Study on the Function of ID2 Gene in Granulosa Cells of Ovaries of Hetian Sheep and Its Correlation Analysis with Lambing Traits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Sample Collection
2.2. Ovarian Collection
2.3. Primer Design
2.4. ID2 Gene Cloning
2.5. Bioinformatics Analysis
2.6. ID2 Gene Tissue Expression
2.7. ID2 Genotype Analysis
2.8. Isolation, Culture, and Identification of Ovarian GCs
2.9. Lentivirus Transfection of Eggs and CCK-8 Assay
2.10. Enzyme-Linked Immunosorbent Assay
2.11. RT-PCR Detection of ID2-Related Gene Expression
2.12. Data Processing and Statistical Analysis
- (1)
- Gene frequency and genotype frequency
- (2)
- Hardy–Weinberg equilibrium test: .
- (3)
- Population homozygosity (Ho) test: .
- (4)
- Population heterozygosity (He) test: .
- (5)
- Effective allele (Ne) test: .
- (6)
- Polymorphic Information Content (PIC) Test: .
3. Test Results and Analysis
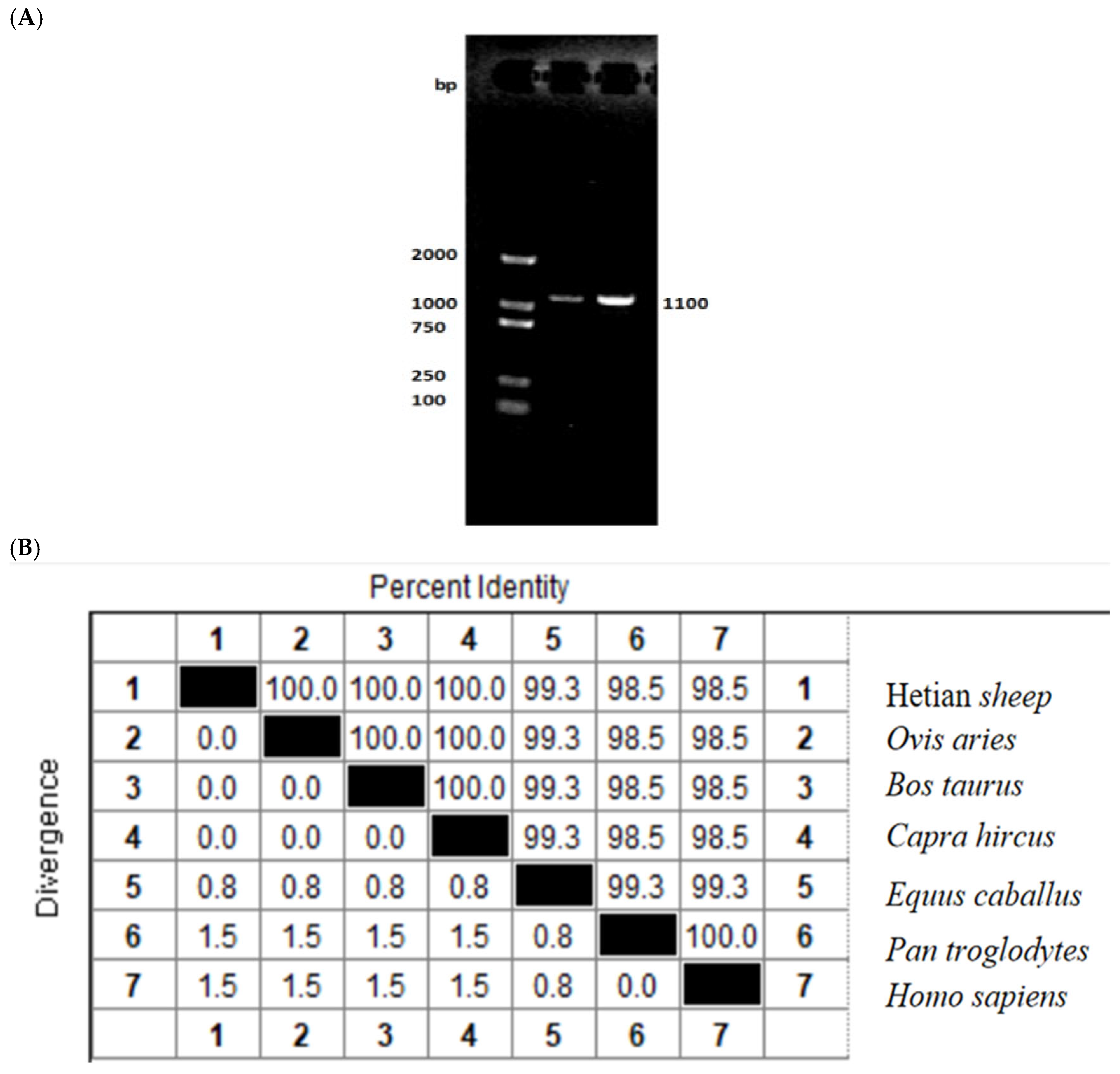
3.1. Cloning and Nucleotide Sequence Analysis of the ID2 Gene of Hetian Sheep
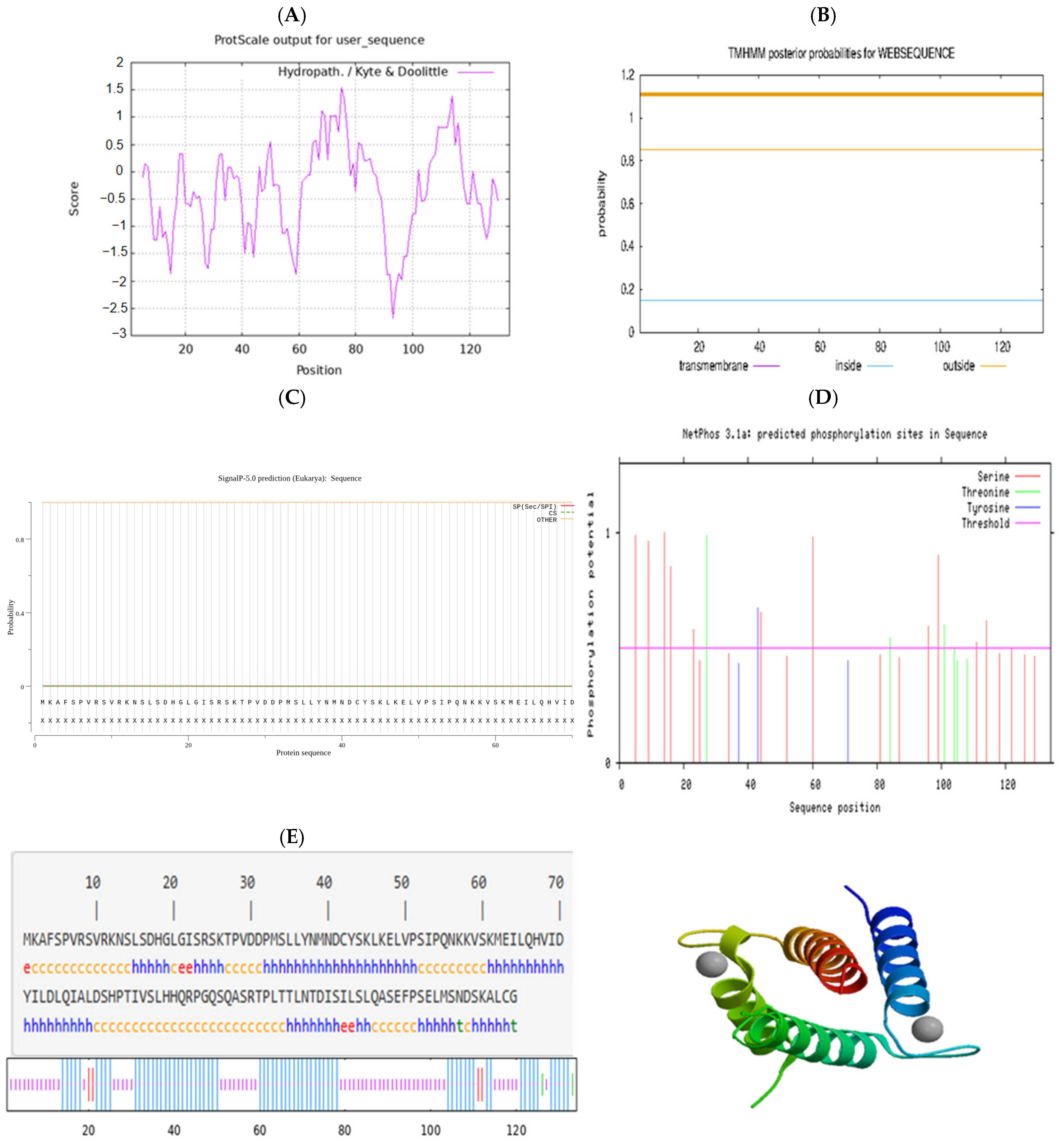
3.2. Prediction and Analysis of the Characteristics of Hetian Sheep ID2 Protein
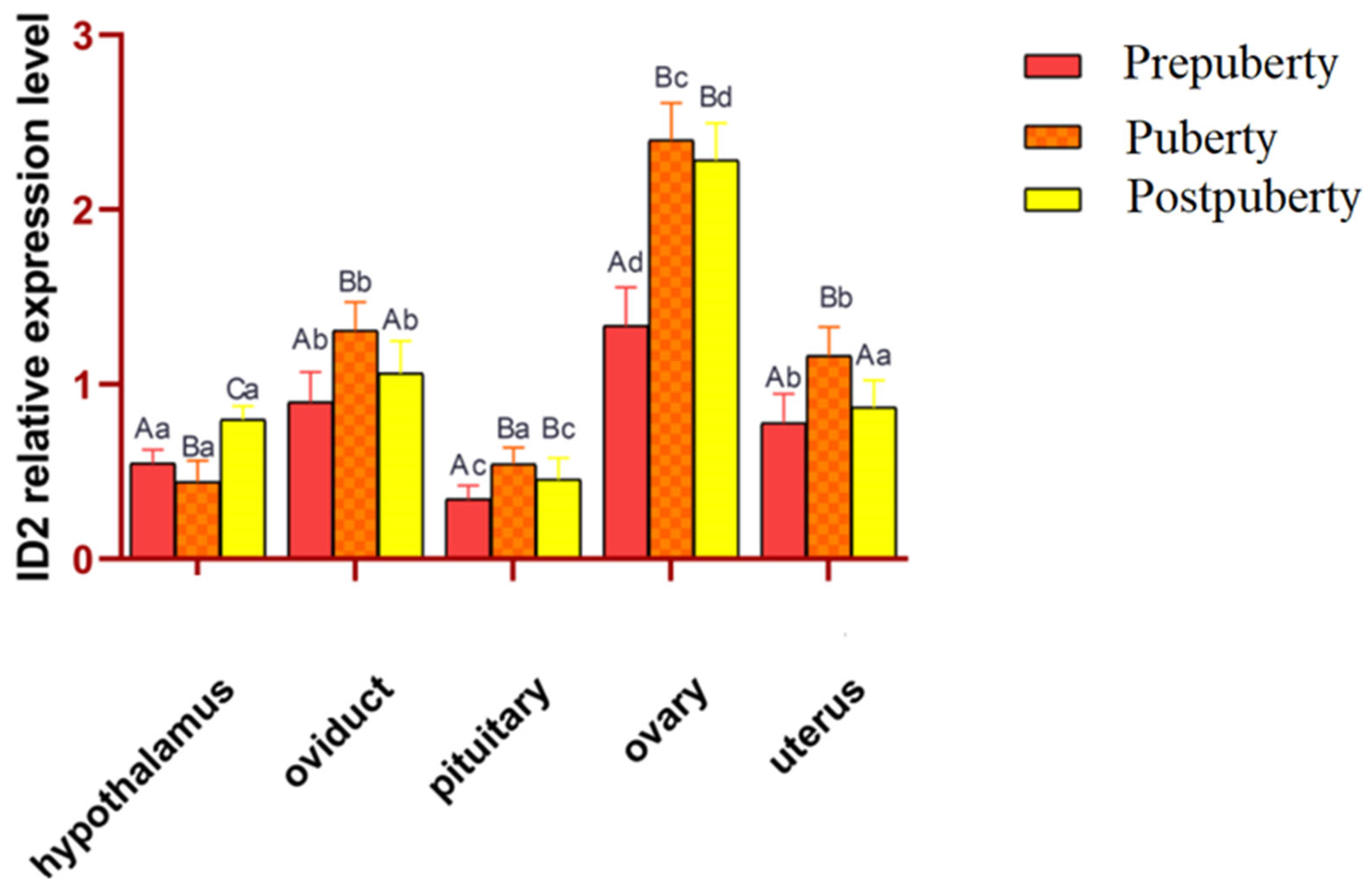
3.3. Analysis of the Expression of the ID2 Gene in Different Tissues of Hetian Sheep at Different Puberty Stages
3.4. Descriptive Characteristics of the Genotyped Ewe Population
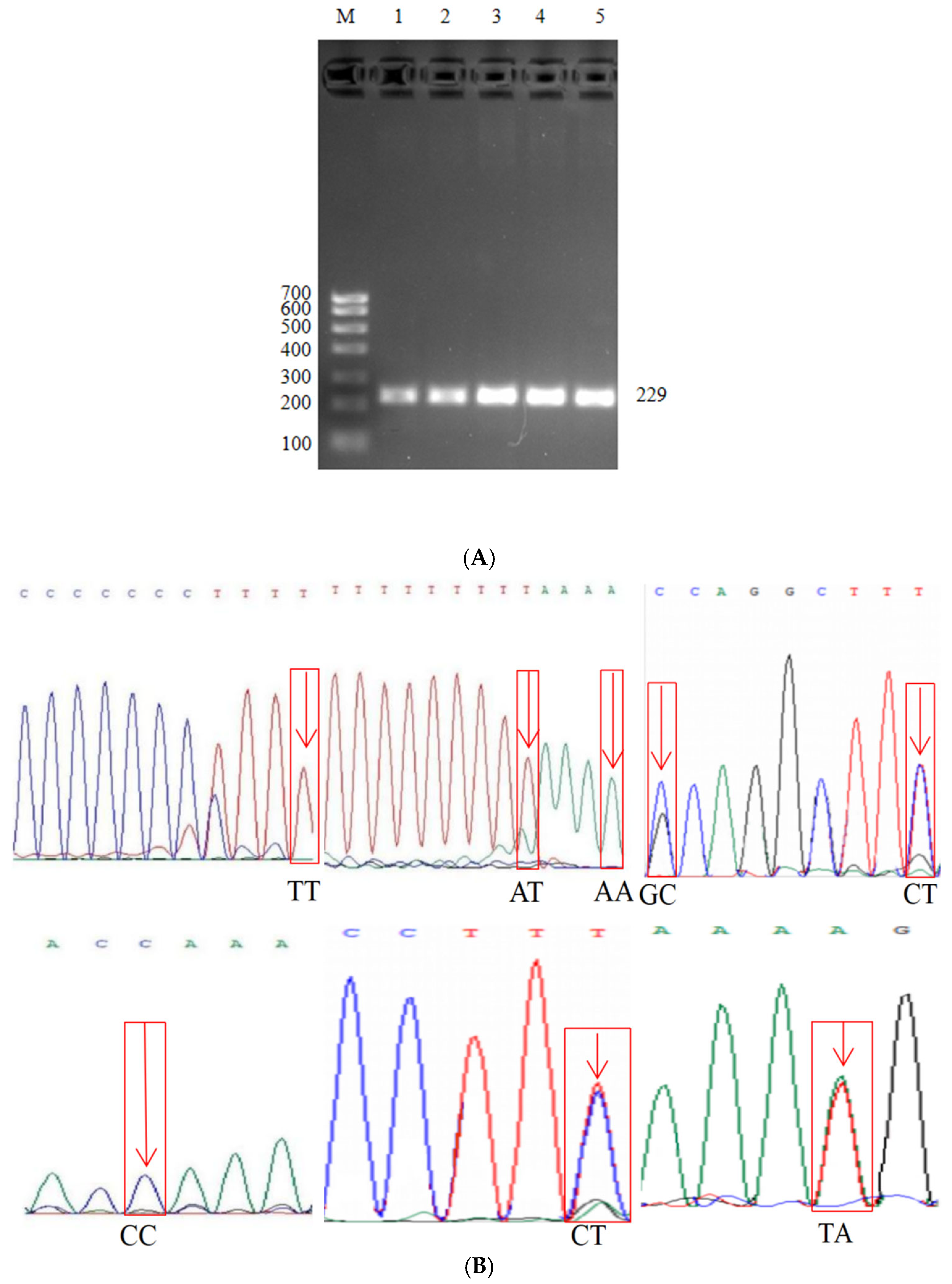
3.5. ID2 Gene PCR Amplification Results and Polymorphic Site Peak Diagram
3.6. Genetic Analysis of SNP Variant Sites in ID2 Gene
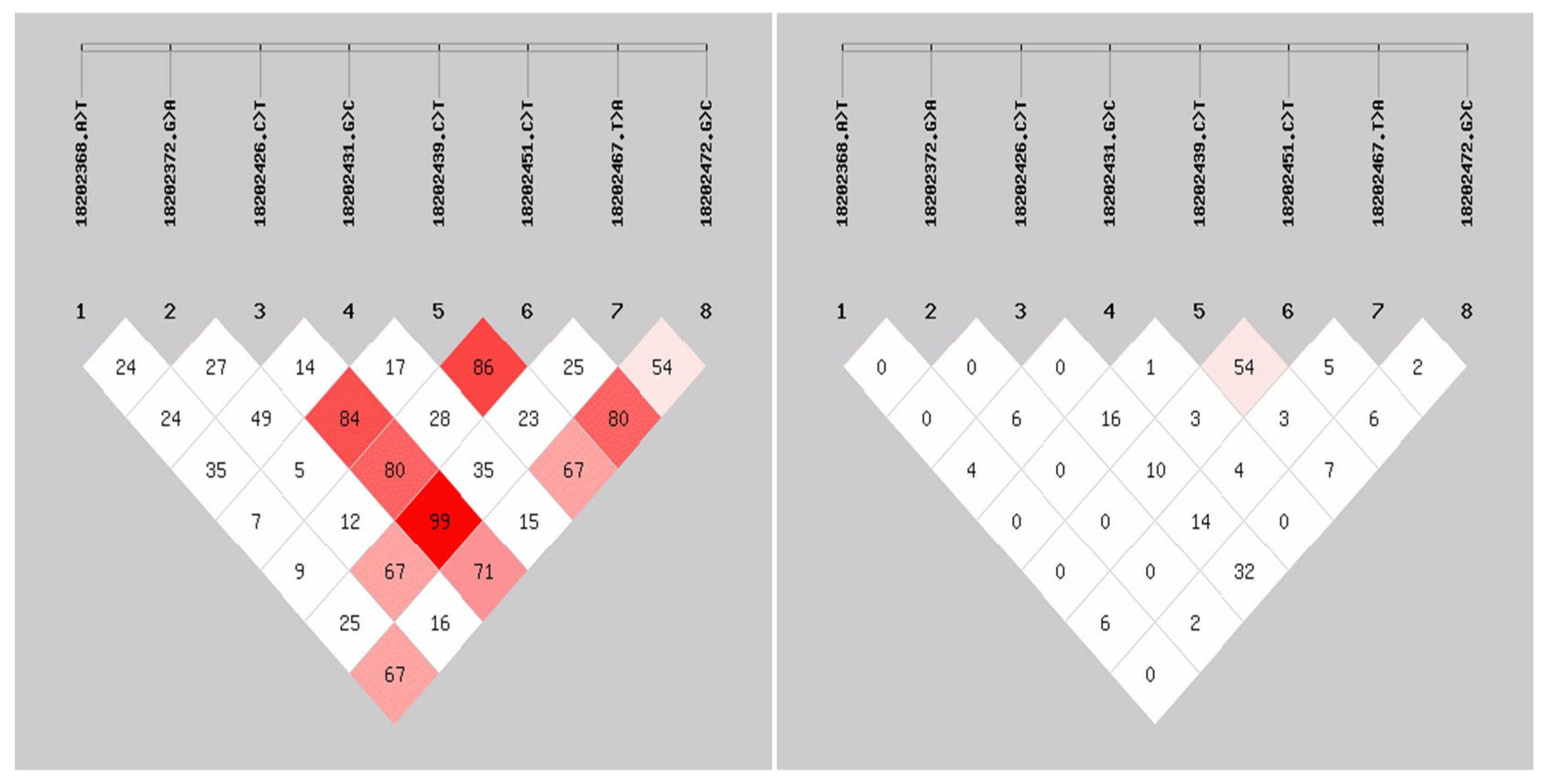
3.7. SNP and Linkage Disequilibrium of ID2 Gene
3.8. Association Analysis Between ID2 Gene Polymorphism and Litter Size
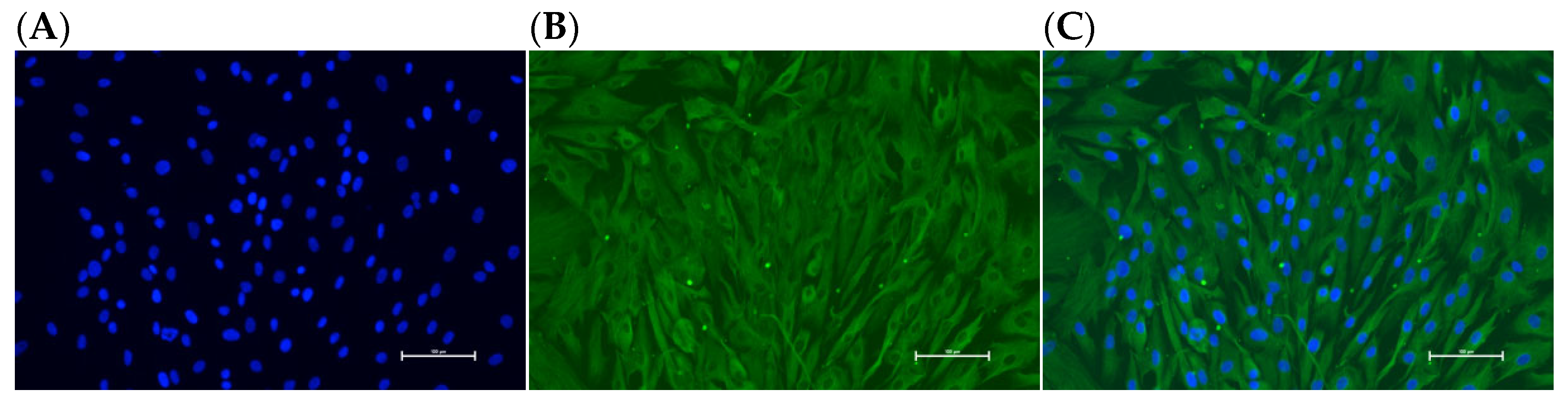
3.9. Identification of Ovarian GCs
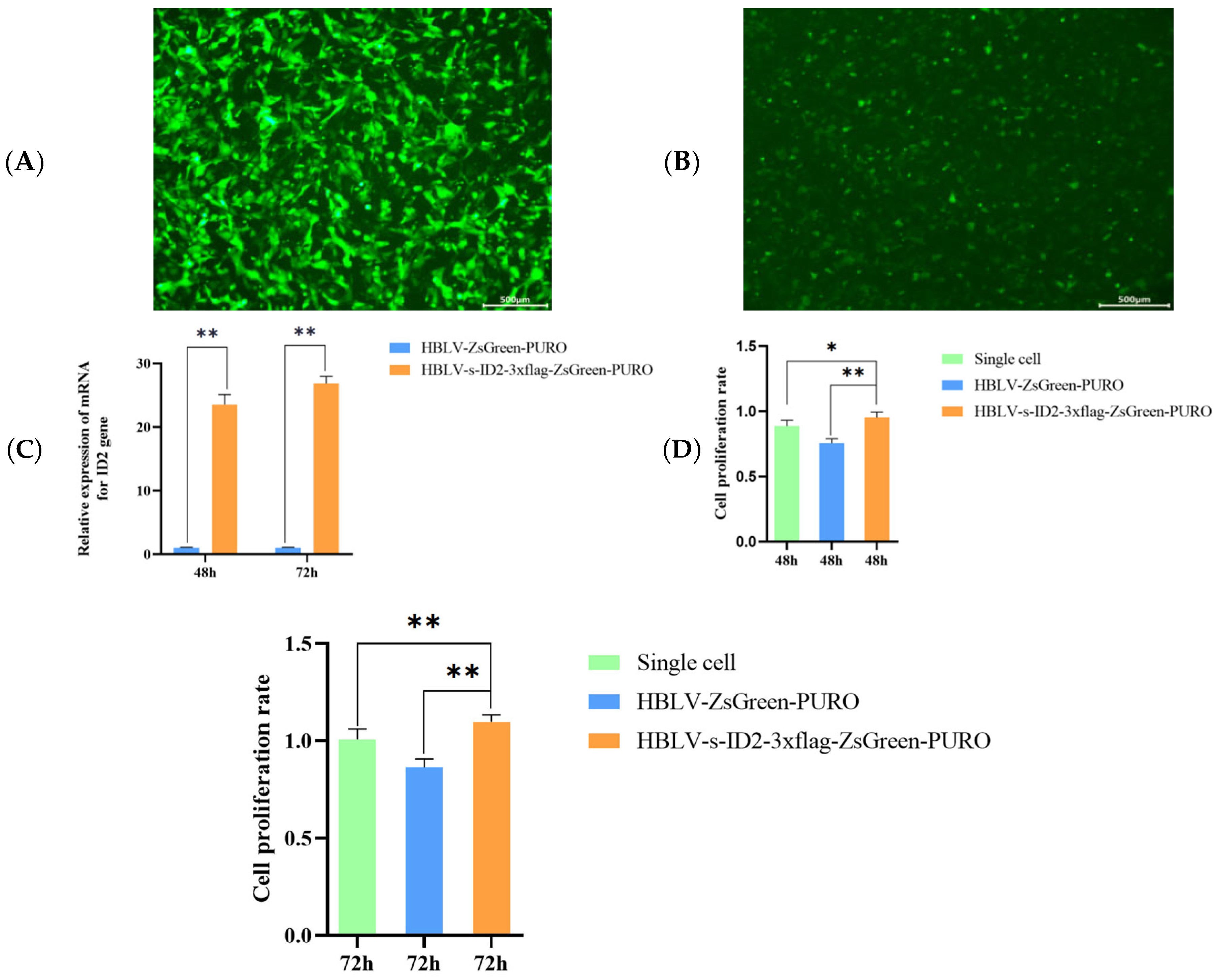
3.10. ID2 Overexpression Enhances Proliferation of Ovarian GCs
3.11. Effects of ID2 Overexpression on P4 and E2 Hormone Production
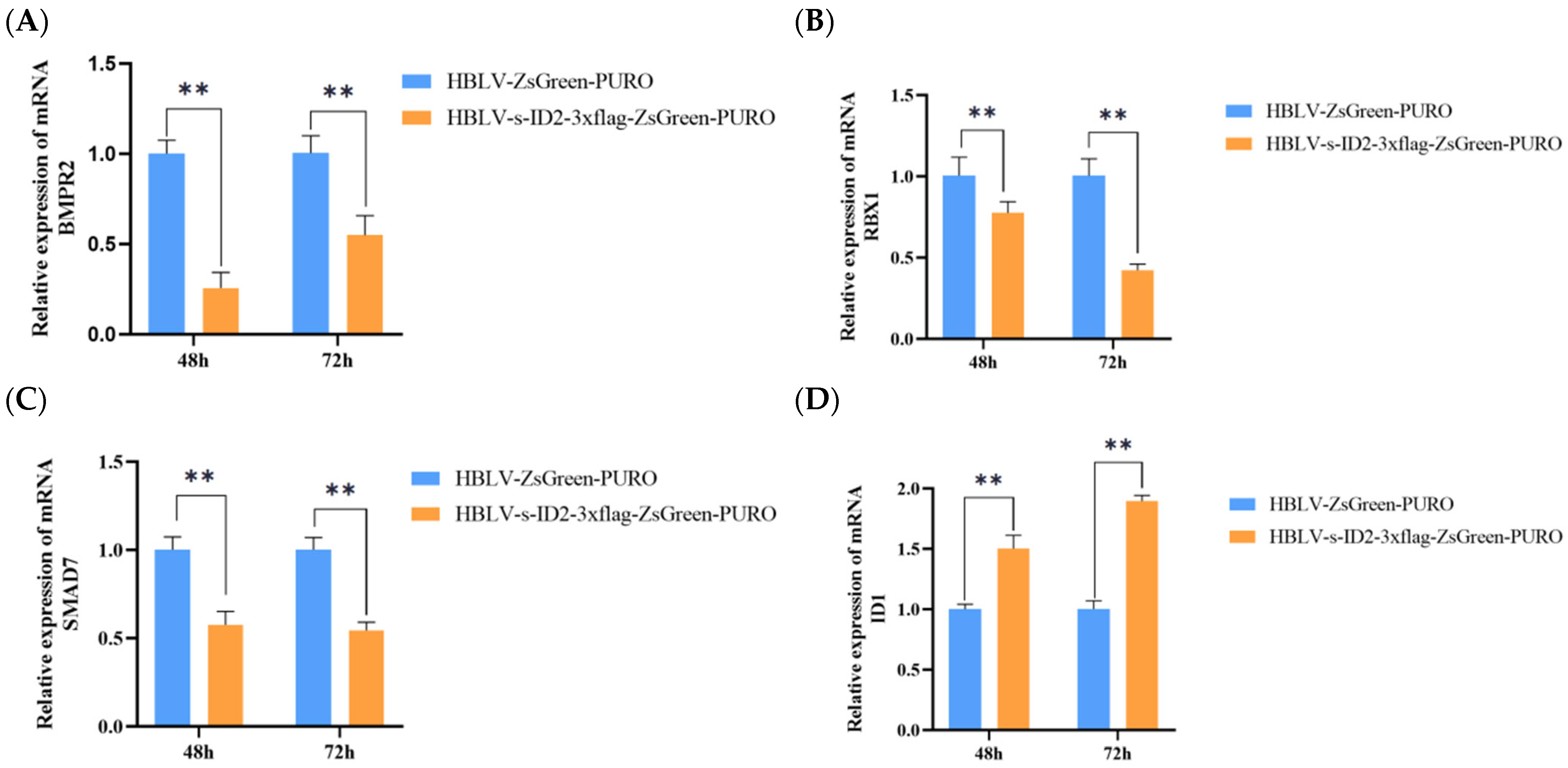
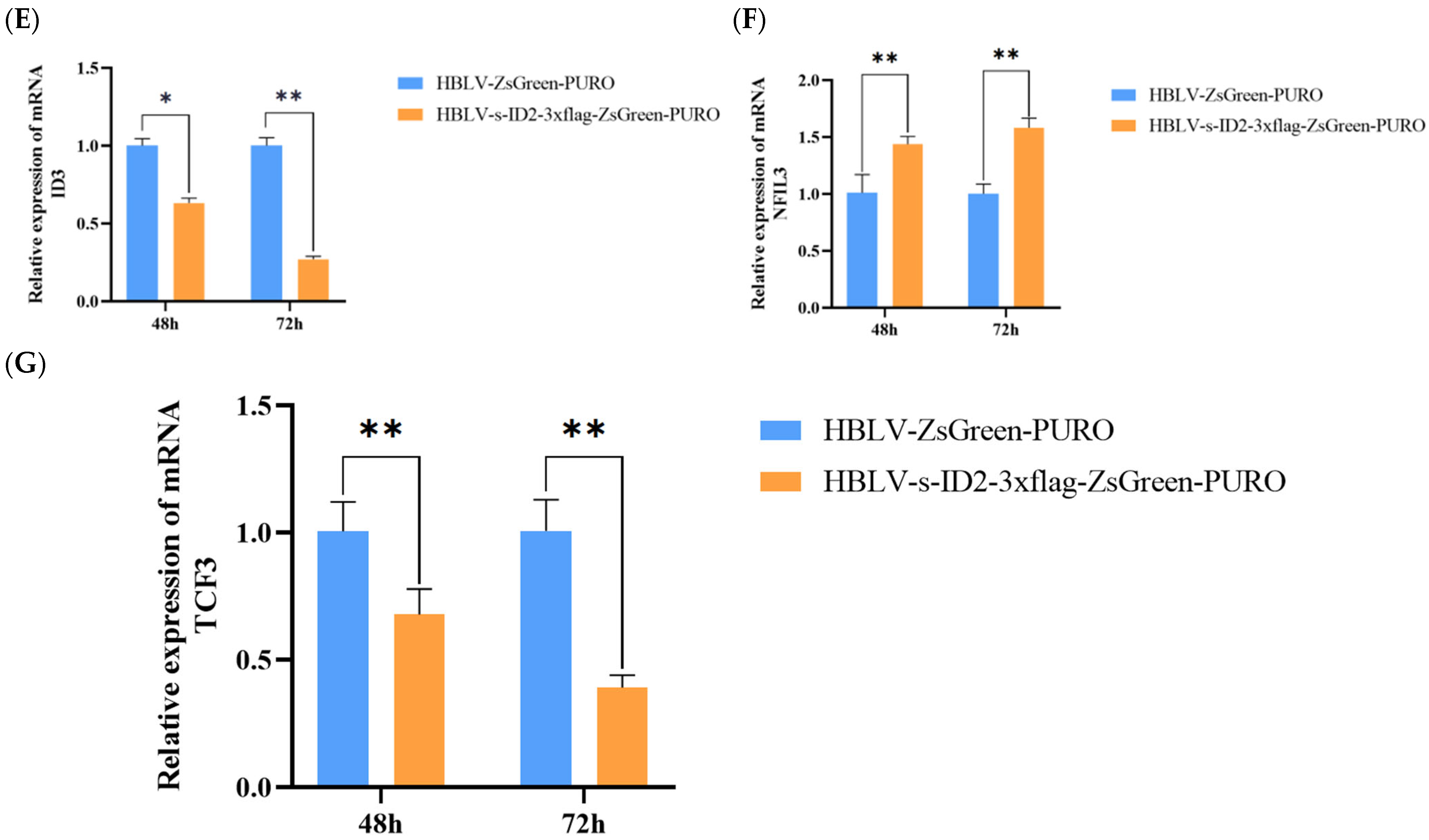
3.12. Effects of ID2 Gene Overexpression on Reproduction-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasimu, A.; Maitusong, A.; Ailijiang, M. Development trends of Hetian sheep in Yutian County: A survey and analysis. Xinjiang Anim. Husb. 2013, 190 (Suppl. S2), 20–21. (In Chinese) [Google Scholar]
- Ajafar, M.H.; Kadhim, A.H.; Al-Thuwaini, T.M. The reproductive traits of sheep and their influencing factors. Rev. Agric. Sci. 2022, 10, 82–89. [Google Scholar] [CrossRef]
