Morphological Differences in Feeding and Digestive Organs, the Diversity of Intestinal Microorganisms, and Variations in Digestive Enzyme Activity Promote the Differentiation of Nutritional Niches in Schizothoracinae Species
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Trait Data Collection
2.2. Observation of Major Feeding Organs and Intestinal Morphology
2.3. Analysis of Food Composition
2.4. Determination of Intestinal Digestive Enzyme Activity
2.5. Extraction of Gut Microbial DNA and Sequencing
2.6. High-Throughput Sequencing Data Analysis
2.7. Statistical Analysis
3. Results
3.1. Feeding Organs and Intestinal Morphology
3.2. Diet Composition
3.3. Intestinal Digestive Enzyme Activities
3.4. Intestinal Microbial Diversity
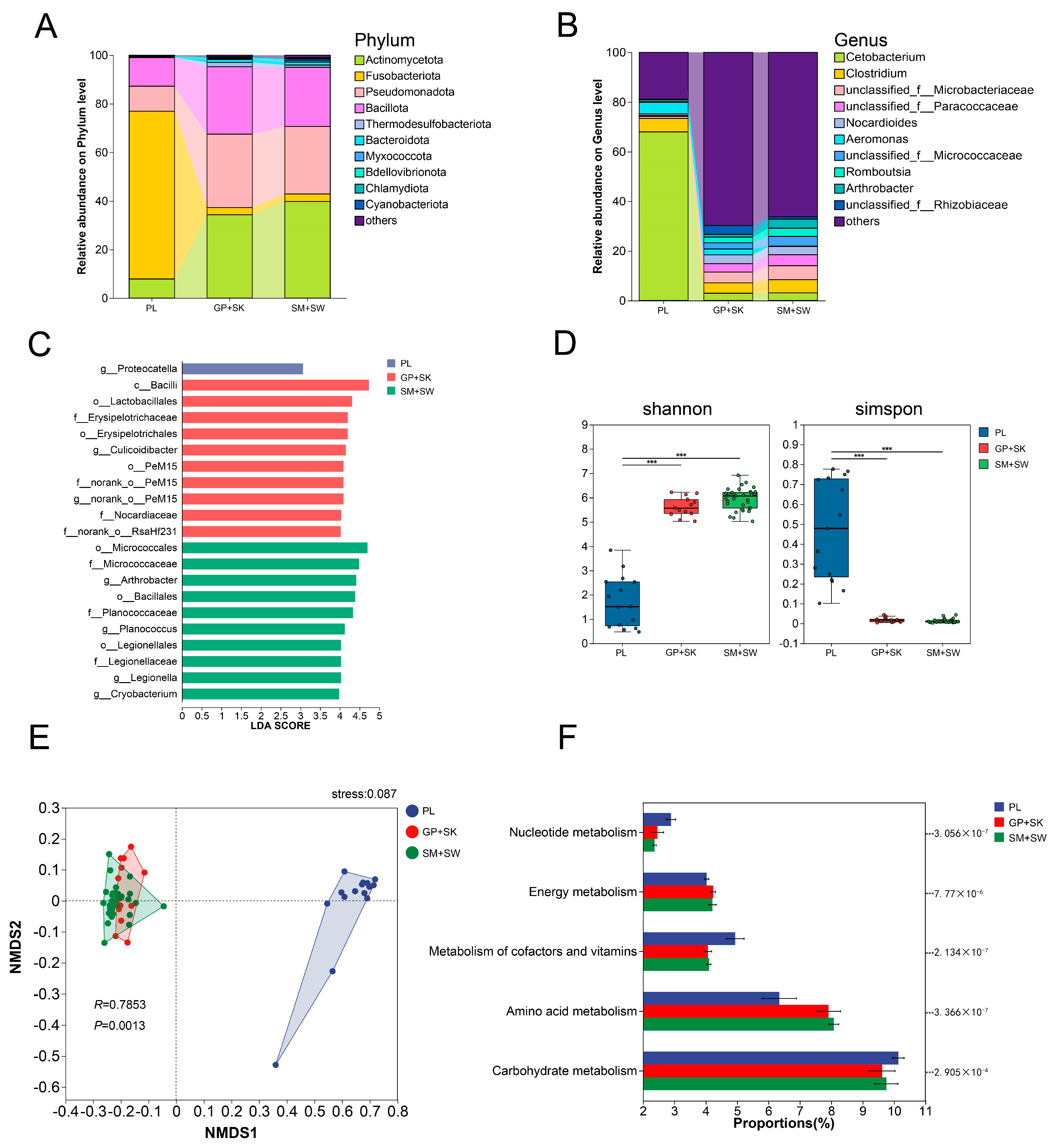
3.4.1. Intestinal Microbial Composition and Differential Analysis
3.4.2. α and β Diversity
3.4.3. Functional Prediction
4. Discussion
4.1. The Differences in Feeding Organs and Intestinal Morphology
4.2. Gut Digestive Enzyme Activity
4.3. Gut Microbial Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.T.; Xue, Z.F.; Tao, Y.; Kan, Z.H.; Zhou, X.B.; Liu, H.L.; Zhang, Y.M. Spatiotemporal Patterns of Leaf Nutrients of Wild Apples in a Wild Fruit Forest Plot in the Ili Valley, China. BMC Plant Biol. 2024, 24, 684. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro-Arias, I.; Isla, J.; Jordano, P.; Benítez-López, A. Fine-scale Coexistence between Mediterranean Mesocarnivores Is Mediated by Spatial, Temporal, and Trophic Resource Partitioning. Ecol. Evol. 2021, 11, 15520–15533. [Google Scholar] [CrossRef]
- Valencia-Rodríguez, D.; Jiménez-Segura, L.; Rogéliz, C.A.; Parra, J.L. Ecological Niche Modeling as an Effective Tool to Predict the Distribution of Freshwater Organisms: The Case of the Sabaleta (Brycon Henni) (Eigenmann, 1913). PLoS ONE 2021, 16, e0247876. [Google Scholar] [CrossRef]
