Oleic Acid Improves Goat Sperm Quality by Enhancing the MBOAT2/ACSL3 Pathway to Attenuate Ferroptosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Semen Collection
2.3. Semen Processing
2.4. Experiment Design
2.5. Measured Parameters
2.6. Preparation of the Stock of BSA Dissolved Oleic Acid
2.7. Evaluation of Sperm Motility by Computer-Assisted Sperm Analysis (CASA) System
2.8. Evaluation of Acrosomal Integrity and Viable Spermatozoa Preserved
2.9. Evaluation of Mitochondrial Activity
2.10. Evaluation of Sperm ROS Content
2.11. Measurement of Sperm MDA Levels
2.12. Measurement of Sperm LPO Levels
2.13. Measurement of MBOAT2 Enzymatic Activity
2.14. Measurement of Ferrous Iron and Total Iron Contents
2.15. Immunofluorescence Localization
2.16. Western Blotting
2.17. Statistical Analysis
3. Results
3.1. Addition of OA Improved Sperm Motility Parameters
3.2. Addition of OA Improved the Sperm Acrosomal Integrity and Viable Spermatozoa Preserved
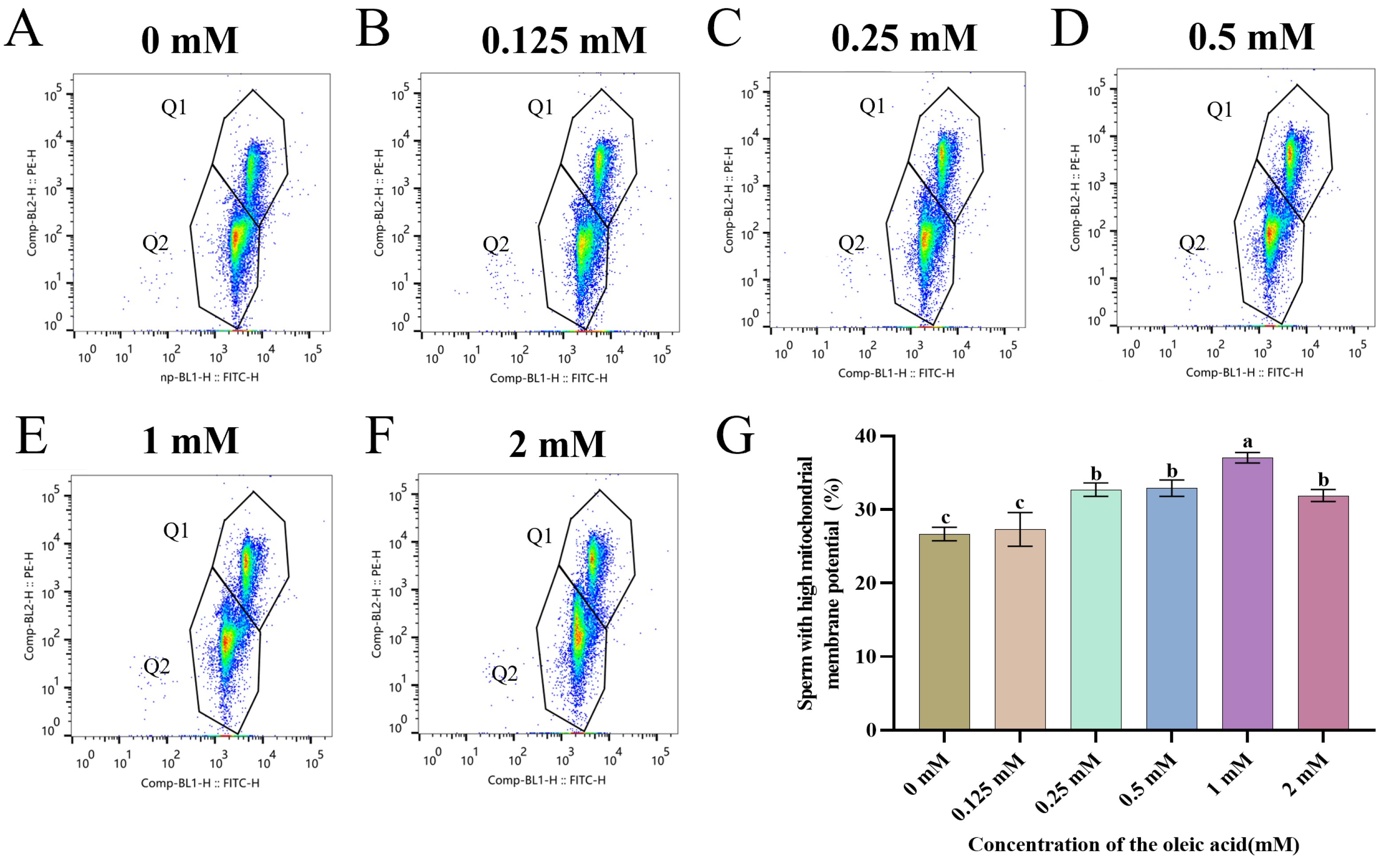
3.3. Addition of OA Improved Sperm Mitochondrial Activity
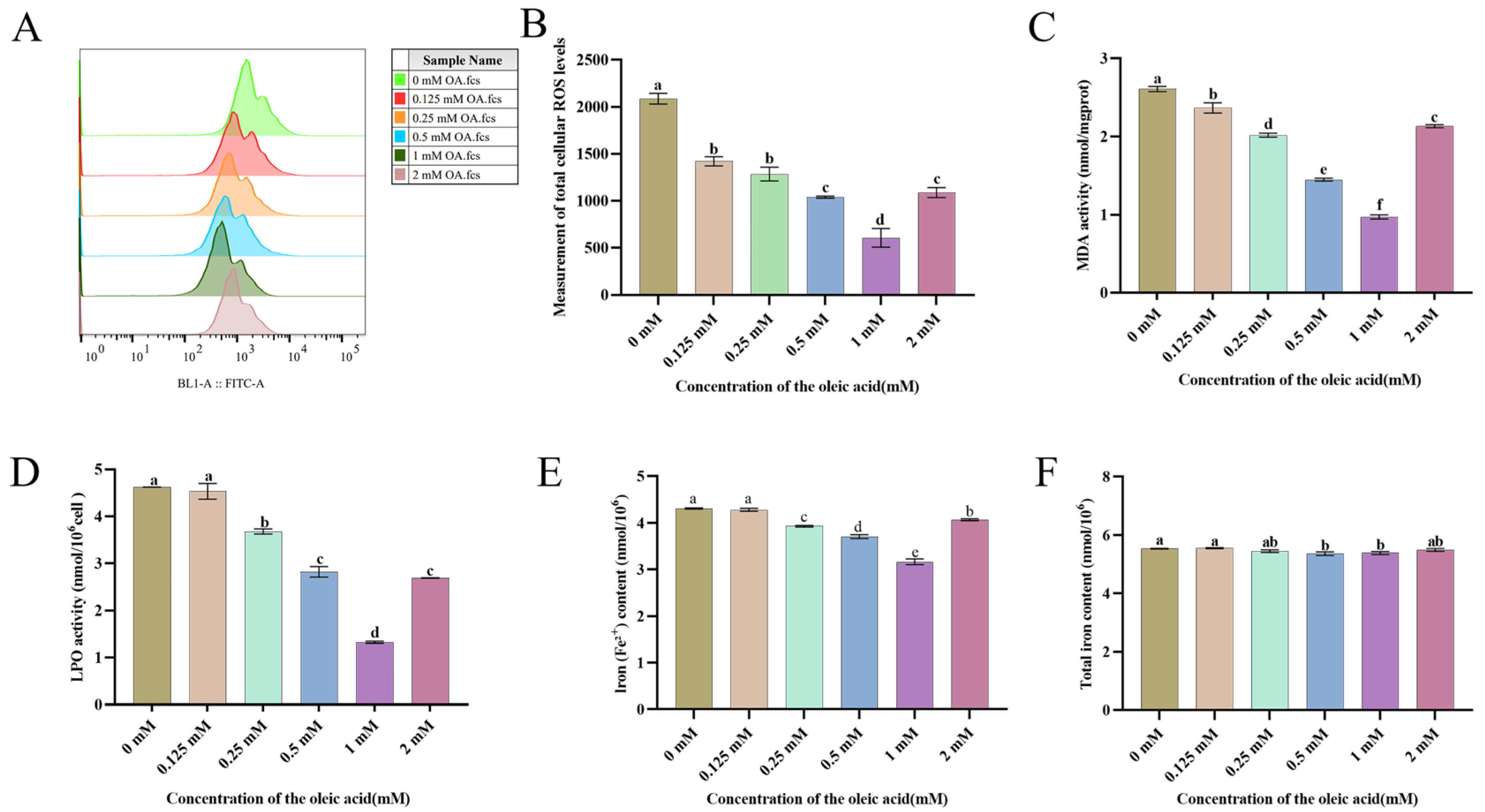
3.4. OA Attenuates Chilling-Induced Oxidative Damage in Goat Sperm by Suppressing ROS Generation and Lipid Peroxidation
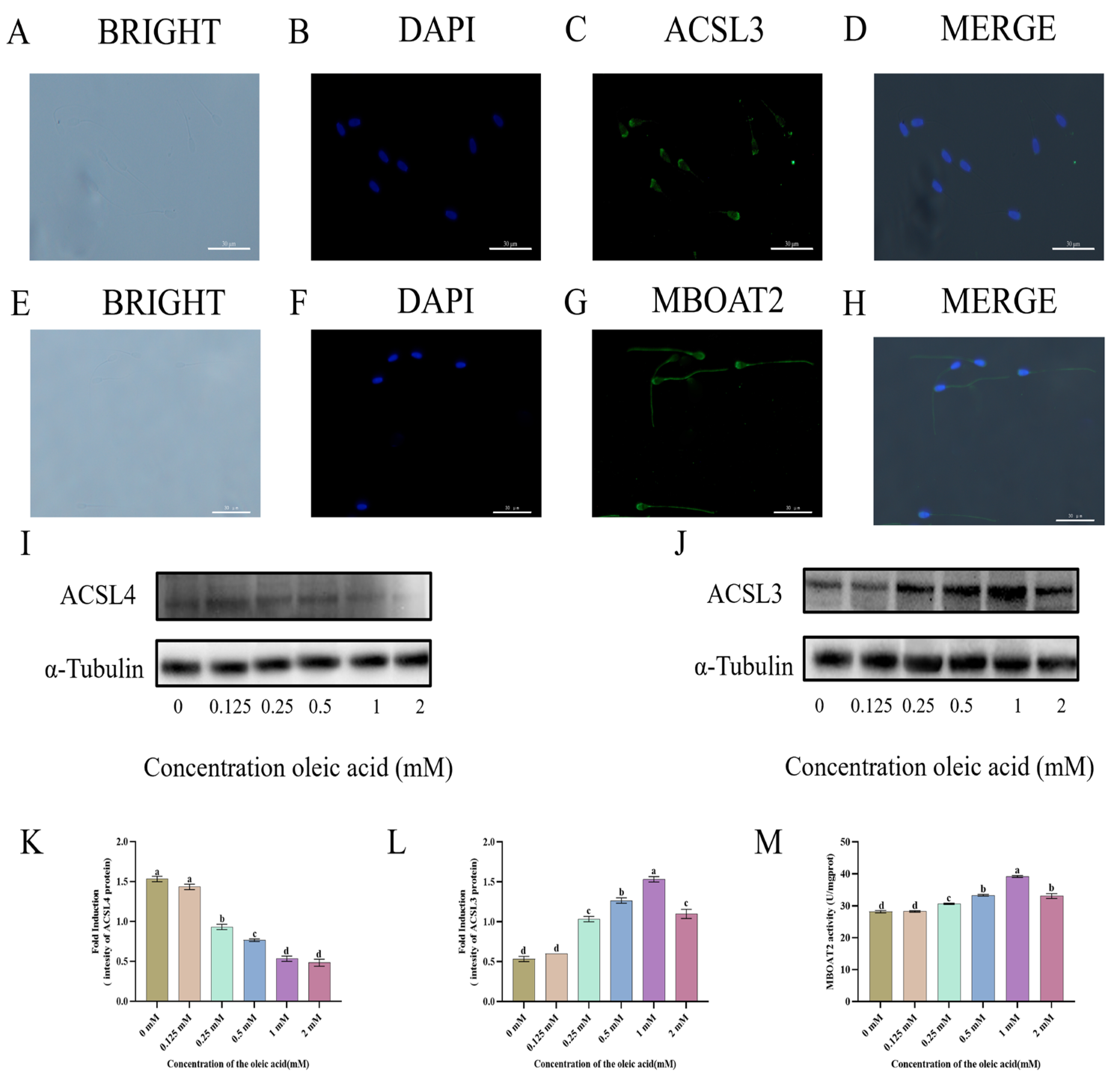
3.5. Localization and Expression of ACSL3 and MBOAT2 Pathways in Goat
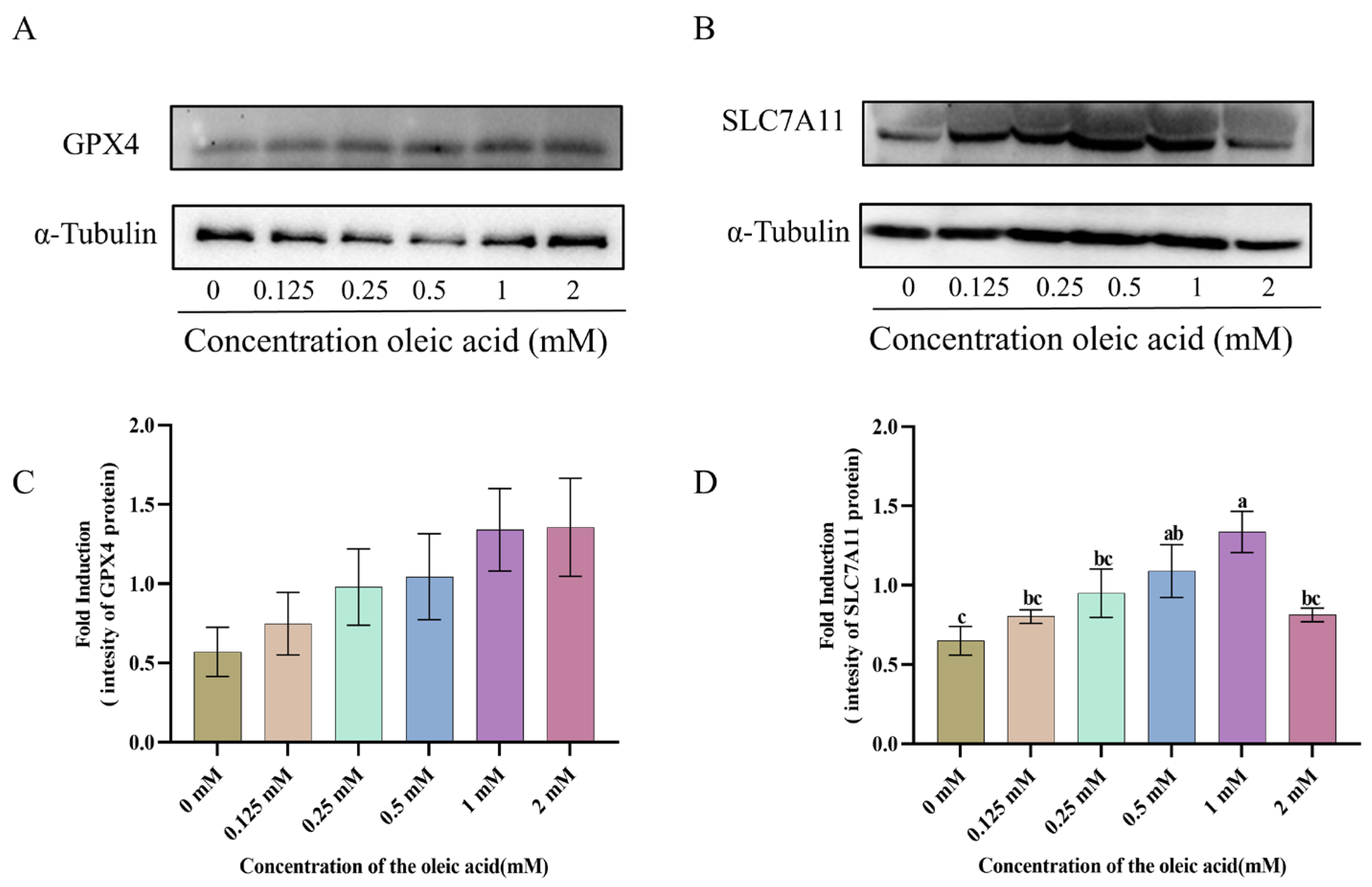
3.6. The Expression of GPX4 and SLC7A11 Proteins
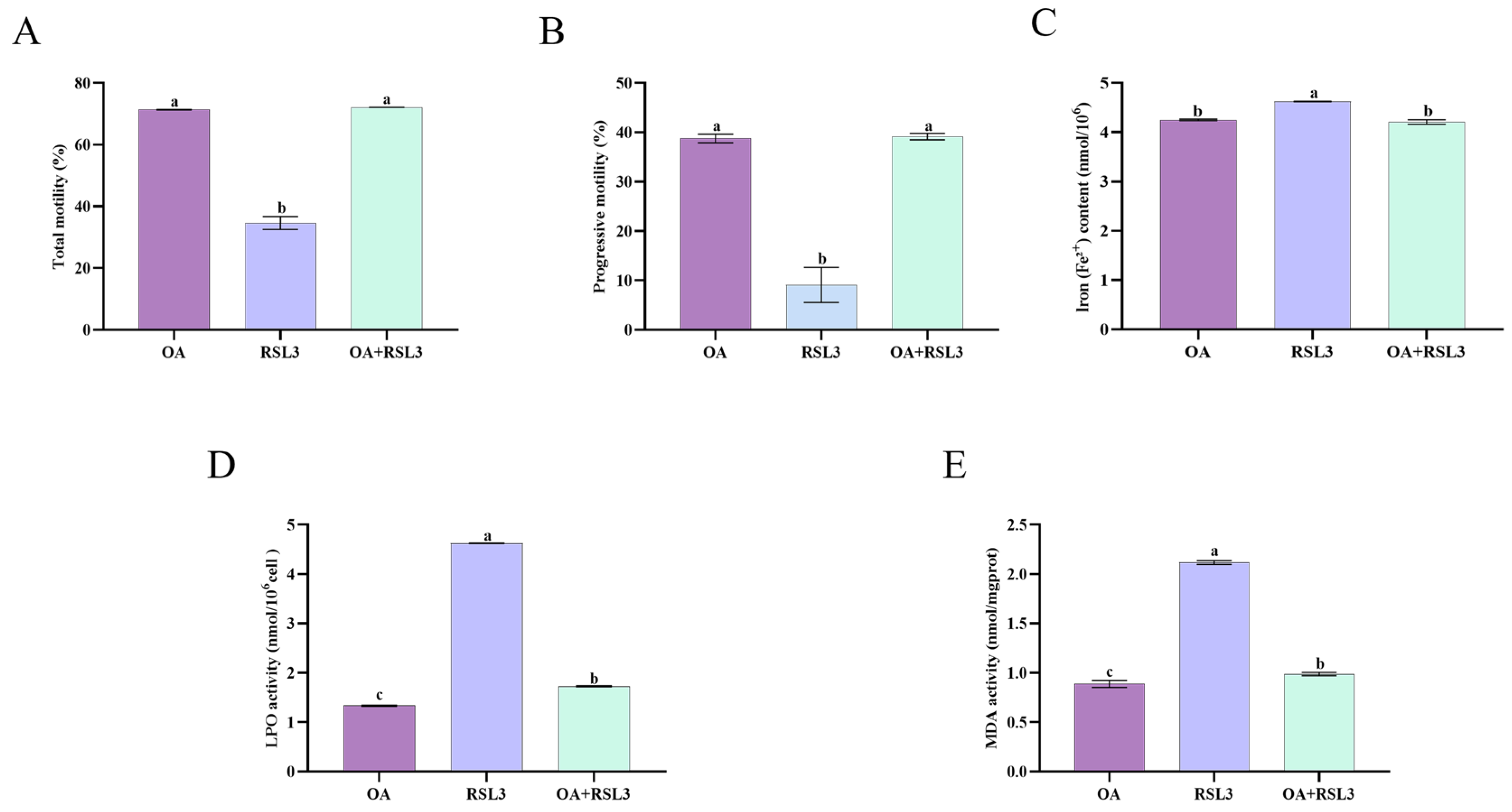
3.7. Oleic Acid Prevents RSL3-Mediated Ferroptosis Through Lipid Peroxidation Inhibition in Sperm Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vinokurov, N.; Vladimirov, L.; Machakhtyrov, G.; Machakhtyrova, V.; Sleptsov, E. PSXV-11 Using assisted reproductive technologies for obtaining new breeding forms by hybridization in sheep breeding. J. Anim. Sci. 2021, 99, 262–263. [Google Scholar] [CrossRef]
- El Amiri, B.; Rahim, A. Exploring Endogenous and Exogenous Factors for Successful Artificial Insemination in Sheep: A Global Overview. Vet. Sci. 2024, 11, 86. [Google Scholar] [CrossRef]