- Al-Jaryan, I.L.; Al-Thuwaini, T.M.; Merzah, L.H.; Alkhammas, A.H. Reproductive physiology and advanced technologies in sheep reproduction. Rev. Agric. Sci. 2023, 11, 171–180. [Google Scholar] [CrossRef]
- Notter, D.R. Genetic improvement of reproductive efficiency of sheep and goats. Anim. Reprod. Sci. 2012, 130, 147–151. [Google Scholar] [CrossRef]
- Tai, Y.; Yang, X.; Han, D.; Xu, Z.; Cai, G.; Hao, J.; Zhang, B.; Deng, X. Transcriptomic diversification of granulosa cells during follicular development between White Leghorn and Silky Fowl hens. Front. Genet. 2022, 13, 965414. [Google Scholar] [CrossRef] [PubMed]
- Hogg, K.; Etherington, S.L.; Young, J.M.; McNeilly, A.S.; Duncan, W.C. Inhibitor of differentiation (Id) genes are expressed in the steroidogenic cells of the ovine ovary and are differentially regulated by members of the transforming growth factor-beta family. Endocrinology 2010, 151, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, G.; Mishra, S.R.; Paul, A.; Punetha, M.; Vidyalakshmi, G.; Narayanan, K.; Bag, S.; Bhure, S.; Chouhan, V.S.; Maurya, V.; et al. Transcriptional and translational abundance of BMP2, 4, 6, 7 and their receptors BMPR1A, 1B and BMPR2 in buffalo ovarian follicle and the role of BMP4 and BMP7 on estrogen production and survival of cultured granulosa cells. Res. Vet. Sci. 2018, 118, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Mamsen, L.S.; Bøtkjær, J.A.; Kristensen, S.G.; Pors, S.E.; Jeppesen, J.V.; Kumar, A.; Kalra, B.; Ernst, E.; Andersen, C.Y. High variability of molecular isoforms of AMH in follicular fluid and granulosa cells from human small antral follicles. Front. Endocrinol. 2021, 12, 617523. [Google Scholar] [CrossRef]
- He, J.; Ao, C.; Li, M.; Deng, T.; Zheng, S.; Zhang, K.; Tu, C.; Ouyang, Y.; Lang, R.; Jiang, Y.; et al. Clusterin-carrying extracellular vesicles derived from human umbilical cord mesenchymal stem cells restore the ovarian function of premature ovarian failure mice through activating the PI3K/AKT pathway. Stem Cell Res. Ther. 2024, 15, 300. [Google Scholar] [CrossRef]
- Kumar, S.; Rajput, P.K.; Bahire, S.V.; Jyotsana, B.; Kumar, V.; Kumar, D. Differential expression of BMP/SMAD signaling and ovarian-associated genes in the granulosa cells of FecB introgressed GMM sheep. Syst. Biol. Reprod. Med. 2020, 66, 185–201. [Google Scholar] [CrossRef]