- Myoung, S.H.; Park, J.M.; Gaston, T.F.; Williamson, J.E. Variability in Resource Use of Sympatric Cowfish (Aracana Aurita) and (a. Ornata) in Southeastern Australian Waters. Reg. Stud. Mar. Sci. 2024, 77, 103696. [Google Scholar] [CrossRef]
- Shelton, J.; Bird, M.; Marr, S. Evidence for Diet Partitioning among Three Coexisting Native Freshwater Fishes in South Africa’s Cape Fold Ecoregion. Afr. J. Aquat. Sci. 2018, 43, 89–100. [Google Scholar] [CrossRef]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V. Fishes of the World; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 1-119-22082-3. [Google Scholar]
- Qi, D.; Chao, Y.; Guo, S.; Zhao, L.; Li, T.; Wei, F.; Zhao, X. Convergent, Parallel and Correlated Evolution of Trophic Morphologies in the Subfamily Schizothoracinae from the Qinghai-Tibetan Plateau. PLoS ONE 2012, 7, e34070. [Google Scholar] [CrossRef]
- Yang, X.F.; Xie, C.X.; Yang, R.B. Comparison on the Morphology of Feeding Apparatus of Six Piscivorous Fishes in Liangzi Lake. J. Huazhong Agric. Univ. 2003, 22, 257–259. [Google Scholar] [CrossRef]
- Jiao, F.; Zhang, L.; Limbu, S.M.; Yin, H.; Xie, Y.; Yang, Z.; Shang, Z.; Kong, L.; Rong, H. A Comparison of Digestive Strategies for Fishes with Different Feeding Habits: Digestive Enzyme Activities, Intestinal Morphology, and Gut Microbiota. Ecol. Evol. 2023, 13, e10499. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Zhang, L.; Wang, X.W. Comparative Study on Digestive Enzyme Activity and Intestinal Tissue Morphology of Four Fishes with Different Feeding Habits. Freshw. Fish. 2023, 53, 29–35. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Gooneratne, R.; Lai, R.; Zeng, C.; Zhan, F.; Wang, W. The Gut Microbiome and Degradation Enzyme Activity of Wild Freshwater Fishes Influenced by Their Trophic Levels. Sci. Rep. 2016, 6, 24340. [Google Scholar] [CrossRef]
- Xu, L.; Xiang, P.; Zhang, B.; Yang, K.; Liu, F.; Wang, Z.; Jin, Y.; Deng, L.; Gan, W.; Song, Z. Host Species Influence the Gut Microbiota of Endemic Cold-Water Fish in Upper Yangtze River. Front. Microbiol. 2022, 13, 906299. [Google Scholar] [CrossRef]
