and metabolism in ectotherms with particular reference to teleost fish. Symp. Soc. Exp. Biol. 1987, 41, 67–93. [Google Scholar] [PubMed]
- Tien, N.S.H.; de Leeuw, J.J.; van Rijssel, J.C.; van der Hammen, T.; Volwater, J.J.J. Temperature-related increase in growth rate in four freshwater lake fish species. J. Fish Biol. 2024, 104, 2044–2055. [Google Scholar] [CrossRef]
- Lahnsteiner, F. Seasonal differences in thermal stress susceptibility of diploid and triploid brook trout, Salvelinus fontinalis (Teleostei, Pisces). J. Fish Biol. 2022, 101, 276–288. [Google Scholar] [CrossRef]
- Réalis-Doyelle, E.; Pasquet, A.; De Charleroy, D.; Fontaine, P.; Teletchea, F. Strong Effects of Temperature on the Early Life Stages of a Cold Stenothermal Fish Species, Brown Trout (Salmo trutta L.). PLoS ONE 2016, 11, e0155487. [Google Scholar] [CrossRef]
- Mayer, N.B.; Hinch, S.G.; Eliason, E.J. Thermal tolerance in Pacific salmon: A systematic review of species, populations, life stages and methodologies. Fish Fish. 2024, 25, 283–302. [Google Scholar] [CrossRef]
- Roessig, J.M.; Woodley, C.M.; Cech, J.J., Jr.; Hansen, L.J. Effects of global climate change on marine and estuarine fishes and fisheries. Rev. Fish Biol. Fish. 2004, 14, 251–275. [Google Scholar] [CrossRef]
- Miranda, L.E.; Coppola, G.; Boxrucker, J. Reservoir Fish Habitats: A perspective on coping with climate change. Rev. Fish. Sci. Aquac. 2020, 28, 478–498. [Google Scholar] [CrossRef]
- Culp, J.M.; Goedkoop, W.; Christensen, T.; Christoffersen, K.S.; Fefilova, E.; Liljaniemi, P.; Novichkova, A.A.; Olafsson, J.S.; Sandoy, S.; Zimmerman, C.E.; et al. Arctic freshwater biodiversity: Establishing baselines, trends, and drivers of ecological change. Freshw. Biol. 2022, 67, 1–13. [Google Scholar] [CrossRef]
- Ryan, S.N. The effect of chronic heat-stress on cortisol-levels in the antarctic fish pagothenia-borchgrevinki. Experientia 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Sleadd, I.M.; Lee, M.; Hassumani, D.O.; Stecyk, T.M.A.; Zeitz, O.K.; Buckley, B.A. Sub-lethal heat stress causes apoptosis in an Antarctic fish that lacks an inducible heat shock response. J. Therm. Biol. 2014, 44, 119–125. [Google Scholar] [CrossRef]
- Bing, L.; Sun, S.M.; Jian, Z.; Su, Y.L.; Zhang, W.X.; Ge, X.P. Transcriptome profiling and histology changes in juvenile blunt snout bream (Megalobrama amblycephala) liver tissue in response to acute thermal stress. Genomics 2019, 111, 242–250. [Google Scholar] [CrossRef]
- Roh, H.; Kim, A.; Kim, N.; Lee, Y.; Kim, D.-H. Multi-omics analysis provides novel insight into immuno-physiological pathways and development of thermal resistance in rainbow trout exposed to acute thermal stress. Int. J. Mol. Sci. 2020, 21, 9198. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, Y.; Zhou, L.; Chi, C.; Sun, X.; Wu, B.; Liu, Z.; Wang, Y. Transcriptome sequencing reveals the response to acute thermal stress in the Pacific Abalone, Haliotis discus hannai. Aquac. Res. 2023, 2023, 7621215. [Google Scholar] [CrossRef]
- Liu, C.; Wen, H.; Zheng, Y.; Zhang, C.; Zhang, Y.; Wang, L.; Sun, D.; Zhang, K.; Qi, X.; Li, Y. Integration of mRNA and miRNA analysis sheds new light on the muscle response to heat stress in Spotted Sea Bass (Lateolabrax maculatus). Int. J. Mol. Sci. 2024, 25, 12098. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Tian, Y.; Li, Z.; Chen, S.; Li, L.; Wang, X.; Wang, L.; Liu, Y.; Zhai, J.; Li, W.; et al. Comparative transcriptome analysis of hybrid Jinhu grouper (Epinephelus fuscoguttatus ♀ × Epinephelus tukula ♂) and Epinephelus fuscoguttatus under temperature stress. Aquaculture 2024, 578, 740037. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.S.; Ye, S.W.; Bureau, D.P.; Liu, H.B.; Yin, J.S.; Mou, Z.B.; Lin, H.; Hao, F.H. Global metabolic responses of the lenok (Brachymystax lenok) to thermal stress. Comp. Biochem. Physiol. D-Genom. Proteom. 2019, 29, 308–319. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Bai, Y.; Xu, S.; Yang, X.; Cheng, B. Intestinal metabolomics of juvenile lenok (Brachymystax lenok) in response to heat stress. Fish Physiol. Biochem. 2022, 48, 1389–1400. [Google Scholar] [CrossRef]
