Molecular Mechanisms Underpinning Astaxanthin-Induced Body Coloration in the Lutjanus erythropterus Revealed by Phenotypic, Physiological and Transcriptomic Analyses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sample Collection
2.2. Fish Growth Indice
2.3. Body Color Measurement
2.4. Total Carotenoid Content Extraction and Full-Wavelength Scanning of Various Tissues
2.5. Determination of Antioxidant Enzyme Activity in Liver
2.6. Transcriptome Sequencing and Data Processing
2.7. Differential Gene Expression Analysis, GO and KEGG Enrichment Analysis
2.8. RT-qPCR
2.9. Statistical Analysis
3. Results
3.1. The Effect of Astaxanthin on the Growth Performance of L. erythropterus
3.2. The Effect of Astaxanthin on Color Change of L. erythropterus
3.3. The Effect of Astaxanthin on Total Carotenoid Content in Various Tissues of the L. erythropterus
3.4. The Effect of Astaxanthin on Antioxidant Enzyme Activity in the Liver of the L. erythropterus
3.5. Transcriptome Sequencing Results and Reference Genome Alignments
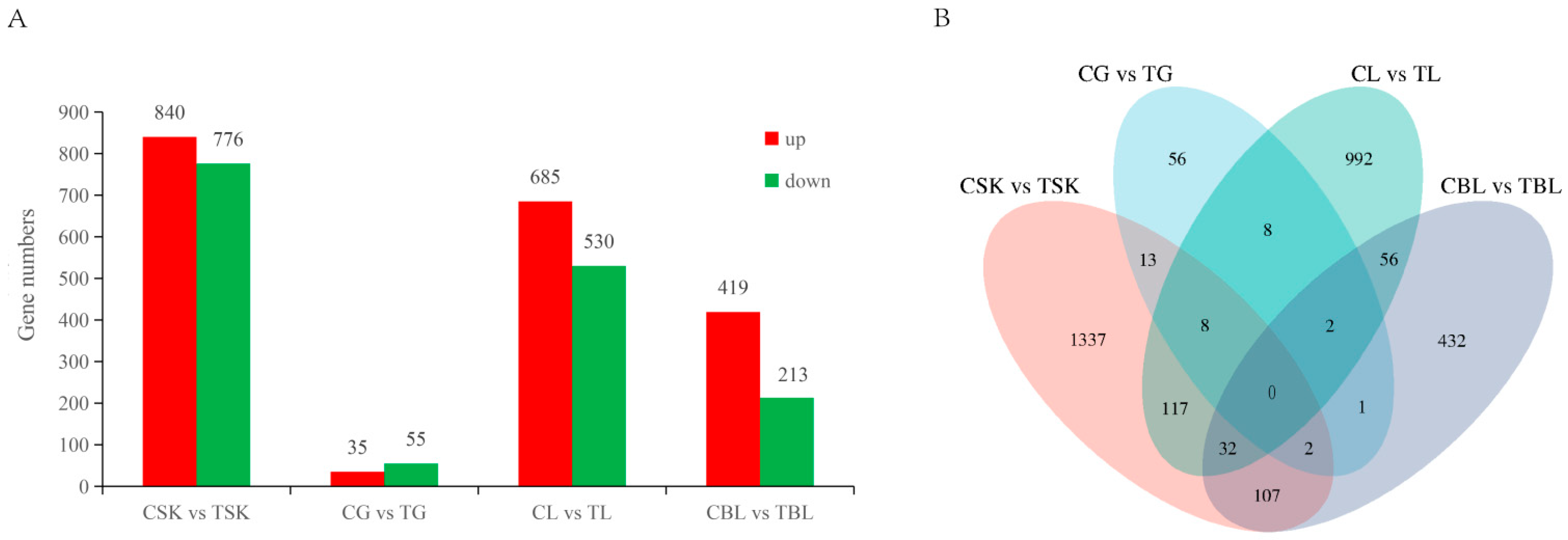
3.6. Differentially Expressed Gene Analysis
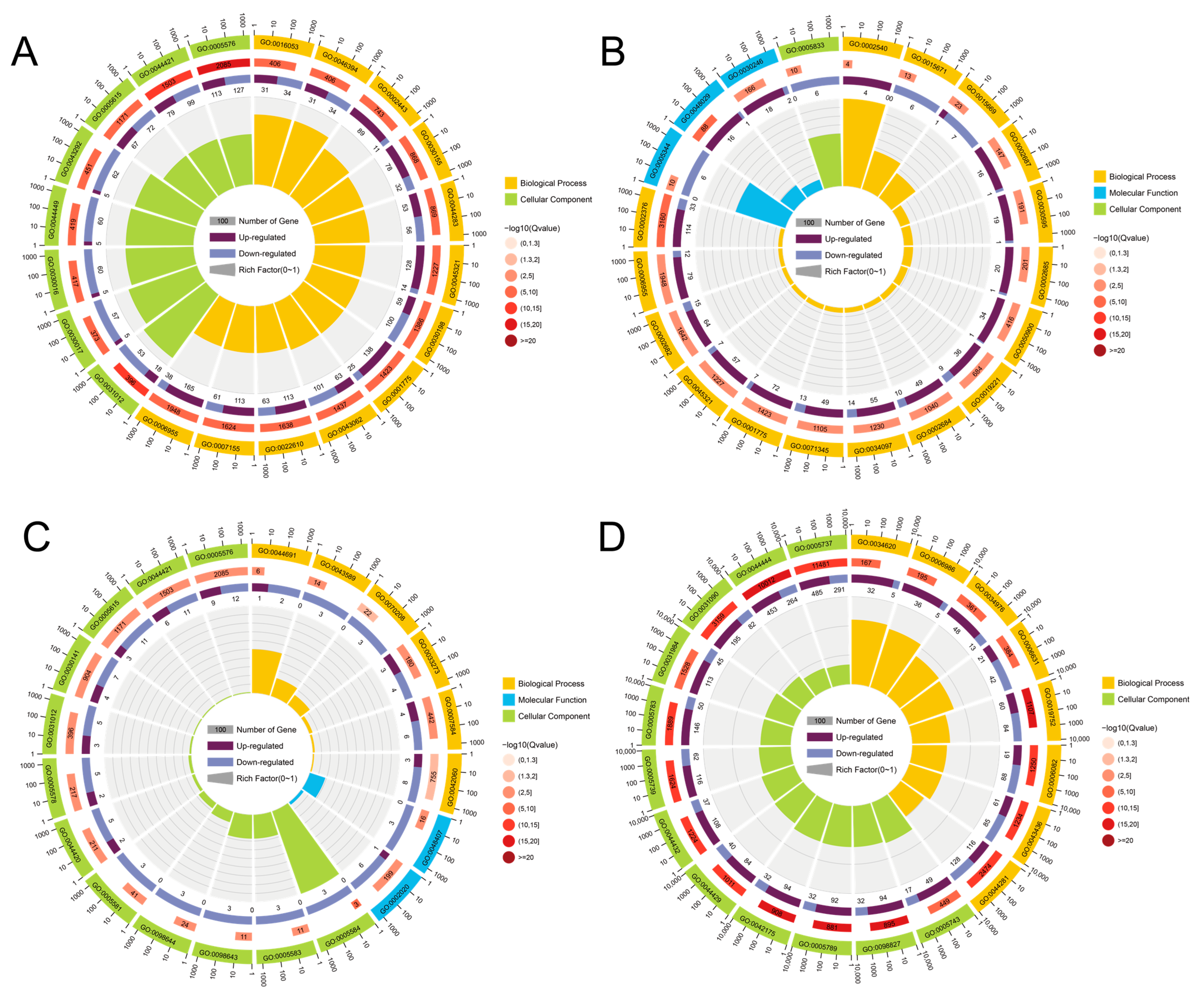
3.7. GO Enrichment Analysis
3.8. KEGG Enrichment Analysis
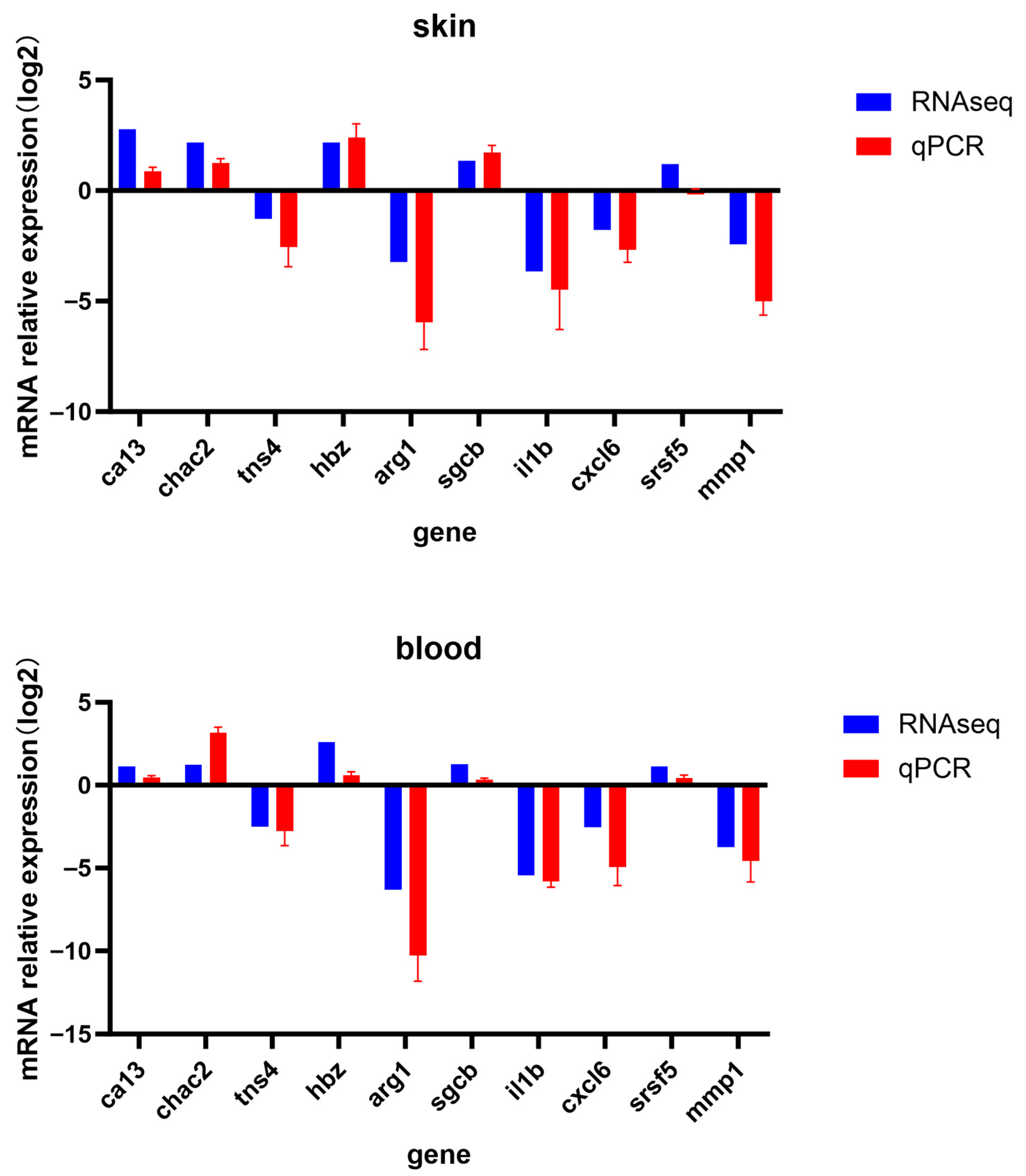
3.9. Validation with RT- qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food Agriculture Organization Ofunited. The State of World Fisheries and Aquaculture 2010. In State of World Fisheries and Aquaculture, Arabic ed.; FAO: Rome, Italy, 2010. [Google Scholar]
- World Bank. Fish to 2030: Prospects for Fisheries and Aquaculture; CGIAR: Montpellier, France, 2013. [Google Scholar]
- Halim, L.J.; Rahim, I.; Mahboob, S.; Al-Ghanim, K.; Amat, A.; Naim, D.M. Phylogenetic Relationships of the Commercial Red Snapper (Lutjanidae sp.) from Three Marine Regions. J. King Saud. Univ. Sci. 2022, 34, 101756. [Google Scholar] [CrossRef]
- Harnessing Hue: Advances and Applications of Fish Skin Pigmentation Genetics in Aquaculture. Available online: https://www.mdpi.com/2410-3888/9/6/220 (accessed on 4 November 2025).