- Snoeck, P.P.N.; Moura, L.C.O.; Silva, M.C.; Machado-Neves, M.; Melo, M.I.V.; Heneine, L.G.D.; Henry, M. Effect of storage conditions on the LDL effectiveness in ovine sperm cryopreservation. Cryobiology 2017, 75, 88–90. [Google Scholar] [CrossRef]
- Fernandes, M.; Hernández, P.R.; Simões, J.; Barbas, J.P. Effects of Three Semen Extenders, Breeding Season Month and Freezing–Thawing Cycle on Spermatozoa Preservation of Portuguese Merino Sheep. Animals 2021, 11, 2619. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, J.; Nartey, M.A.; Mo, X.; Ye, J.; Zhang, Y.; Zeng, C.; Zhang, M.; Fang, Y.; Zhou, G. Ram sperm cryopreservation disrupts metabolism of unsaturated fatty acids. Theriogenology 2023, 204, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Pastrana, Y.M.; Marcon, J.L.; Amaral, A.P.d.; Santos, F.B.P.; Lima, E.S.; Acho, L.D.R.; Souza, R.O.S.d.; Grando, C.C.; Streit Junior, D.P.; Godoy, L. Catalase and Uric Acid Prevent Morphological Damage to the Sperm Flagella of Colossoma macropomum During 96 Hours at Low Storage Temperatures. Biopreservation Biobanking 2024, 22, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, X.; Li, H. Mechanisms of oxidative stress-induced sperm dysfunction. Front. Endocrinol. 2025, 16, 1520835. [Google Scholar] [CrossRef]
- Kafi, A.; Khalid, M.; Herath, T.; Kershaw, C. Cysteine supplementation pre-freeze and post-thaw improves integrity and reduces oxidative stress in cryopreserved ram spermatozoa. Cryobiology 2024, 114, 104854. [Google Scholar] [CrossRef]
- Piri, M.; Mahdavi, A.H.; Hajian, M.; Nasr-Esfahani, M.H.; Soltani, L.; Vash, N.T. Effects of nano-berberine and berberine loaded on green synthesized selenium nanoparticles on cryopreservation and in vitro fertilization of goat sperm. Sci. Rep. 2024, 14, 24171. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Y.; Jiang, C.; Sohail, T.; Sun, X.; Wang, J.; Li, Y. Damage to Mitochondria During the Cryopreservation, Causing ROS Leakage, Leading to Oxidative Stress and Decreased Quality of Ram Sperm. Reprod. Domest. Anim. 2024, 59, e14737. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef]