- Loos, B.; Salas-Bastos, A.; Nordin, A.; Debbache, J.; Stierli, S.; Cheng, P.F.; Rufli, S.; Wyss, C.; Levesque, M.P.; Dummer, R.; et al. TGFβ signaling sensitizes MEKi-resistant human melanoma to targeted therapy-induced apoptosis. Cell Death Dis. 2024, 15, 925. [Google Scholar] [CrossRef]
- Meng, Z.; Li, T.; Li, J.; Ding, S.; Liu, Y.; Zhao, G.; Chen, C.; Zhao, P.; Zhou, L. LncRNAPVT1 is associated with cancer-associated fibroblast proliferation through regulating TGF-β in oral squamous cell carcinoma. Immunol. Investig. 2024, 53, 1250–1263. [Google Scholar] [CrossRef]
- Du, X.; Zhang, L.; Li, X.; Pan, Z.; Liu, H.; Li, Q. TGF-β signaling controls FSHR signaling-reduced ovarian granulosa cell apoptosis through the SMAD4/miR-143 axis. Cell Death Dis. 2016, 7, e2476. [Google Scholar] [CrossRef]
- Scobey, M.J.; Fix, C.A.; Walker, W.H. The Id2 transcriptional repressor is induced by follicle-stimulating hormone and cAMP. J. Biol. Chem. 2004, 279, 16064–16070. [Google Scholar] [CrossRef]
- Chaudhary, J.; Johnson, J.; Kim, G.; Skinner, M.K. Hormonal regulation and differential actions of the helix-loop-helix transcriptional inhibitors Id1, Id2, Id3, and Id4 in Sertoli cells. Endocrinology 2001, 142, 1727–1736. [Google Scholar] [CrossRef]
- Guirgis, F.W.; Jacob, V.; Wu, D.; Henson, M.; Daly-Crews, K.B.; Hopson, C.; Black, L.P.; DeVos, E.L.; Sulaiman, D.; Labilloy, G.; et al. DHCR7 expression predicts poor outcomes and mortality from sepsis. Crit. Care Explor. 2023, 5, e0929. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiang, H.; Wang, D.; Rehman, S.U.; Li, Z.; Song, X.; Cui, K.; Luo, X.; Yang, C.; Liu, Q. Exploration of transcriptional regulation network between buffalo oocytes and granulosa cells and its impact on different diameter follicles. BMC Genom. 2024, 25, 1004. [Google Scholar] [CrossRef]
- Chermuła, B.; Brązert, M.; Jeseta, M.; Ożegowska, K.; Sujka-Kordowska, P.; Konwerska, A.; Bryja, A.; Kranc, W.; Jankowski, M.; Nawrocki, M.J.; et al. The unique mechanisms of cellular proliferation, migration and apoptosis are regulated through oocyte maturational development—A complete transcriptomic and histochemical study. Int. J. Mol. Sci. 2018, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Nishikawa, S.I.; Yokota, Y. Lactation defect in mice lacking the helix-loop-helix inhibitor Id2. EMBO J. 2000, 19, 5772–5781. [Google Scholar] [CrossRef]