- Xiong, M.H.; Dong, W.W.; Liu, X.; Zhu, B.; Song, Y.; Hu, X.; Zeng, C.; Yu, D.; Shao, K. Species Composition and Community Structure of Fish in the Main Stream and Some Tributaries of the Yalong River below Ganzi from 2017 to 2022. J. Lake Sci. 2024, 36, 223–235. [Google Scholar] [CrossRef]
- Tong, C.; Tian, F.; Zhao, K. Genomic Signature of Highland Adaptation in Fish: A Case Study in Tibetan Schizothoracinae Species. BMC Genom. 2017, 18, 948. [Google Scholar] [CrossRef]
- Menon, A. Taxonomy of Fishes of the Genus Schizothorax Heckel with the Description of a New Species from Kumaon Himalayas. Rec. Zool. Surv. India 1965, 63, 195–207. [Google Scholar] [CrossRef]
- Wu, Y. The Fishes of the Qinghai-Xizang Plateu; Sichuan Publishing House of Science and Technology: Chengdu, China, 1992; ISBN 7-5364-2168-0. [Google Scholar]
- Guo, Y.; Zhang, R.M.; Cai, L.G. Fish Fauna of Xinjiang; Xinjiang Science and Technology Press: Urumqi, China, 2012; ISBN 978-7-5466-1529-5. [Google Scholar]
- Li, X.Q.; Zeng, R.K.; Ni, D.F.; Feng, G.; Wan, Z.K.; Wang, J.; Wu, T.F.; Lu, T.X.; Deng, H.T.; Yao, W.Z. Food Organism Compositions and Feeding Characteristics of Gymnodiptychus Pachycheilus in the Middle Reach of Yalong River. J. Fish. China 2025, 49, 039311. [Google Scholar]
- Yang, C.J. Population Genetic Structure and Gut Microbial Diversity of the Schizopygopsis malacanthus. Master’s Thesis, Qinghai University, Qinghai, China, 2022. [Google Scholar]
- Ding, R.H. The Fishes of Sichuan, China; Sichuan Publishing House of Science and Technology: Chengdu, China, 1994; ISBN 7-5364-2295-4. [Google Scholar]
- Yue, P.Q. Fauna Sinica Osteichthyes Cypriniformes III; Science Press: Beijing, China, 2000; ISBN 7-03-007575-7. [Google Scholar]
- Hubbs, C.; Lagler, K.F. Fishes of the Great Lakes Region, Revised Edition; University of Michigan Press: Ann Arbor, MI, USA, 2004; ISBN 978-0-472-11371-2. [Google Scholar]