- Cheng, T.; Chen, J.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S.; Xie, S.; Zhang, W.; Deng, J.; Tan, B.; et al. Protective effect of steroidal saponins on heat stress in the liver of largemouth bass (Micropterus salmoides) revealed by metabolomic analysis. Aquac. Rep. 2023, 33, 101875. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Li, P.; Liu, Y.; Song, M.; Li, F.; Ou, J.; Lai, J. Integrated metabolomic and transcriptomic responses to heat stress in a high-altitude fish, Triplophysa siluroides. Fish Shellfish Immunol. 2023, 142, 109118. [Google Scholar] [CrossRef]
- Noori, R.; Bateni, S.M.; Saari, M.; Almazroui, M.; Torabi Haghighi, A. Strong warming rates in the surface and bottom layers of a boreal lake: Results from approximately six decades of measurements (1964–2020). Earth Space Sci. 2022, 9, e2021EA001973. [Google Scholar] [CrossRef]
- Noori, R.; Woolway, R.I.; Saari, M.; Pulkkanen, M.; Kløve, B. Six Decades of thermal change in a pristine lake situated north of the arctic circle. Water Resour. Res. 2022, 58, e2021WR031543. [Google Scholar] [CrossRef]
- Wu, Y.F.; Wu, C.Z. The Fishes of the Qinghai—Xizang Plateau; Science and Technology Press: Chengdu, China, 1992. [Google Scholar]
- Chen, Y.F.; Cao, W.Y. “Schizothoracinae,” in Fauna Sinica, Osteichthyes, Cypriniformes, 3rd ed.; Yue, P.Q., Ed.; Science Press: Beijing, China, 2000; pp. 273–390. [Google Scholar]
- Qi, D.; Chao, Y.; Wu, R.; Xia, M.; Chen, Q.; Zheng, Z. Transcriptome analysis provides insights into the adaptive responses to hypoxia of a Schizothoracine fish (Gymnocypris eckloni). Front. Physiol. 2018, 9, 1326. [Google Scholar] [CrossRef]
- Yan, S. Tudy on the Biology of Gymnocypris eckloni and the Research of Reproductive Property, Embryo and Artificial Cultivation; China West Normal University: Nanchong, China, 2016. [Google Scholar]
- Yang, L.; Fang, J.; Peng, X.; Cui, H.; He, M.; Zuo, Z.; Zhou, Y.; Yang, Z. Study on the morphology, histology and enzymatic activity of the digestive tract of Gymnocypris eckloni Herzenstein. Fish Physiol. Biochem. 2017, 43, 1175–1185. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.H.; Tian, F.; Liu, S.J.; Feng, C.G.; Zhao, K. Mitochondrial genome and phylogenetic relationship of Gymnocypris eckloni (Schizothoracinae) in Qaidam river basin. Genomics 2020, 112, 4316–4321. [Google Scholar] [CrossRef]
- Nie, M.; Ni, W.; Wang, L.; Gao, Q.; Liu, D.; Tian, F.; Wang, Z.; Zhang, C.; Qi, D. Insights into mirna-mrna regulatory mechanisms of cold adaptation in Gymnocypris eckloni: Ubiquitin-mediated proteolysis is pivotal for adaptive energy metabolism. Front. Genet. 2022, 13, 903995. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Liu, D.; Gao, Q.; Nie, M.; Zhu, S.; Chao, Y.; Yang, X.; Yang, C.; Zhang, C.; et al. Chromosome-level genome assembly provides insight into the evolution of chromosomes and the globin gene superfamily in Gymnocypris eckloni. Sci. Data 2022, 9, 464. [Google Scholar] [CrossRef]
- Zhao, G.; Tian, S.; Chen, R.; Cao, Y.; Zhang, Y.; Han, B. Effect of global climate change on the sustainability of cold-water fish habitat in the alpine region: A case study on the Gymnocypris eckloni in the source region of the Yellow River. J. Environ. Manag. 2024, 367, 121926. [Google Scholar] [CrossRef]