- He, X.; Wang, W.; Du, X.; Chu, M. Association between single-nucleotide polymorphism in PIK3CD gene and litter size in Small Tail Han sheep. Anim. Biotechnol. 2023, 34, 3337–3342. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, H.; Yang, H.; Zhao, Z.; Blair, H.T.; Zhai, M.; Yu, Q.; Wu, P.; Fang, C.; Xie, M. Polymorphisms and association of GRM1, GNAQ and HCRTR1 genes with seasonal reproduction and litter size in three sheep breeds. Reprod. Domest. Anim. 2022, 57, 532–540. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Q.; Di, R.; Hu, W.; Wang, X.; He, X.; Ma, L.; Chu, M. Single nucleotide polymorphisms in BMP2 and BMP7 and the association with litter size in Small Tail Han sheep. Anim. Reprod. Sci. 2019, 204, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, X.; Muhatai, G.; Wang, L. Expression profile analysis of sheep ovary after superovulation and estrus synchronisation treatment. Vet. Med. Sci. 2022, 8, 1276–1287. [Google Scholar] [CrossRef]
- Vasu, M.; Ahlawat, S.; Choudhary, V.; Kaur, R.; Arora, R.; Sharma, R.; Sharma, U.; Chhabra, P.; Mir, M.; Singh, M.K. Identification and validation of stable reference genes for expression profiling of target genes in diverse ovine tissues. Gene 2024, 897, 148067. [Google Scholar] [CrossRef]
- Hsueh, A.J.; Kawamura, K.; Cheng, Y.; Fauser, B.C. Intraovarian control of early folliculogenesis. Endocr. Rev. 2015, 36, 1–24. [Google Scholar] [CrossRef]
- Zhang, H.; McClatchie, T.; Baltz, J.M. L-Serine transport in growing and maturing mouse oocytes. J. Cell. Physiol. 2020, 235, 8585–8600. [Google Scholar] [CrossRef]
- Sun, X.; Terakawa, J.; Clevers, H.; Barker, N.; Daikoku, T.; Dey, S.K. Ovarian LGR5 is critical for successful pregnancy. FASEB J. 2014, 28, 2380–2389. [Google Scholar] [CrossRef]
- Deshpande, G.; Willis, E.; Chatterjee, S.; Fernandez, R.; Dias, K.; Schedl, P. BMP signaling and the maintenance of primordial germ cell identity in Drosophila embryos. PLoS ONE 2014, 9, e88847. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, M.; Mfoundou, J.D.L.; Wang, X. Expression and distribution patterns of VEGF, TGF-β1 and HIF-1α in the ovarian follicles of Tibetan sheep. Vet. Med. Sci. 2022, 8, 2223–2229. [Google Scholar] [CrossRef]
- Quezada, M.; Wang, J.; Hoang, V.; McGee, E.A. Smad7 is a transforming growth factor-beta–inducible mediator of apoptosis in granulosa cells. Fertil. Steril. 2012, 97, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wen, H.; Wang, C.; Li, Q. SMAD7 antagonizes key TGFβ superfamily signaling in mouse granulosa cells in vitro. Reproduction 2013, 146, 1–11. [Google Scholar] [CrossRef]