- Ahi, E.P.; Lecaudey, L.A.; Ziegelbecker, A.; Steiner, O.; Glabonjat, R.; Goessler, W.; Hois, V.; Wagner, C.; Lass, A.; Sefc, K.M. Comparative Transcriptomics Reveals Candidate Carotenoid Color Genes in an East African Cichlid Fish. BMC Genom. 2020, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Poon, Z.W.J.; Shen, X.; Uichanco, J.A.; Terence, C.; Chua, S.W.G.; Domingos, J.A. Comparative Transcriptome Analysis Reveals Factors Involved in the Influence of Dietary Astaxanthin on Body Colouration of Malabar Snapper (Lutjanus malabaricus). Aquaculture 2023, 562, 738874. [Google Scholar] [CrossRef]
- Tu, N.P.C.; Ha, N.N.; Linh, N.T.T.; Tri, N.N. Effect of Astaxanthin and Spirulina Levels in Black Soldier Fly Larvae Meal-Based Diets on Growth Performance and Skin Pigmentation in Discus Fish, Symphysodon sp. Aquaculture 2022, 553, 738048. [Google Scholar] [CrossRef]
- David, M.A. Effect of Dietary Astaxanthin on Growth, Physiology, Body Color, Transcriptome and Metabolome Profiling of Juvenile Blood Parrotfish (Vieja Melanurus ♀ × Amphilophus Citrinellus ♂). Aquac. Rep. 2022, 24, 101142. [Google Scholar]
- Wang, M.; Ding, H.; Wu, S.; Wang, M.; Ma, J.; Xiao, J.; Bao, Z.; Wang, B.; Hu, J. Comparative Transcriptome Analysis Provides New Insights into the Protective Effect of Astaxanthin on the Liver of Leopard Coral Grouper (Plectropomus leopardus). Aquaculture 2023, 565, 739118. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, H.-L.; Chen, M.-D.; Yao, R.; Wang, Z.-Q.; Niu, J. Effects of Synthetic Astaxanthin and Haematococcus pluvialis on Growth, Antioxidant Capacity, Immune Response, and Hepato-Morphology of Oncorhynchus mykiss under Cage Culture with Flowing Freshwater. Aquaculture 2023, 562, 738860. [Google Scholar] [CrossRef]
- Yi, X.; Xu, W.; Zhou, H.; Zhang, Y.; Luo, Y.; Zhang, W.; Mai, K. Effects of Dietary Astaxanthin and Xanthophylls on the Growth and Skin Pigmentation of Large Yellow Croaker Larimichthys Croceus. Aquaculture 2014, 433, 377–383. [Google Scholar] [CrossRef]
- Nogueira, N.; Canada, P.; Caboz, J.; Andrade, C.; Cordeiro, N. Effect of Different Levels of Synthetic Astaxanthin on Growth, Skin Color and Lipid Metabolism of Commercial Sized Red Porgy (Pagrus pagrus). Anim. Feed. Sci. Technol. 2021, 276, 114916. [Google Scholar] [CrossRef]
- Liu, F.; Sun, F.; Kuang, G.Q.; Wang, L.; Yue, G.H. Identification of Pmel17 for Golden Skin Color Using Linkage Mapping in Mozambique Tilapia. Aquaculture 2022, 548, 737703. [Google Scholar] [CrossRef]
- Fang, W.; Huang, J.; Li, S.; Lu, J. Identification of Pigment Genes (Melanin, Carotenoid and Pteridine) Associated with Skin Color Variant in Red Tilapia Using Transcriptome Analysis. Aquaculture 2022, 547, 737429. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, L.; Dong, Z.; Chen, X.; Song, F.; Liu, N.; Yang, H.; Fu, J. Comparative Transcriptome Analysis Identifies Candidate Genes Related to Skin Color Differentiation in Red Tilapia. Sci. Rep. 2016, 6, 31347. [Google Scholar] [CrossRef] [PubMed]