- Mattioli, S.; Dimauro, C.; Cesarani, A.; Dal Bosco, A.; Bartolini, D.; Galli, F.; Migni, A.; Sebastiani, B.; Signorini, C.; Oger, C.; et al. A Dynamic Model for Estimating the Interaction of ROS–PUFA–Antioxidants in Rabbit. Antioxidants 2022, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Pierantoni, R. Mitochondrial Reactive Oxygen Species (ROS) Production Alters Sperm Quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.G.; Storey, B.T. Assessment of cell damage caused by spontaneous lipid peroxidation in rabbit spermatozoa. Biol. Reprod. 1984, 30, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Kagan, V.E.; Bayir, H.; Pagnussat, G.C.; Head, B.; Traber, M.G.; Stockwell, B.R. Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev. 2018, 32, 602–619. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Zeng, Y.; Mo, X.; Hong, S.; He, H.; Li, J.; Fatima, S.; Liu, Q. Oxidative stress induces mitochondrial iron overload and ferroptotic cell death. Sci. Rep. 2023, 13, 15515. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, J.-Y.; Oh, M.; Lee, E.-W. An integrated view of lipid metabolism in ferroptosis revisited via lipidomic analysis. Exp. Mol. Med. 2023, 55, 1620–1631. [Google Scholar] [CrossRef]
- Bebber, C.M.; Müller, F.; Prieto Clemente, L.; Weber, J.; von Karstedt, S. Ferroptosis in Cancer Cell Biology. Cancers 2020, 12, 164. [Google Scholar] [CrossRef]
- Xin, S.; Schick, J.A. PUFAs dictate the balance of power in ferroptosis. Cell Calcium 2023, 110, 102703. [Google Scholar] [CrossRef]