- Lian, G.Y.; Wang, Q.M.; Tang, P.M.; Zhou, S.; Huang, X.R.; Lan, H.Y. Combination of Asiatic Acid and Naringenin Modulates NK Cell Anti-cancer Immunity by Rebalancing Smad3/Smad7 Signaling. Mol. Ther. 2018, 26, 2255–2266. [Google Scholar] [CrossRef]
- da Silveira, J.C.; Carnevale, E.M.; A Winger, Q.; Bouma, G.J. Regulation of ACVR1 and ID2 by cell-secreted exosomes during follicle maturation in the mare. Reprod. Biol. Endocrinol. 2014, 12, 44. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, Q.; Niu, M.; Hou, J.; Zhu, Z.; Sun, M.; Li, Z.; He, Z. BMP4 promotes mouse iPS cell differentiation to male germ cells via Smad1/5, Gata4, Id1 and Id2. Reproduction 2017, 153, 211–220. [Google Scholar] [CrossRef]
- Abir, R.; Ben-Haroush, A.; Melamed, N.; Felz, C.; Krissi, H.; Fisch, B. Expression of bone morphogenetic proteins 4 and 7 and their receptors IA, IB, and II in human ovaries from fetuses and adults. Fertil. Steril. 2008, 89, 1430–1440. [Google Scholar] [CrossRef]
- Stephens, C.S.; Johnson, P.A. Bone morphogenetic protein 15 may promote follicle selection in the hen. Gen. Comp. Endocrinol. 2016, 235, 170–176. [Google Scholar] [CrossRef]
- Kalous, J.; Aleshkina, D.; Anger, M. A role of PI3K/Akt signaling in oocyte maturation and early embryo development. Cells 2023, 12, 1830. [Google Scholar] [CrossRef] [PubMed]
- Baddela, V.S.; Michaelis, M.; Tao, X.; Koczan, D.; Brenmoehl, J.; Vanselow, J. Comparative analysis of PI3K-AKT and MEK-ERK1/2 signaling-driven molecular changes in granulosa cells. Reproduction 2025, 169, e240317. [Google Scholar] [CrossRef]
- Baumgarten, S.C.; Convissar, S.M.; Zamah, A.M.; Fierro, M.A.; Winston, N.J.; Scoccia, B.; Stocco, C. FSH regulates IGF-2 expression in human granulosa cells in an AKT-dependent manner. J. Clin. Endocrinol. Metab. 2015, 100, E1046–E1055. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Mao, Y.; Zhou, W.; Jiang, Y. Dynamic changes in the follicular transcriptome and promoter DNA methylation pattern of steroidogenic genes in chicken follicles throughout the ovulation cycle. PLoS ONE 2015, 10, e0146028. [Google Scholar] [CrossRef] [PubMed]