- Ji, Q. The study on the morphology of feeding organs and the feeding habits of six schizothoracine fishes. Master’s Thesis, Huazhong University, Wuhan, China, 2008. [Google Scholar]
- Zhang, Z.S.; Huang, X.F. Research Methods on Freshwater Plankton; Science Press: Beijing, China, 1994; ISBN 7-03-001038-8. [Google Scholar]
- Weng, J.Z. Atlas of Common Freshwater Planktonic Algae in China; Shanghai Scientific & Technical Publishers: Shanghai, China, 2010; ISBN 978-7-5478-0287-8. [Google Scholar]
- Hu, H.J.; Li, Y.Y. The Freshwater Algae of China; Science Press: Beijing, China, 2006; ISBN 7-03-016633-7. [Google Scholar]
- Popova, O.; Reshetnikov, Y.S. On Complex Indices in Investigation of Fish Feeding. J. Ichthyol. 2011, 51, 686–691. [Google Scholar] [CrossRef]
- Natarajan, A.; Jhingran, A. Index of PREPONDERANCE— a Method of Grading the Food Elements in the Stomach Analysis of Fishes. Indian J. Fish. 1961, 8, 54–59. [Google Scholar]
- Li, Y.J.; Chen, Q. Effects of Dietary Alanyl-Glutamine Supplementation on Growth Performance, Serum Biochemical Indices, Liver Antioxidant Capacity, Intestinal Digestive Enzyme Activities and Morphological Structure of Paramisgurnus dabyranus. Chin. J. Anim. Nutr. 2024, 36, 3219–3230. [Google Scholar] [CrossRef]
- Legrand, T.; Wos-Oxley, M.; Wynne, J.; Weyrich, L.; Oxley, A. Dead or Alive: Microbial Viability Treatment Reveals Both Active and Inactive Bacterial Constituents in the Fish Gut Microbiota. J. Appl. Microbiol. 2021, 131, 2528–2538. [Google Scholar] [CrossRef]
- Fung, C.; Rusling, M.; Lampeter, T.; Love, C.; Karim, A.; Bongiorno, C.; Yuan, L. Automation of QIIME2 Metagenomic Analysis Platform. Curr. Protocol. 2021, 1, e254. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Bohórquez-Herrera, J.; Cruz-Escalona, V.H.; Adams, D.C.; Peterson, M.S. Feeding Ecomorphology of Seven Demersal Marine Fish Species in the Mexican Pacific Ocean. Environ. Biol. Fishes 2015, 98, 1459–1473. [Google Scholar] [CrossRef]