- Shan, K.; Fu, G.; Li, J.; Qi, Y.; Feng, N.; Li, Y.; Chen, Y.Q. Cis-monounsaturated fatty acids inhibit ferroptosis through downregulation of transferrin receptor 1. Nutr. Res. 2023, 118, 29–40. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2020, 17, 2054–2081. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Jiang, Y.; Glandorff, C.; Sun, M. GSH and Ferroptosis: Side-by-Side Partners in the Fight against Tumors. Antioxidants 2024, 13, 697. [Google Scholar] [CrossRef] [PubMed]
- Hai, E.; Li, B.; Song, Y.; Zhang, J.; Zhang, J. Inhibiting ferroptosis mitigates sheep sperm freezing damage. Front. Vet. Sci. 2025, 12, 1526474. [Google Scholar] [CrossRef] [PubMed]
- Hai, E.; Li, B.; Song, Y.; Zhang, J.; Zhang, J. Ferroptosis emerges as the predominant form of regulated cell death in goat sperm cryopreservation. J. Anim. Sci. Biotechnol. 2025, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Reznik, E.; Santer, M.; Fongheiser, M.A.; Smith, N.; Hirschhorn, T.; Zandkarimi, F.; Soni, R.K.; Dafré, A.L.; Miranda-Vizuete, A.; et al. Ferroptosis inhibition by oleic acid mitigates iron-overload-induced injury. Cell Chem. Biol. 2023, 31, 249–264.e7. [Google Scholar] [CrossRef]
- Cao, Y.; Li, J.; Chen, Y.; Wang, Y.; Liu, Z.; Huang, L.; Liu, B.; Feng, Y.; Yao, S.; Zhou, L.; et al. Monounsaturated fatty acids promote cancer radioresistance by inhibiting ferroptosis through ACSL3. Cell Death Dis. 2025, 16, 184. [Google Scholar] [CrossRef]
- Perez, M.A.; Magtanong, L.; Dixon, S.J.; Watts, J.L. Dietary Lipids Induce Ferroptosis in Caenorhabditiselegans and Human Cancer Cells. Dev. Cell 2020, 54, 447–454.e4. [Google Scholar] [CrossRef]
- Zadeh Hashem, E.; Haddad, R.; Eslami, M. Evaluation of ram semen enrichment with oleic acid on different spermatozoa parameters during low temperature liquid storage. Small Rumin. Res. 2017, 150, 30–39. [Google Scholar] [CrossRef]
- Palacín, I.; Vicente-Fiel, S.; Santolaria, P.; Yániz, J.L. Standardization of CASA sperm motility assessment in the ram. Small Rumin. Res. 2013, 112, 128–135. [Google Scholar] [CrossRef]