- Li, J.T.; Duan, Y.T.; Kong, W.Q.; Gao, H.; Fu, S.X.; Li, H.J.; Zhou, Y.H.; Liu, H.P.; Yuan, D.Y.; Zhou, C.W. Heat stress affects swimming performance and induces biochemical, structural, and transcriptional changes in the heart of Gymnocypris eckloni. Aquac. Rep. 2024, 35, 101998. [Google Scholar] [CrossRef]
- Duan, Y.T.; Li, H.J.; Li, J.T.; Bai, S.H.; Fu, S.X.; Zhou, Y.H.; Liu, S.D.; Li, R.D.; Liu, H.P.; Zhou, C.W.; et al. Integrated transcriptome and 16S rDNA analyses reveal that acute heat stress induces intestinal damage in Gymnocypris eckloni. Front. Mar. Sci. 2024, 11, 1448313. [Google Scholar] [CrossRef]
- Zhou, C.; Duan, Y.; Li, J.; Fu, S.; Bai, S.; Zhuang, Y.; Li, H.; Zhou, Y.; Shen, J.; Zhou, R.; et al. Decoding the intestinal response to heat stress in Gymnocypris eckloni: Insights from a thorough analysis of microbiome and transcriptome. Aquaculture 2024, 591, 741112. [Google Scholar] [CrossRef]
- Zhou, C.; Duan, Y.; Li, J.; Fu, S.; Bai, S.; Zhuang, Y.; Liu, S.; Li, H.; Zhou, Y.; Wang, Q.; et al. A study on the hepatic response to heat stress in Gymnocypris eckloni through an approach combining metabolomic and transcriptomic profiling. Aquac. Rep. 2024, 39, 102392. [Google Scholar] [CrossRef]
- Ma, D.; Fan, J.; Zhu, H.; Su, H.; Jiang, P.; Yu, L.; Liao, G.; Bai, J. Histologic examination and transcriptome analysis uncovered liver damage in largemouth bass from formulated diets. Aquaculture 2020, 526, 735329. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, H.; Yan, X.; Li, P.; Wang, C.; Zhang, C.; Ji, H.; Gao, Q.; Dong, S. Selenium improved mitochondrial quality and energy supply in the liver of high-fat diet-fed grass carp (Ctenopharyngodon idella) after heat stress. Fish Physiol. Biochem. 2022, 48, 1701–1716. [Google Scholar] [CrossRef]
- Jiao, S.; Nie, M.; Song, H.; Xu, D.; You, F. Physiological responses to cold and starvation stresses in the liver of yellow drum (Nibea albiflora) revealed by LC-MS metabolomics. Sci. Total Environ. 2020, 715, 136940. [Google Scholar] [CrossRef]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, H.; Liu, X.; Wang, Z.; Li, Y.; Ren, Y.; Gao, C.; Jiao, T.; Cai, Y.; Zhao, S. Transcriptome and metabolome integration provides new insights into the regulatory networks of Tibetan pig alveolar type ii epithelial cells in response to hypoxia. Front. Genet. 2022, 13, 812411. [Google Scholar] [CrossRef] [PubMed]
- Meeuwig, M.H.; Dunham, J.B.; Hayes, J.P.; Vinyard, G.L. Effects of constant and cyclical thermal regimes on growth and feeding of juvenile cutthroat trout of variable sizes. Ecol. Freshw. Fish 2004, 13, 208–216. [Google Scholar] [CrossRef]
- Morissette, J.; Swart, S.; MacCormack, T.J.; Currie, S.; Morash, A.J. Thermal variation near the thermal optimum does not affect the growth, metabolism or swimming performance in wild Atlantic salmon (Salmo salar). J. Fish Biol. 2021, 98, 1585–1589. [Google Scholar] [CrossRef]
- Lu, D.L.; Ma, Q.; Wang, J.; Li, L.Y.; Han, S.L.; Limbu, S.M.; Li, D.L.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. Fasting enhances cold resistance in fish through stimulating lipid catabolism and autophagy. J. Physiol. 2019, 597, 1585–1603. [Google Scholar] [CrossRef]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef]
- Duan, C.; Tian, C.; Guan, Y.; Xu, H.; Yang, L.; Chen, Y.; Liu, Y.; Shen, Y.; Zhang, Y.; Cao, S.; et al. Long-term thermal stress induces hepatic injury and alters the thermotolerance response in Hong Kong catfish (Clarias fuscus). Aquaculture 2024, 590, 741041. [Google Scholar] [CrossRef]