- Djurdjevič, I.; Furmanek, T.; Miyazawa, S.; Bajec, S.S. Comparative Transcriptome Analysis of Trout Skin Pigment Cells. BMC Genom. 2019, 20, 359. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, J.; Li, Y.; Zhao, L.; Liu, Z. Analysis of Yellow Mutant Rainbow Trout Transcriptomes at Different Developmental Stages Reveals Dynamic Regulation of Skin Pigmentation Genes. Sci. Rep. 2022, 12, 256. [Google Scholar] [CrossRef]
- Schmeisser, J.; Verlhac-Trichet, V.; Madaro, A.; Lall, S.P.; Torrissen, O.; Olsen, R.E. Molecular Mechanism Involved in Carotenoid Metabolism in Post-Smolt Atlantic Salmon: Astaxanthin Metabolism During Flesh Pigmentation and Its Antioxidant Properties. Mar. Biotechnol. 2021, 23, 653–670. [Google Scholar] [CrossRef]
- Hoekstra, H.E. Genetics, Development and Evolution of Adaptive Pigmentation in Vertebrates. Heredity 2006, 97, 222–234. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms Involved in the Intestinal Absorption of Dietary Vitamin A and Provitamin A Carotenoids. Biochim. Biophys. Acta 2012, 1821, 70–77. [Google Scholar] [CrossRef]
- Bohn, T.; Desmarchelier, C.; El, S.N.; Keijer, J.; van Schothorst, E.; Rühl, R.; Borel, P. β-Carotene in the Human Body: Metabolic Bioactivation Pathways—From Digestion to Tissue Distribution and Excretion. Proc. Nutr. Soc. 2019, 78, 68–87. [Google Scholar] [CrossRef]
- von Lintig, J.; Moon, J.; Lee, J.; Ramkumar, S. Carotenoid Metabolism at the Intestinal Barrier. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158580. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Liang, Q.L.; Xu, Z.M.; Wang, Z.D.; Guo, Y.S. Development of morphology and chromatophores in larval, juvenile and young Crimson Snapper (Lutjanus erythropterus). J. Guangdong Ocean. Univ. 2022, 42, 116–122. [Google Scholar]
- Zhang, Y.-P.; Wang, Z.-D.; Guo, Y.-S.; Liu, L.; Yu, J.; Zhang, S.; Liu, S.-J.; Liu, C.-W. Morphological Characters and Transcriptome Profiles Associated with Black Skin and Red Skin in Crimson Snapper (Lutjanus erythropterus). Int. J. Mol. Sci. 2015, 16, 26991–27004. [Google Scholar] [CrossRef]
- He, S.Q.; Li, R.M.; Yang, Q.H.; Tan, B.P.; Dong, X.H.; Chi, S.Y.; Zhang, S.; Liu, H.Y. Effects of zinc on growth, non-specific immune parameters, disease resistance and intestinal microbiota structure of Litopenaeus vannamei. J. Fish. China 2021, 45, 1726–1739. [Google Scholar]
- Luo, M.; Lu, G.; Yin, H.; Wang, L.; Atuganile, M.; Dong, Z. Fish Pigmentation and Coloration: Molecular Mechanisms and Aquaculture Perspectives. Rev. Aquac. 2021, 13, 2395–2412. [Google Scholar] [CrossRef]
- Fujii, R. The Regulation of Motile Activity in Fish Chromatophores. Pigment. Cell Res. 2000, 13, 300–319. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Karim, M.; Natrah, F.M.I. Carotenoids Modulate Stress Tolerance and Immune Responses in Aquatic Animals. Rev. Aquac. 2023, 15, 872–894. [Google Scholar] [CrossRef]
- Jiang, Z.; Cui, P.; Qin, Q.; Liu, F.; Gao, X.; Tian, Q.; Zhou, X. Deposition and distribution of carotenoids in tissues and organs of koi carp. J. Dalian Ocean. Univ. 2012, 27, 22–26. [Google Scholar] [CrossRef]