- Eser, A.; Alakuş, A.; Bağcı, K.; Cihangiroğlu, A.Ç.; Yağcıoğlu, S.; Arıcı, R.; Demir, K. Precursor A-Kinase Anchor Protein 4 as a Predictive Biomarker of Post-Thaw Semen Quality in Goats. Vet. Sci. 2025, 12, 1003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, S.; Wang, Q.; Min, L.; Bastaw, E.M.; Zhu, Z. Long-chain fatty acids promote ATP production in post-thaw boar sperm through mitochondrial β-oxidation. Anim. Reprod. Sci. 2025, 281, 107985. [Google Scholar] [CrossRef] [PubMed]
- Gwo, J.C.; Lei, S.Y. Evaluation of cryodamage in small abalone, Haliotis diversicolor, spermatozoa by using a flow cytometer. J. World Aquac. Soc. 2022, 54, 181–192. [Google Scholar] [CrossRef]
- Zezeski, A.L.; Hamilton, L.E.; Geary, T.W. Improved Evaluation of Mitochondrial Membrane Potential in Bovine Spermatozoa Using JC-1 with Flow Cytometry. Biol. Reprod. 2025, 113, 141–153. [Google Scholar] [CrossRef]
- Zhu, Z.; Zeng, Y.; Zeng, W. Cysteine improves boar sperm quality via glutathione biosynthesis during the liquid storage. Anim. Biosci. 2021, 35, 166–176. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, W.; Yang, Q.; Zhao, H.; Zhang, W.; Adetunji, A.O.; Hoque, S.A.M.; Kou, X.; Min, L. Pyrroloquinoline Quinone Improves Ram Sperm Quality through Its Antioxidative Ability during Storage at 4 °C. Antioxidants 2024, 13, 104. [Google Scholar] [CrossRef]
- Li, R.; Wu, X.; Zhu, Z.; Lv, Y.; Zheng, Y.; Lu, H.; Zhou, K.; Wu, D.; Zeng, W.; Dong, W.; et al. Polyamines Protect Boar Sperm from Oxidative Stress in vitro. J. Anim. Sci. 2022, 100, skac069. [Google Scholar] [CrossRef]
- Liou, G.-G.; Hsieh, C.-C.; Lee, Y.-J.; Li, P.-H.; Tsai, M.-S.; Li, C.-T.; Wang, S.-H. N-Acetyl Cysteine Overdose Inducing Hepatic Steatosis and Systemic Inflammation in Both Propacetamol-Induced Hepatotoxic and Normal Mice. Antioxidants 2021, 10, 442. [Google Scholar] [CrossRef]