- Mininni, A.N.; Milan, M.; Ferraresso, S.; Petochi, T.; Di Marco, P.; Marino, G.; Livi, S.; Romualdi, C.; Bargelloni, L.; Patarnello, T. Liver transcriptome analysis in gilthead sea bream upon exposure to low temperature. BMC Genom. 2014, 15, 1–12. [Google Scholar] [CrossRef]
- Mennigen, J.A. Micromanaging metabolism—A role for miRNAs in teleost energy metabolism. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2016, 199, 115–125. [Google Scholar] [CrossRef]
- Raza, S.H.A.; Abdelnour, S.A.; Alotaibi, M.A.; AlGabbani, Q.; Naiel, M.A.E.; Shokrollahi, B.; Noreldin, A.E.; Jahejo, A.R.; Shah, M.A.; Alagawany, M.; et al. MicroRNAs mediated environmental stress responses and toxicity signs in teleost fish species. Aquaculture 2022, 546, 737310. [Google Scholar] [CrossRef]
- Blödorn, E.B.; Domingues, W.B.; Nunes, L.S.; Komninou, E.R.; Pinhal, D.; Campos, V.F. MicroRNA roles and their potential use as selection tool to cold tolerance of domesticated teleostean species: A systematic review. Aquaculture 2021, 540, 736747. [Google Scholar] [CrossRef]
- Ikert, H.; Craig, P.M. Chronic exposure to venlafaxine and increased water temperature reversibly alters microRNA in zebrafish gonads (Danio rerio). Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 33, 100634. [Google Scholar] [CrossRef]
- Bao, J.-W.; Qiang, J.; Tao, Y.-F.; Li, H.-X.; He, J.; Xu, P.; Chen, D.-J. Responses of blood biochemistry, fatty acid composition and expression of microRNAs to heat stress in genetically improved farmed tilapia (Oreochromis niloticus). J. Therm. Biol. 2018, 73, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Y.; Ma, F.; Kang, Y.; Liu, Z.; Wang, J. Identification and characterization of microRNAs in the liver of rainbow trout in response to heat stress by high-throughput sequencing. Gene 2018, 679, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Dietzen, D.J. 13—Amino acids, peptides, and proteins. In Principles and Applications of Molecular Diagnostics; Rifai, N., Horvath, A.R., Wittwer, C.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 345–380. [Google Scholar]
- Qaid, M.M.; Al-Garadi, M.A. Protein and amino acid metabolism in poultry during and after heat stress: A Review. Animals 2021, 11, 1167. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Lai, J.; Liu, Y.; Song, M.; Li, F.; Gong, Q. Integrated biochemical, transcriptomic and metabolomic analyses provide insight into heat stress response in Yangtze sturgeon (Acipenser dabryanus). Ecotoxicol. Environ. Saf. 2023, 249, 114366. [Google Scholar] [CrossRef]
- Herrera, M.; Herves, M.A.; Giráldez, I.; Skar, K.; Mogren, H.; Mortensen, A.; Puvanendran, V. Effects of amino acid supplementations on metabolic and physiological parameters in Atlantic cod (Gadus morhua) under stress. Fish Physiol. Biochem. 2017, 43, 591–602. [Google Scholar] [CrossRef]
- Jin, M.; Yang, F.; Yang, I.; Yin, Y.; Luo, J.J.; Wang, H.; Yang, X.F. Uric acid, hyperuricemia and vascular diseases. Front. Biosci. 2012, 17, 656–669. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Di Micoli, V.; Angeloni, C.; Giovannini, M.; Borghi, C. Purine metabolism dysfunctions: Experimental methods of detection and diagnostic potential. Int. J. Mol. Sci. 2023, 24, 7027. [Google Scholar] [CrossRef] [PubMed]
- Garavito, M.F.; Narváez-Ortiz, H.Y.; Zimmermann, B.H. Pyrimidine metabolism: Dynamic and versatile pathways in pathogens and cellular development. J. Genet. Genom. 2015, 42, 195–205. [Google Scholar] [CrossRef]