- Pessanha, A.L.M.; Araújo, F.G.; Oliveira, R.E.M.; Silva, A.F.D.; Sales, N.S. Ecomorphology and Resource Use by Dominant Species of Tropical Estuarine Juvenile Fishes. Neotrop. Ichthyol. 2015, 13, 401–412. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, M.D.; Zhu, F.Y. Comparative Study of Three Species of Schizothoracine on Feeding and Digestive Organs in Upper Nujiang River. Chin. J. Zool. 2019, 54, 207–221. [Google Scholar] [CrossRef]
- Du, Z.J. Biological Study on Artificial Culture of Schizothorax prenanti. China Fish. 2003, 4, 81. [Google Scholar] [CrossRef]
- Zhang, F.B. Study on Fish Community Structure and Nutritional Relationships of Main Fish Species in Chishui River. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2018. [Google Scholar]
- Wang, M. Study on Fish Community Structure and Food Composition in Lhasa River. Master’s Thesis, Hebei University, Hebei, China, 2024. [Google Scholar]
- Silva, N.C.D.S.; Costa, A.J.L.D.; Louvise, J.; Soares, B.E.; Reis, V.C.E.S.; Albrecht, M.P.; Caramaschi, E.P. Resource Partitioning and Ecomorphological Variation in Two Syntopic Species of Lebiasinidae (Characiformes) in an Amazonian Stream. Acta Amazonica 2016, 46, 25–36. [Google Scholar] [CrossRef]
- Dumay, O.; Tari, P.S.; Tomasini, J.A.; Mouillot, D. Functional Groups of Lagoon Fish Species in Languedoc Roussillon, Southern France. J. Fish Biol. 2004, 64, 970–983. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Tan, H.; Yang, C.; Ren, L.; Liu, Q.; Wang, S.; Hu, F.; Xiao, J.; Zhao, R.; et al. Genetic Effects on the Gut Microbiota Assemblages of Hybrid Fish from Parents with Different Feeding Habits. Front. Microbiol. 2018, 9, 2972. [Google Scholar] [CrossRef] [PubMed]
- Herder, F.; Freyhof, J. Resource Partitioning in a Tropical Stream Fish Assemblage. J. Fish Biol. 2006, 69, 571–589. [Google Scholar] [CrossRef]