- Sun, L.; Li, Y.; Yang, A.; Xie, M.; Xiong, R.; Huang, C. Astaxanthin: A Comprehensive Review of Synthesis, Biological Activities and Applications. Food Chem. 2025, 488, 144847. [Google Scholar] [CrossRef]
- Bharti, A.; Hooda, V.; Jain, U.; Chauhan, N. Astaxanthin: A Nature’s Versatile Compound Utilized for Diverse Applications and Its Therapeutic Effects. 3 Biotech. 2025, 15, 88. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, Z.; Li, Q.; Yang, H. Current Challenges and Issues in the Application of Astaxanthin. Fishes 2025, 10, 159. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, S. Effect of Dietary Astaxanthin on the Growth Performance and Nonspecific Immunity of Red Swamp Crayfish Procambarus Clarkii. Aquaculture 2019, 512, 734341. [Google Scholar] [CrossRef]
- Wang, W.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Hossain, M.S.; Moss, A.S. Effects of Dietary Astaxanthin Supplementation on Juvenile Kuruma Shrimp, Marsupenaeus japonicus. Aquaculture 2018, 491, 197–204. [Google Scholar] [CrossRef]
- Harith, Z.T.; Sukri, S.M.; Remlee, N.F.S.; Sabir, F.N.M.; Zakaria, N.N.A. Effects of Dietary Astaxanthin Enrichment on Enhancing the Colour and Growth of Red Tilapia, Oreochromis sp. Aquac. Fish. 2024, 9, 52–56. [Google Scholar] [CrossRef]
- Niu, J.; Wen, H.; Li, C.-H.; Liu, Y.-J.; Tian, L.-X.; Chen, X.; Huang, Z.; Lin, H.-Z. Comparison Effect of Dietary Astaxanthin and β-Carotene in the Presence and Absence of Cholesterol Supplementation on Growth Performance, Antioxidant Capacity and Gene Expression of Penaeus monodon under Normoxia and Hypoxia Condition. Aquaculture 2014, 422–423, 8–17. [Google Scholar] [CrossRef]
- Montero, D.; Tort, L.; Robaina, L.; Vergara, J.M.; Izquierdo, M.S. Low Vitamin E in Diet Reduces Stress Resistance of Gilthead Seabream (Sparus aurata) Juveniles. Fish. Shellfish. Immunol. 2001, 11, 473–490. [Google Scholar] [CrossRef]
- Tocher, D.; Mourente, G.; Van der Eecken, A.; Evjemo, J.; Diaz, E.; Wille, M.; Bell, J.; Olsen, Y. Comparative Study of Antioxidant Defence Mechanisms in Marine Fish Fed Variable Levels of Oxidised Oil and Vitamin E. Aquac. Int. 2003, 11, 195–216. [Google Scholar] [CrossRef]
- Wang, C.; Wachholtz, M.; Wang, J.; Liao, X.; Lu, G. Analysis of the Skin Transcriptome in Two Oujiang Color Varieties of Common Carp. PLoS ONE 2014, 9, e90074. [Google Scholar] [CrossRef]
- Madaro, A.; Torrissen, O.; Whatmore, P.; Lall, S.P.; Schmeisser, J.; Trichet, V.V.; Olsen, R.E. Red and White Chinook Salmon (Oncorhynchus tshawytscha): Differences in the Transcriptome Profile of Muscle, Liver, and Pylorus. Mar. Biotechnol. 2020, 22, 581–593. [Google Scholar] [CrossRef]
- Gao, G.-Q.; Song, L.-S.; Tong, B.; Li, G.-P. Expression Levels of GSTA2 and APOD Genes Might Be Associated with Carotenoid Coloration in Golden Pheasant (Chrysolophus pictus) Plumage. Zool. Res. 2016, 37, 144–150. [Google Scholar] [CrossRef]
- Zhu, X.; Hao, R.; Tian, C.; Zhang, J.; Zhu, C.; Li, G. Integrative Transcriptomics and Metabolomics Analysis of Body Color Formation in the Leopard Coral Grouper (Plectropomus leopardus). Front. Mar. Sci. 2021, 8, 726102. [Google Scholar] [CrossRef]