- Sahu, U.; Villa, E.; Reczek, C.R.; Zhao, Z.; O’Hara, B.P.; Torno, M.D.; Mishra, R.; Shannon, W.D.; Asara, J.M.; Gao, P.; et al. Pyrimidines maintain mitochondrial pyruvate oxidation to support de novo lipogenesis. Science 2024, 383, 1484–1492. [Google Scholar] [CrossRef]



| miRNA | Gene | KEGG Pathway |
|---|---|---|
| miR-181b-5p | nhp2 | Ribosome biogenesis in eukaryotes |
| miR-125b-2-3p | ranbp2 | RNA transport |
| calr | Phagosome, protein processing in endoplasmic reticulum | |
| novel_582 | rpl34 | Ribosome |
| agl | Starch and sucrose metabolism | |
| miR-132-3p | ugt1a1 | Starch and sucrose metabolism, porphyrin and chlorophyll metabolism, ascorbate and aldarate metabolism, pentose and glucuronate interconversions, retinol metabolism, steroid hormone biosynthesis, drug metabolism—cytochrome P450, metabolism of xenobiotics by cytochrome P450 |
| miR-205-5p | rgn | Carbon metabolism, ascorbate and aldarate metabolism, pentose phosphate pathway |
| pdxp | Vitamin B6 metabolism | |
| adcy8 | Purine metabolism, progesterone-mediated oocyte maturation, GnRH signaling pathway, vascular smooth muscle contraction, gap junction, melanogenesis, oocyte meiosis | |
| miR-196a-5p | pepck | Pyruvate metabolism, TCA cycle, PPAR signaling pathway, glycolysis/gluconeogenesis, adipocytokine signaling pathway, FoxO signaling pathway, insulin signaling pathway |
| miR-133a-3p | bpnt1 | Sulfur metabolism |
| gne | Amino sugar and nucleotide sugar metabolism |
| Metabolic Pathway | p Value | Association with Transcriptome | Metabolites |
|---|---|---|---|
| Steroid hormone biosynthesis | 0.002999 | Yes | Cortisol, 5-beta-Androstane-3,17-dione, deoxycorticosterone, hydrocortisone, corticosterone, tetrahydrocortisone, tetrahydrocorticosterone |
| Tryptophan metabolism | 0.01117 | Yes | Indole, N-Formylkynurenine, L-Tryptophan, indole-3-acetic acid |
| Pyruvate metabolism | 0.03314 | Yes | acetyl phosphate, phosphoenolpyruvate |
| Aldosterone-regulated sodium reabsorption | 0.03314 | No | Hydrocortisone, cortisol |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 0.03928 | No | Phosphoenolpyruvate, L-Tyrosine, L-tryptophan, indole |
| cGMP-PKG signaling pathway | 0.00276 | No | Guanosine monophosphate, adenosine, adenosine 5′-monophosphate, |
| AMPK signaling pathway | 0.00998 | No | NAD+, adenosine 5′-monophosphate, D-Fructose 6-phosphate |
| cAMP signaling pathway | 0.02040 | No | adenosine 5′-monophosphate, adenosine |
| Longevity regulating pathway | 0.02040 | No | NAD+, adenosine 5′-monophosphate |
| Olfactory transduction | 0.02040 | No | Guanosine monophosphate, adenosine 5′-monophosphate |
| Purine metabolism | 0.03753 | Yes | XMP, adenosine 5′-monophosphate, adenine, adenosine, deoxyguanosine, guanosine monophosphate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, M.; Ni, W.; Wang, Z.; Liu, D.; Gao, Q.; Zhang, C.; Qi, D. Physiological Responses to Thermal Stress in the Liver of Gymnocypris eckloni Revealed by Multi-Omics. Animals 2025, 15, 3272. https://doi.org/10.3390/ani15223272
Nie M, Ni W, Wang Z, Liu D, Gao Q, Zhang C, Qi D. Physiological Responses to Thermal Stress in the Liver of Gymnocypris eckloni Revealed by Multi-Omics. Animals. 2025; 15(22):3272. https://doi.org/10.3390/ani15223272
Chicago/Turabian StyleNie, Miaomiao, Weilin Ni, Zhenji Wang, Dan Liu, Qiang Gao, Cunfang Zhang, and Delin Qi. 2025. "Physiological Responses to Thermal Stress in the Liver of Gymnocypris eckloni Revealed by Multi-Omics" Animals 15, no. 22: 3272. https://doi.org/10.3390/ani15223272
APA StyleNie, M., Ni, W., Wang, Z., Liu, D., Gao, Q., Zhang, C., & Qi, D. (2025). Physiological Responses to Thermal Stress in the Liver of Gymnocypris eckloni Revealed by Multi-Omics. Animals, 15(22), 3272. https://doi.org/10.3390/ani15223272