- Xiang, X.; Ye, Y.T.; Zhou, X.H. Study of Digestive Function for Silurus asotus. Feed Ind. 2005, 26, 24–27. [Google Scholar] [CrossRef]
- Al-Tameemi, R.; Aldubaikul, A.; Salman, N.A. Comparative Study of α-Amylase Activity in Three Cyprinid Species of Different Feeding Habits from Southern Iraq. Turk. J. Fish. Aquat. Sci. 2010, 10, 411–414. [Google Scholar] [CrossRef]
- Gioda, C.R.; Pretto, A.; Freitas, C.D.S.; Leitemperger, J.; Loro, V.L.; Lazzari, R.; Lissner, L.A.; Baldisserotto, B.; Salbego, J. Different Feeding Habits Influence the Activity of Digestive Enzymes in Freshwater Fish. Cienc. Rural 2017, 47, e20160113. [Google Scholar] [CrossRef]
- Day, R.D.; German, D.P.; Manjakasy, J.M.; Farr, I.; Hansen, M.J.; Tibbetts, I.R. Enzymatic Digestion in Stomachless Fishes: How a Simple Gut Accommodates Both Herbivory and Carnivory. J. Comp. Physiol. B 2011, 181, 603–613. [Google Scholar] [CrossRef]
- Chakrabarti, I.; Gani, M.A.; Chaki, K.; Sur, R.; Misra, K. Digestive Enzymes in 11 Freshwater Teleost Fish Species in Relation to Food Habit and Niche Segregation. Comp. Biochem. Physiol. A Physiol. 1995, 112, 167–177. [Google Scholar] [CrossRef]
- Liu, M.D.; Zhu, F.Y.; Zhu, T.B. Status of Aquatic Organisms Resources and Their Environments in Xizang (2017–2021). J. Fish. China 2025, 49, 116–139. [Google Scholar] [CrossRef]
- Solovyev, M.M.; Kashinskaya, E.N.; Izvekova, G.I.; Gisbert, E.; Glupov, V.V. Feeding Habits and Ontogenic Changes in Digestive Enzyme Patterns in Five Freshwater Teleosts. J. Fish Biol. 2015, 85, 1395–1412. [Google Scholar] [CrossRef]
- Finegold, S.M.; Vaisanen, M.-L.; Molitoris, D.R.; Tomzynski, T.J.; Song, Y.; Liu, C.; Collins, M.D.; Lawson, P.A. Cetobacterium Somerae Sp. Nov. from Human Feces and Emended Description of the Genus Cetobacterium. Syst. Appl. Microbiol. 2003, 26, 177–181. [Google Scholar] [CrossRef]