- Yi, X.; Qiu, Y.; Tang, X.; Lei, Y.; Pan, Y.; Raza, S.H.A.; Althobaiti, N.A.; Albalawi, A.E.; Al Abdulmonem, W.; Makhlof, R.T.M.; et al. Effect of Five Different Antioxidants on the Effectiveness of Goat Semen Cryopreservation. Reprod. Sci. 2024, 31, 1958–1972. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Moura, A.K.; Zuo, R.; Wang, Z.; Roudbari, K.; Hu, J.Z.; Wang, M.; Li, P.L.; Zhang, Y.; Li, X. Defective lipid droplet biogenesis exacerbates oleic acid-induced cellular homeostasis disruption and ferroptosis in mouse cardiac endothelial cells. Cell Death Discov. 2025, 11, 374. [Google Scholar] [CrossRef]
- Dong, J.; Qi, F.; Qie, H.; Du, S.; Li, L.; Zhang, Y.; Xu, K.; Li, D.; Xu, Y. Oleic Acid Inhibits SDC4 and Promotes Ferroptosis in Lung Cancer Through GPX4/ACSL4. Clin. Respir. J. 2024, 18, e70014. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, R.; Liu, S.; Duan, T.; Zhai, L.; Zhang, M.; Han, X.; Xiang, Y.; Huang, X.; Lin, H.; et al. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front. Pharmacol. 2018, 9, 1371. [Google Scholar] [CrossRef]
- Magtanong, L.; Ko, P.-J.; To, M.; Cao, J.Y.; Forcina, G.C.; Tarangelo, A.; Ward, C.C.; Cho, K.; Patti, G.J.; Nomura, D.K.; et al. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem. Biol. 2019, 26, 420–432.e9. [Google Scholar] [CrossRef]

| Sperm Parameters | Time (h) | 0 mM | 0.125 mM | 0.25 mM | 0.5 mM | 1 mM | 2 mM |
|---|---|---|---|---|---|---|---|
| Total motility (%) | 24 | 80.2 ± 1.1 | 80.9 ± 1.1 | 80.3 ± 1.1 | 80.7 ± 0.1 | 81.4 ± 0.7 | 79.8 ± 0.7 |
| 48 | 71.1 ± 0.2 c | 72.8 ± 0.6 bc | 73.5 ± 0.7 b | 73.5.9 ± 0.3 b | 78.5 ± 1.0 a | 74.3 ± 0.7 b | |
| 72 | 70.8 ± 0.3 e | 70.8 ± 0.3 e | 73.4 ± 0.5 c | 75.2 ± 0.2 b | 76.5 ± 0.5 a | 71.9 ± 0.1 d | |
| 96 | 60.5 ± 0.9d | 64.5 ± 1.4 cd | 66.0 ± 0.3 bc | 70.4 ± 3.3 ab | 72.5 ± 0.2 a | 66.6 ± 1.1 bc | |
| Progressive motility (%) | 24 | 47.9 ± 1.5 bc | 48.2 ± 1.1 bc | 49.8 ± 1.9 bc | 51.2 ± 1.8 ab | 55.6 ± 1.8 a | 45.8 ± 1.3 c |
| 48 | 43.8 ± 2.6 | 47 ± 0.2 | 47.4 ± 1.7 | 50.2 ± 3.4 | 50.5 ± 4.6 | 40.5 ± 1.8 | |
| 72 | 40.8 ± 0.5 b | 42.1 ± 1.7 b | 44.7 ± 1.3 ab | 45.5 ± 0.6 ab | 50.2 ± 4.9 a | 39.8 ± 0.9 b | |