- Kowanetz, M.; Valcourt, U.; Bergström, R.; Heldin, C.H.; Moustakas, A. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor beta and bone morphogenetic protein. Mol. Cell. Biol. 2004, 24, 4241–4254. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Miyazono, K. Regulation of TGF-β family signaling by inhibitory Smads. Cold Spring Harb. Perspect. Biol. 2017, 9, a022095. [Google Scholar] [CrossRef] [PubMed]
- Sakata-Goto, T.; Takahashi, K.; Kiso, H.; Huang, B.; Tsukamoto, H.; Takemoto, M.; Hayashi, T.; Sugai, M.; Nakamura, T.; Yokota, Y.; et al. Id2 controls chondrogenesis acting downstream of BMP signaling during maxillary morphogenesis. Bone 2012, 50, 69–78. [Google Scholar] [CrossRef]
- Male, V.; Nisoli, I.; Kostrzewski, T.; Allan, D.S.; Carlyle, J.R.; Lord, G.M.; Wack, A.; Brady, H.J. The transcription factor E4bp4/Nfil3 controls commitment to the NK lineage and directly regulates Eomes and Id2 expression. J. Exp. Med. 2014, 211, 635–642. [Google Scholar] [CrossRef] [PubMed]


| Genes | Primer Sequence (5′→3′) | Amplicon Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| ID2 | F: CTTCCCTCCTCCCAATCGCT R: GCAGGCAATCACCATTCAATAAAC | 1100 | 56 |
| ID2-1 | F: GCTGTGGAAGGAGTCGTGT R: GCGCTTTGCTGTCATTTG | 207 | 58 |
| QID2 | F: TCCAGTGAGGTCCGTTAGG R: TGATGTCGGTGTTGAGGGT | 317 | 58 |
| ACTB | F: GCAGATGTGGATCAGCAAGC R: TCTCGTTTTCTGCGCAAGTT | 113 | 58 |
| Genes | Primer Sequence (5′→3′) | Amplicon Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| ID1 | F: CCAGAACCGCAAGGTGAGCA R: CGCCGATTTCGCCATTGA | 161 | 58 |
| ID3 | F: CTCTGGCTTTCTCCTTCCTT R: CGAGTAGCAGTGGTTCATGTC | 195 | 58 |
| RBX1 | F: CAGCGATGGATGTGGATACC R: CTCGCTGGCTCAAAACACG | 370 | 58 |
| BMPR2 | F: GCAGGTTCTGGTGTCTAGGG R: GTGACAGGTTGCGCTCATTC | 251 | 58 |
| SMAD7 | F: GTGCTCAAGAAACTGAAGGAAC R: TCGCAGAGTCGGCTAAGGT | 314 | 58 |
| NFIL3 | F: CCGAAGACTTGACGACAGG R: GCCGCTTTTCCCAATACAT | 135 | 58 |
| TCF3 | F: AAGCCACGGGACTATTTTG R: GTGCTGAACAACAGGAACTTTA | 180 | 58 |
| ACTB | F: GCAGATGTGGATCAGCAAGC R: TCTCGTTTTCTGCGCAAGTT | 133 | 58 |
| Amino Acids | Quantity | Proportion (%) | Amino Acids | Quantity | Proportion (%) |
|---|---|---|---|---|---|
| Alanine (A) | 5 | 3.7% | Leucine (L) | 17 | 12.7% |
| Arginine (R) | 5 | 3.7% | Lysine (K) | 9 | 6.7% |
| Asparagine (N) | 6 | 4.5% | Methionine (M) | 5 | 3.7% |
| Asparticacid (D) | 9 | 6.7% | Phenylalanine (F) | 2 | 1.5% |
| Cysteine (C) | 2 | 1.5% | Proline (P) | 9 | 6.7% |
| Glutamine (Q) | 7 | 5.2% | Serine (S) | 20 | 14.9% |
| Glutamate (E) | 4 | 3.0% | Threonine (T) | 6 | 4.5% |
| Glycine (G) | 4 | 3.0% | Tryptophan (W) | 0 | 0% |
| Histidine (H) | 5 | 3.7% | Tyrosine (Y) | 3 | 2.2% |