- Thejaswini, S.; Jojy, S.; Vijayan, A.; Martin Paul, A. Isolation of Gut Actinobacteria from Fishes; Springer: New York, NY, USA, 2022; ISBN 978-1-0716-1728-1. [Google Scholar]
- Gong, L.; Liu, B.; Wu, H.; Feng, J.; Jiang, T. Seasonal Dietary Shifts Alter the Gut Microbiota of Avivorous Bats: Implication for Adaptation to Energy Harvest and Nutritional Utilization. Msphere 2021, 6, e0046721. [Google Scholar] [CrossRef]
- Li, Z.; Lv, J.; Chen, J.; Sun, F.; Sheng, R.; Qin, Y.; Rao, L.; Lu, T.; Sun, L. Comparative Study of Gut Content Microbiota in Freshwater Fish with Different Feeding Habits: A Case Study of an Urban Lake. J. Fish Biol. 2025, 106, 823–835. [Google Scholar] [CrossRef]
- Wang, X.; Hao, J.; Zhang, C.; Zhu, P.; Gao, Q.; Liu, D.; Nie, M.; Jia, J.; Qi, D. Differences and Correlation Analysis of Feeding Habits and Intestinal Microbiome in Schizopygopsis Microcephalus and Ptychobarbus Kaznakovi in the Upper Reaches of Yangtze River. Front. Microbiol. 2025, 16, 1513401. [Google Scholar] [CrossRef]
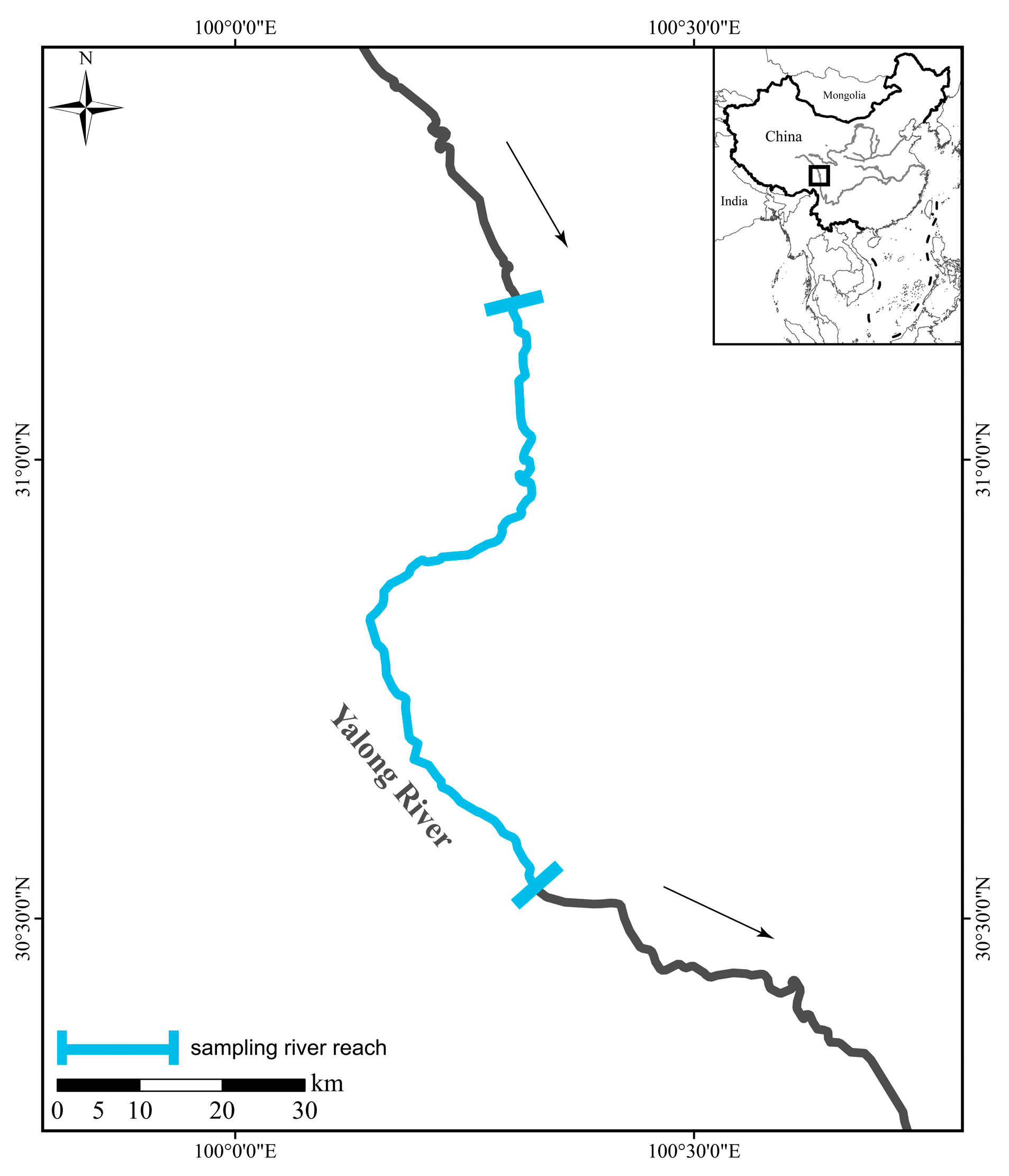

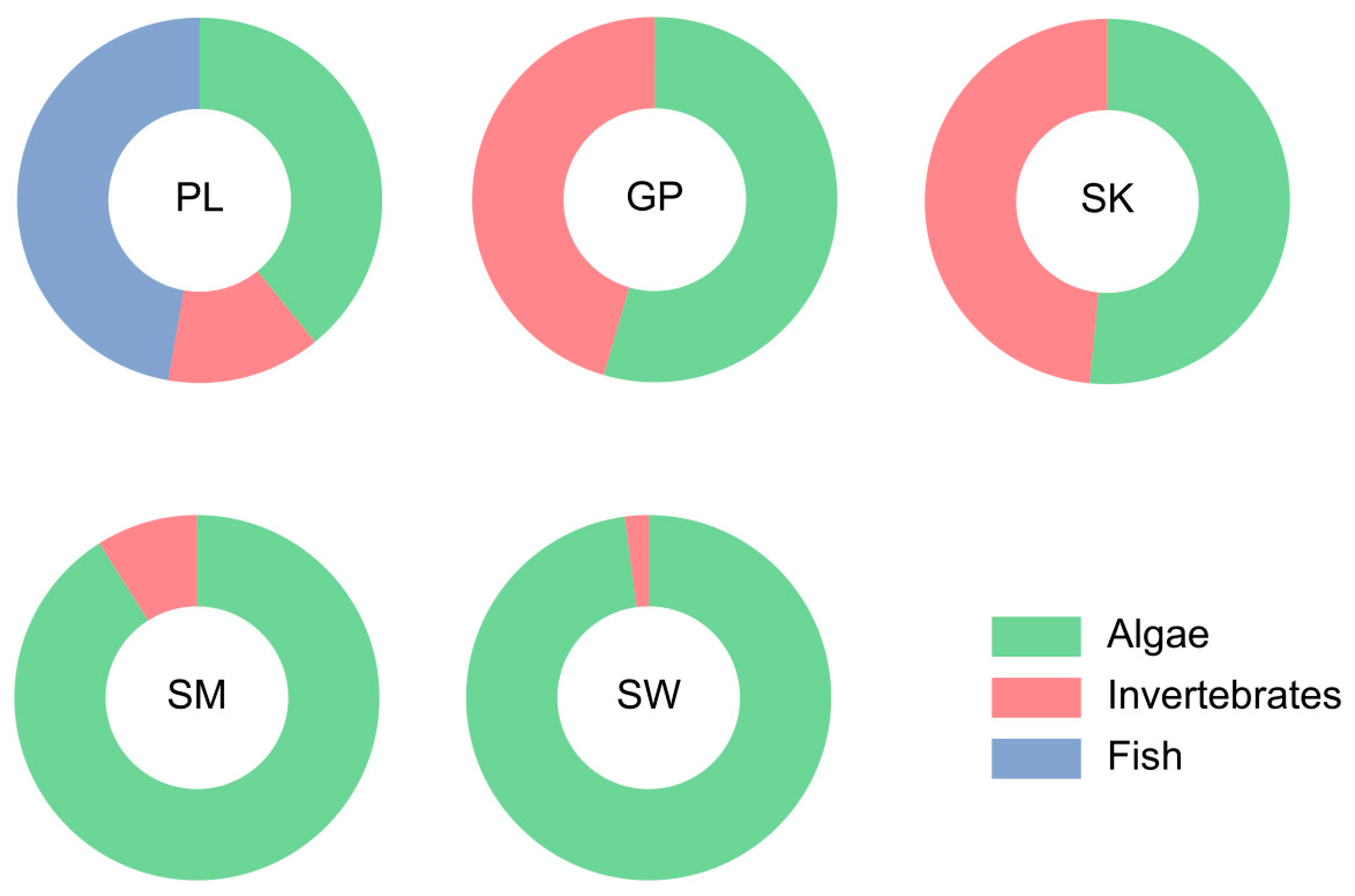

| Species Name | Ptychobarbus leptosomus (n = 15) | Gymnodiptychus pachycheilus (n = 7) | Schizothorax kozlovi (n = 6) | Schizopygopsis malacanthus (n = 20) | Schizothorax wangchiachii (n = 13) |
|---|---|---|---|---|---|
| Mouth position | Subterminal | Subterminal | Subterminal | Subterminal | Subterminal |
| Anterior margin of lower jaw | No keratin, well-developed lips | No keratin, well-developed lips | No keratin, well-developed lips | Sharp keratin | Sharp keratin |
| Pharyngeal tooth formula | 3.4/4.3 | 3.4/4.3 | 2.3.5/5.3.2 | 3.4/4.3 | 2.3.5/5.3.2 |
| Pharyngeal tooth morphology | Pointed apex, narrow chewing surface | Pointed apex, narrow chewing surface | Pointed apex, moderate chewing surface | Pointed apex, narrow chewing surface | Pointed apex, moderate chewing surface |
| Number of outer gill rakers on first gill arch | 11–15 | 16–18 | 13–17 | 17–21 | 20–22 |