| 96 | 30.5 ± 2.5 | 34.6 ± 3.7 | 35.5 ± 2.0 | 37.2 ± 0.9 | 37.7 ± 3.6 | 31.8 ± 2.3 | |
| VCL (μm/s) | 24 | 109.5 ± 14.5 b | 111.8 ± 13.5 b | 125.3 ± 10.5 ab | 139.9 ± 5.4 ab | 156.3 ± 1.2 a | 147.6 ± 10.4 a |
| 48 | 108.8 ± 8.7 d | 112.4 ± 1.2 cd | 124.5 ± 3.9 bc | 131.5 ± 2.9 ab | 140.0 ± 2.3 a | 127.2 ± 0.4 ab | |
| 72 | 110.8 ± 10.2 | 115.4 ± 11.1 | 124.9 ± 6.7 | 134.4 ± 4.6 | 138.9 ± 3.9 | 112.8 ± 9.4 | |
| 96 | 111.2 ± 9.0 | 132.5 ± 9.3 | 124.5 ± 11.8 | 113.9 ± 10.3 | 134.6 ± 4.1 | 117.6 ± 6.1 | |
| VSL (μm/s) | 24 | 49.4 ± 9.0 c | 48.7 ± 7.8 c | 60.4 ± 4.7 ab | 65.6 ± 2.8 ab | 75.0 ± 1.3 a | 63.0 ± 5.3 ab |
| 48 | 48.7 ± 5.5 b | 49.6 ± 0.2 b | 63.3 ± 2.5 a | 63.9 ± 4.4 a | 64.0 ± 4.9 a | 57.6 ± 0.6 ab | |
| 72 | 50.9 ± 4.4 b | 53.8 ± 7.4 b | 60.9 ± 3.1 ab | 63.6 ± 0.5 ab | 71.2 ± 3.4 a | 50.9 ± 4.3 b | |
| 96 | 50.3 ± 2.7 | 55.2 ± 3.3 | 57.9 ± 4.8 | 48.5 ± 3.6 | 59.0 ± 2.6 | 49.0 ± 3.5 | |
| VAP (μm/s) | 24 | 63.4 ± 9.1 b | 63.1 ± 7.9 b | 73.7 ± 5.7 ab | 79.7 ± 3.3 ab | 90.9 ± 0.5 a | 80.0 ± 6.0 ab |
| 48 | 65.2 ± 4.9 c | 68.6 ± 3.5 bc | 73.0 ± 2.0 abc | 76.6 ± 4.5 ab | 80.1 ± 0.5 a | 71.6 ± 0.3 abc | |
| 72 | 64.4 ± 5.2 b | 66.5 ± 7.1 b | 73.2 ± 3.5 ab | 77.5 ± 0.7 ab | 84.9 ± 1.7 a | 63.9 ± 4.6 b | |
| 96 | 61.4 ± 4.2 | 69.5 ± 3.9 | 70.5 ± 6.1 | 61.3 ± 4.6 | 74.2 ± 2.0 | 61.7 ± 3.6 | |
| BCF (Hz) | 24 | 32.8 ± 2.0 a | 32.6 ± 1.0 a | 26.5 ± 0.3 b | 30.0 ± 0.9 ab | 29.3 ± 0.4 ab | 28.0 ± 1.9 b |
| 48 | 32.1 ± 2.5 a | 28.8 ± 1.2 bc | 29.2 ± 0.5 bc | 26.6 ± 1.7 b | 29.5 ± 0.1 bc | 29.2 ± 0.2 bc | |
| 72 | 33.6 ± 2.4 a | 30.4 ± 0.6 ab | 28.4 ± 1.2 ab | 30.3 ± 0.4 ab | 27.2 ± 1.1 b | 30.5 ± 2.4 ab | |
| 96 | 22.4 ± 1.1 b | 23.2 ± 1.4 b | 28.9 ± 1.4 a | 23.8 ± 1.2 b | 29.1 ± 0.8 a | 23.1 ± 0.1 b | |
| LIN (%) | 24 | 43.3 ± 2.9 bc | 42.1 ± 2.1 c | 48.9 ± 0.5 a | 47.5 ± 0.9 ab | 49.2 ± 0.8 a | 43.4 ± 0.7 bc |
| 48 | 43.3 ± 1.2 | 44.9 ± 0.2 | 46.6 ± 1.2 | 47.3 ± 2.7 | 47.8 ± 2.5 | 43.3 ± 0.6 | |