| Isoleucine (I) | 9 | 6.7% | Valine (V) | 7 | 5.2% |
| Variable | Value | Notes |
|---|---|---|
| Sample size | 157 | All genotyped animals included in the SNP–litter size analysis; all selected ewes delivered live lamb(s). |
| Sex | Female (100%) | All ewes with lambing records. |
| Herd origin | Single commercial flock | Same farm and uniform management to minimize environmental heterogeneity. |
| Age at sampling | Mean ± SD: 3.4 ± 0.7; Range: 3.0–5.0 | Age recorded at sampling. |
| Parity distribution | 1st: 45 (28.7%); 2nd: 62 (39.5%); ≥3: 50 (31.8%) | Parity = number of previous lambings at sampling. |
| Mating method | artificial insemination | artificial insemination |
| Ewes lambed | 157 (100.0%) | All sampled ewes delivered live lamb(s). |
| Total live-born lambs | 236 (total) | Sum of live lambs born within 24 h across 157 ewes. |
| Lambs per ewe | 1.50 ± 0.50 | Calculated as 236/157 ≈ 1.50. (236/157 ≈ 1.50) |
| Litter size distribution | Single (1 lamb): 78 (49.7%) Twin (2 lambs): 79 (50.3%) Triplet (3 lambs): 0 (0%)—none observed | Categories based on live-born lambs within 24 h; totals sum to 157 ewes and 236 lambs. |
| Fertility | 100.0% | Fertility = proportion of mated ewes that delivered live lamb(s). |
| Fecundity | 150.3% | Fecundity = total live lambs ÷ number of mated ewes × 100 = (236/157) × 100 ≈ 150.3%. |
| Prolificacy | 50.3% | Prolificacy reported both as mean lambs per lambed ewe (1.50) and proportion of ewes producing multiples (50.3%). |
| Location | Genotype | Genotype Frequency (Sample Size) | Allele | Allele Frequency | X2 (p-Value) |
|---|---|---|---|---|---|
| g.18202368 A>T | AA | 0.96 | A | 0.98 | 0.08 (p > 0.5) |
| AT | 0.04 | T | 0.02 | ||
| g.18202372 G>A | GG | 0.59 | G | 0.77 | 0.85 (p > 0.5) |
| GA | 0.37 | A | 0.23 | ||
| AA | 0.04 | ||||
| g.18202426 C>T | CC | 0.71 | C | 0.84 | 0.37 (p > 0.5) |
| CT | 0.26 | T | 0.16 | ||
| TT | 0.03 | ||||
| g.18202431 G>C | GG | 0.87 | G | 0.94 | 0.73 (p > 0.5) |
| GC | 0.13 | C | 0.06 | ||
| g.18202439 C>T | CC | 0.93 | C | 0.96 | 0.21 (p > 0.5) |
| CT | 0.07 | T | 0.04 | ||
| g.18202451 C>T | CC | 0.95 | C | 0.97 | 0.11 (p > 0.5) |
| CT | 0.05 | T | 0.03 | ||
| g.18202467 T>A | TT | 0.96 | T | 0.98 | 0.08 (p > 0.5) |
| TA | 0.04 | A | 0.02 | ||
| g.18202472 G>C | GG | 0.58 | G | 0.77 | 2.85 (p > 0.5) |
| GC | 0.38 | C | 0.23 | ||
| CC | 0.04 |