- Toomey, M.B.; Lopes, R.J.; Araújo, P.M.; Johnson, J.D.; Gazda, M.A.; Afonso, S.; Mota, P.G.; Koch, R.E.; Hill, G.E.; Corbo, J.C.; et al. High-Density Lipoprotein Receptor SCARB1 Is Required for Carotenoid Coloration in Birds. Proc. Natl. Acad. Sci. USA 2017, 114, 5219–5224. [Google Scholar] [CrossRef]
- Widjaja-Adhi, M.A.K.; Lobo, G.P.; Golczak, M.; Von Lintig, J. A Genetic Dissection of Intestinal Fat-Soluble Vitamin and Carotenoid Absorption. Hum. Mol. Genet. 2015, 24, 3206–3219. [Google Scholar] [CrossRef]
- Reboul, E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef]




| Ingredient | A0 | A1 |
|---|---|---|
| Red fish meal | 71 | 71 |
| Soybean meal | 6 | 6 |
| Peanut meal | 5 | 5 |
| Corn gluten powder | 5 | 5 |
| Bread flour | 2 | 2 |
| Fish oil | 5 | 5 |
| Soybean lecithin | 3.5 | 3.5 |
| Ca(H2PO4)2 | 1 | 1 |
| Mineral premix a | 0.5 | 0.5 |
| Feeding attractant | 0.05 | 0.05 |
| Vitamin C | 0.05 | 0.05 |
| Antioxidants | 0.05 | 0.05 |
| Choline chloride | 0.5 | 0.5 |
| Microcrystalline cellulose | 0.35 | 0.15 |
| (10%) Astaxanthin b | 0 | 0.2 |
| Total | 100 | 100 |
| Nutrient levels | ||
| Moisture | 10.1 | 10.7 |
| Crude protein | 53.06 | 52.54 |
| Crude fat | 11.1 | 11.2 |
| Ash | 14.3 | 14.7 |
| Gene | Primer (5′-3′) |
|---|---|
| rab10 | F: GAGGGTCGTACCAAAAGCCA |
| R: GTTGGCCTTAGCACTCGTCT | |
| arg1 | F: ATCGGCTCCATCCACGGTCAC |
| R: ACACCTTCACACCCAGGAGCTT | |
| il1b | F: AAAACCTGCTCAACATCATGCT |
| R: GTTAGTTCCTTCACTGCCTCCC | |
| mmp1 | F: CATCGCCAGTTTCTCCACGTT |
| R: CGCTGTAGATCCTTGTGAACCTC | |
| cxcl6 | F: GCTGATTCTGCCTAACTCACAC |
| R: GACTTTCTTCACCCAGGGAGC | |
| tns4 | F: GACTGATATTCCTGTGCTGCT |
| R: AATGTTCCTGCTGTCTTGTCC | |
| srsf5 | F: ACTTGTCCTCTCGTGTCAGC |
| R: ACTTCTCGACCTCTTCTTGGC | |
| hbz | F: GACCAAGACTTACTTCGCCCACT |
| R: AGCAGCCAGAAACTTGTCCAC | |
| ca13 | F: CCAACCCCAGGATTCAGAGAGT |
| R: AGCCTCTCCTTCTGCAGTGA | |
| chac2 | F: ATCGGCTACATTAAAGGCTTC |
| R: CCGTGATGACCTGATAACCAC | |
| sgcb | F: ACTACACAAGAGCACCGTA |
| R: TCCCCTTTAATGTTCAGGTCA |
| Parameter | C4 | T4 |
|---|---|---|
| Initial body length/cm | 4.81 ± 0.32 | 4.81 ± 0.32 |
| Initial weight/g | 3.29 ± 0.17 | 3.29 ± 0.17 |
| Final body length/cm | 6.76 ± 0.38 a | 7.57 ± 0.67 b |
| Final weight/g | 5.59 ± 0.96 a | 8.21 ± 2.35 b |
| Body length growth rate/% PLG | 16.85 ± 6.71 a | 31.01 ± 11.72 b |
| Weight gain rate/% WGR | 69.88 ± 29.52 a | 149.46 ± 72.41 b |
| Specific growth rate/(%/d) SGR | 1.84 ± 0.61 a | 3.13 ± 0.98 b |
| Condition factor CF | 3.13 ± 0.30 | 3.20 ± 0.18 |
| Survival rate/% SR | 90.0 | 95.6 |
| Location | Chromaticity Value | C4 | T4 | T6 |
|---|---|---|---|---|
| Ventral skin | L* | 77.86 ± 20.75 a | 82.30 ± 9.65 b | 85.55 ± 6.27 b |
| a* | −5.97 ± 10.47 a | 12.76 ± 5.81 b | 16.30 ± 4.54 b | |
| b* | 1.48 ± 18.92 a | 19.70 ± 6.61 b | 19.52 ± 6.73 b | |
| Dorsal skin | L* | 69.55 ± 4.60 a | 62.30 ± 4.98 a | 61.92 ± 3.10 a |
| a* | 3.33 ± 0.94 a | 4.34 ± 1.29 b | 5.46 ± 1.37 b | |
| b* | 9.58 ± 2.22 a | 6.31 ± 1.72 a | 7.04 ± 1.35 a | |
| Gill cover | L* | 14.39 ± 5.52 a | 54.59 ± 37.19 b | 40.00 ± 39.76 b |
| a* | −90.83 ± 20.42 a | −17.08 ± 37.92 b | −30.30 ± 44.15 b | |