| 72 | 44.0 ± 0.8 | 45.6 ± 2.8 | 48.6 ± 1.9 | 47.9 ± 1.4 | 51.7 ± 3.8 | 44.2 ± 1.2 | |
| 96 | 45.5 ± 1.3 | 42.0 ± 0.9 | 46.4 ± 0.5 | 43.2 ± 2.9 | 43.9 ± 2.5 | 41.8 ± 0.8 | |
| STR (%) | 24 | 73.8 ± 3.6 bc | 73.5 ± 3.4 c | 81.0 ± 0.2 ab | 81.0 ± 0.3 ab | 82.3 ± 1.3 a | 77.7 ± 0.6 abc |
| 48 | 73.2 ± 2.9 b | 76.7 ± 0.8 ab | 81.5 ± 1.1 a | 82.0 ± 1.3 a | 80.5 ± 1.9 a | 78.9 ± 0.6 a | |
| 72 | 74.7 ± 1.5 | 77.8 ± 3.3 | 81.0 ± 1.6 | 80.8 ± 1.0 | 82.4 ± 2.5 | 76.4 ± 2.5 | |
| 96 | 81.2 ± 1.5 | 78.6 ± 0.9 | 80.4 ± 0.5 | 78.3 ± 3.3 | 77.6 ± 2.6 | 78.2 ± 0.9 | |
| WOB (%) | 24 | 57.2 ± 1.1 ab | 55.9 ± 0.5 bc | 59.3 ± 0.5 a | 57.5 ± 1.0 ab | 58.9 ± 0.3 a | 54.8 ± 0.4 c |
| 48 | 56.6 ± 0.08 | 56.7 ± 0.4 | 56.2 ± 0.2 | 56.6 ± 0.7 | 58.2 ± 1.8 | 56.2 ± 0.4 | |
| 72 | 57.2 ± 0.6 | 57.2 ± 1.3 | 58.8 ± 1.4 | 58.2 ± 1.4 | 61.6 ± 2.9 | 56.4 ± 0.2 | |
| 96 | 55.3 ± 0.7 ab | 53.1 ± 0.8 b | 56.8 ± 0.5 a | 54.3 ± 1.5 ab | 55.4 ± 1.5 ab | 52.8 ± 0.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, W.; Zhu, Z.; Adetunji, A.O.; Zhao, S.; Ma, D.; Kou, X.; Min, L. Oleic Acid Improves Goat Sperm Quality by Enhancing the MBOAT2/ACSL3 Pathway to Attenuate Ferroptosis. Animals 2025, 15, 3258. https://doi.org/10.3390/ani15223258
Bi W, Zhu Z, Adetunji AO, Zhao S, Ma D, Kou X, Min L. Oleic Acid Improves Goat Sperm Quality by Enhancing the MBOAT2/ACSL3 Pathway to Attenuate Ferroptosis. Animals. 2025; 15(22):3258. https://doi.org/10.3390/ani15223258
Chicago/Turabian StyleBi, Wen, Zhendong Zhu, Adedeji O. Adetunji, Shengyan Zhao, Dongping Ma, Xin Kou, and Lingjiang Min. 2025. "Oleic Acid Improves Goat Sperm Quality by Enhancing the MBOAT2/ACSL3 Pathway to Attenuate Ferroptosis" Animals 15, no. 22: 3258. https://doi.org/10.3390/ani15223258
APA StyleBi, W., Zhu, Z., Adetunji, A. O., Zhao, S., Ma, D., Kou, X., & Min, L. (2025). Oleic Acid Improves Goat Sperm Quality by Enhancing the MBOAT2/ACSL3 Pathway to Attenuate Ferroptosis. Animals, 15(22), 3258. https://doi.org/10.3390/ani15223258