| Location | Genetic Homozygosity | Genetic Heterozygosity | Polymorphic Information Content | Effective Number of Alleles |
|---|---|---|---|---|
| g.18202368 A>T | 0.96 | 0.43 | 0.42 | 1.04 |
| g.18202372 G>A | 0.65 | 0.35 | 0.29 | 1.54 |
| g.18202426 C>T | 0.73 | 0.27 | 0.23 | 1.37 |
| g.18202431 G>C | 0.88 | 0.12 | 0.11 | 0.13 |
| g.18202439 C>T | 0.93 | 0.68 | 0.65 | 1.07 |
| g.18202451 C>T | 0.95 | 0.50 | 0.50 | 1.05 |
| g.18202467 T>A | 0.96 | 0.04 | 0.04 | 1.04 |
| g.18202472 G>C | 0.65 | 0.35 | 0.29 | 1.53 |
| Location | Genotype | Sample Size | Number of Lambs Litter Size |
|---|---|---|---|
| g.18202368 A>T | AA | 150 | 1.52 ± 0.501 a |
| AT | 7 | 1.14 ± 0.378 b | |
| g.18202372 G>A | GG | 92 | 1.27 ± 0.447 A |
| GA | 59 | 1.83 ± 0.378 B | |
| AA | 6 | 1.83 ± 0.408 B | |
| g.18202426 C>T | CC | 112 | 1.47 ± 0.501 |
| CT | 40 | 1.56 ± 0.502 | |
| TT | 5 | 1.8 ± 0.447 | |
| g.18202431 G>C | GG | 137 | 1.47 ± 0.501 a |
| GC | 20 | 1.75 ± 0.444 b | |
| g.18202439 C>T | CC | 146 | 1.49 ± 0.502 |
| CT | 11 | 1.73 ± 0.467 | |
| g.18202451 C>T | CC | 149 | 1.49 ± 0.502 |
| CT | 8 | 1.75 ± 0.463 | |
| g.18202467 T>A | TT | 150 | 1.51 ± 0.501 |
| TA | 7 | 1.29 ± 0.488 | |
| g.18202472 G>C | GG | 92 | 1.41 ± 0.495 a |
| GC | 59 | 1.61 ± 0.492 b | |
| CC | 6 | 1.83 ± 0.408 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Wang, X.; Zhu, L.; Shahbaz, G.M.; Gu, R.; Huang, Q.; Li, W.; Xing, F. Study on the Function of ID2 Gene in Granulosa Cells of Ovaries of Hetian Sheep and Its Correlation Analysis with Lambing Traits. Animals 2025, 15, 3271. https://doi.org/10.3390/ani15223271
Sun H, Wang X, Zhu L, Shahbaz GM, Gu R, Huang Q, Li W, Xing F. Study on the Function of ID2 Gene in Granulosa Cells of Ovaries of Hetian Sheep and Its Correlation Analysis with Lambing Traits. Animals. 2025; 15(22):3271. https://doi.org/10.3390/ani15223271
Chicago/Turabian StyleSun, Huiping, Xinkun Wang, Lexiao Zhu, Gul Muhammad Shahbaz, Ruohuai Gu, Qiaoyan Huang, Wei Li, and Feng Xing. 2025. "Study on the Function of ID2 Gene in Granulosa Cells of Ovaries of Hetian Sheep and Its Correlation Analysis with Lambing Traits" Animals 15, no. 22: 3271. https://doi.org/10.3390/ani15223271
APA StyleSun, H., Wang, X., Zhu, L., Shahbaz, G. M., Gu, R., Huang, Q., Li, W., & Xing, F. (2025). Study on the Function of ID2 Gene in Granulosa Cells of Ovaries of Hetian Sheep and Its Correlation Analysis with Lambing Traits. Animals, 15(22), 3271. https://doi.org/10.3390/ani15223271