| b* | −23.08 ± 11.99 a | −0.71 ± 28.82 b | −12.06 ± 32.60 b |
| Tissue | C4 | T4 | T6 |
|---|---|---|---|
| Skin | 52.38 ± 10.71 a | 79.30 ± 28.16 a | 110.62 ± 5.48 a |
| Muscle | 16.72 ± 0.26 c | 21.40 ± 4.81 b | 17.89 ± 0.57 d |
| Intestine | 23.94 ± 6.17 c | 31.87 ± 2.29 b | 20.85 ± 1.70 d |
| Liver | 44.90 ± 8.35 ab | 31.05 ± 5.26 b | 34.64 ± 3.24 c |
| Eyes | 31.07 ± 4.16 bc | 35.45 ± 3.43 b | 48.17 ± 2.10 b |
| Blood | 18.40 ± 0.98 c | 19.87 ± 1.60 b | 23.46 ± 8.22 d |
| Samples | Clean Reads | Clean Bases (G) | Effective Rate (%) | Q30 | GC Content (%) |
|---|---|---|---|---|---|
| CBL-1 | 72,913,062 | 10.94 | 93.53 | 91.8 | 49.0 |
| CBL-2 | 77,026,538 | 11.55 | 94.06 | 92.0 | 49.3 |
| CBL-3 | 80,300,150 | 12.05 | 93.73 | 91.7 | 49.0 |
| CG-1 | 61,788,340 | 9.27 | 90.70 | 93.6 | 46.7 |
| CG-2 | 59,065,970 | 8.86 | 94.34 | 93.3 | 46.6 |
| CG-3 | 70,387,004 | 10.56 | 95.43 | 92.1 | 47.2 |
| CL-1 | 73,115,202 | 10.97 | 95.34 | 93.8 | 46.7 |
| CL-2 | 74,479,514 | 11.17 | 95.08 | 93.3 | 46.8 |
| CL-3 | 73,981,624 | 11.10 | 94.05 | 93.7 | 46.7 |
| CSK-1 | 5,7442,268 | 8.62 | 94.68 | 92.9 | 46.9 |
| CSK-2 | 56,544,130 | 8.48 | 94.64 | 92.1 | 47.5 |
| CSK-3 | 72,659,662 | 10.90 | 91.54 | 92.5 | 47.8 |
| TBL-1 | 64,366,828 | 9.66 | 92.98 | 91.6 | 49.3 |
| TBL-2 | 69,536,200 | 10.43 | 94.85 | 91.7 | 49.4 |
| TBL-3 | 79,400,088 | 11.91 | 92.55 | 91.7 | 49.6 |
| TG-1 | 53,779,626 | 8.07 | 95.16 | 92.9 | 47.0 |
| TG-2 | 62,145,380 | 9.32 | 95.31 | 92.9 | 47.2 |
| TG-3 | 61,689,482 | 9.25 | 94.35 | 92.5 | 46.4 |
| TL-1 | 65,672,548 | 9.85 | 92.42 | 92.9 | 45.8 |
| TL-2 | 73,566,852 | 11.04 | 93.72 | 92.6 | 47.1 |
| TL-3 | 73,708,750 | 11.06 | 95.50 | 91.9 | 47.7 |
| TSK-1 | 51,675,468 | 7.75 | 96.48 | 92.0 | 48.3 |
| TSK-2 | 60,077,824 | 9.01 | 86.32 | 93.2 | 46.8 |
| TSK-3 | 53,900,786 | 8.09 | 94.14 | 92.5 | 48.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Chen, Z.; Lai, Z.; Feng, W.; Wang, Z.; Guo, Y. Molecular Mechanisms Underpinning Astaxanthin-Induced Body Coloration in the Lutjanus erythropterus Revealed by Phenotypic, Physiological and Transcriptomic Analyses. Animals 2025, 15, 3257. https://doi.org/10.3390/ani15223257
Song L, Chen Z, Lai Z, Feng W, Wang Z, Guo Y. Molecular Mechanisms Underpinning Astaxanthin-Induced Body Coloration in the Lutjanus erythropterus Revealed by Phenotypic, Physiological and Transcriptomic Analyses. Animals. 2025; 15(22):3257. https://doi.org/10.3390/ani15223257
Chicago/Turabian StyleSong, Lei, Zizhao Chen, Zhuoxin Lai, Wenjun Feng, Zhongduo Wang, and Yusong Guo. 2025. "Molecular Mechanisms Underpinning Astaxanthin-Induced Body Coloration in the Lutjanus erythropterus Revealed by Phenotypic, Physiological and Transcriptomic Analyses" Animals 15, no. 22: 3257. https://doi.org/10.3390/ani15223257
APA StyleSong, L., Chen, Z., Lai, Z., Feng, W., Wang, Z., & Guo, Y. (2025). Molecular Mechanisms Underpinning Astaxanthin-Induced Body Coloration in the Lutjanus erythropterus Revealed by Phenotypic, Physiological and Transcriptomic Analyses. Animals, 15(22), 3257. https://doi.org/10.3390/ani15223257