| Number of intestinal flexures | 2 | 6 | 7 | 10 | 13 |
| Body length range (cm) | 28.5 ± 9.3 | 33.4 ± 8.2 | 27.9 ± 10.7 | 29.4 ± 7.6 | 29.3 ± 4.8 |
| Relative gut length | 1.41 ± 0.2 | 1.57 ± 0.1 | 2.69 ± 0.3 | 4.22 ± 0.3 | 5.14 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, T.; Liu, F.; Chang, M.; Yan, R.; Luo, W.; Wen, L.; Ding, W.; Fu, Q.; Wang, X.; Li, X.; et al. Morphological Differences in Feeding and Digestive Organs, the Diversity of Intestinal Microorganisms, and Variations in Digestive Enzyme Activity Promote the Differentiation of Nutritional Niches in Schizothoracinae Species. Animals 2025, 15, 3242. https://doi.org/10.3390/ani15223242
Yan T, Liu F, Chang M, Yan R, Luo W, Wen L, Ding W, Fu Q, Wang X, Li X, et al. Morphological Differences in Feeding and Digestive Organs, the Diversity of Intestinal Microorganisms, and Variations in Digestive Enzyme Activity Promote the Differentiation of Nutritional Niches in Schizothoracinae Species. Animals. 2025; 15(22):3242. https://doi.org/10.3390/ani15223242
Chicago/Turabian StyleYan, Taiming, Fei Liu, Mengna Chang, Ruizhen Yan, Wenjie Luo, Lin Wen, Wenxiang Ding, Qipeng Fu, Xuanyu Wang, Xin Li, and et al. 2025. "Morphological Differences in Feeding and Digestive Organs, the Diversity of Intestinal Microorganisms, and Variations in Digestive Enzyme Activity Promote the Differentiation of Nutritional Niches in Schizothoracinae Species" Animals 15, no. 22: 3242. https://doi.org/10.3390/ani15223242
APA StyleYan, T., Liu, F., Chang, M., Yan, R., Luo, W., Wen, L., Ding, W., Fu, Q., Wang, X., Li, X., Song, H., Gao, K., Wang, X., Xu, C., Zeng, R., Tang, Z., He, Z., & Yang, D. (2025). Morphological Differences in Feeding and Digestive Organs, the Diversity of Intestinal Microorganisms, and Variations in Digestive Enzyme Activity Promote the Differentiation of Nutritional Niches in Schizothoracinae Species. Animals, 15(22), 3242. https://doi.org/10.3390/ani15223242






