Mandibular Shape Variation, Allometry and Modularity in Adult Mesocephalic Dogs (Canis lupus familiaris): Insights into Morphological Integration and Animal Anatomy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample and Selection Criteria
2.3. Digital Image Acquisition
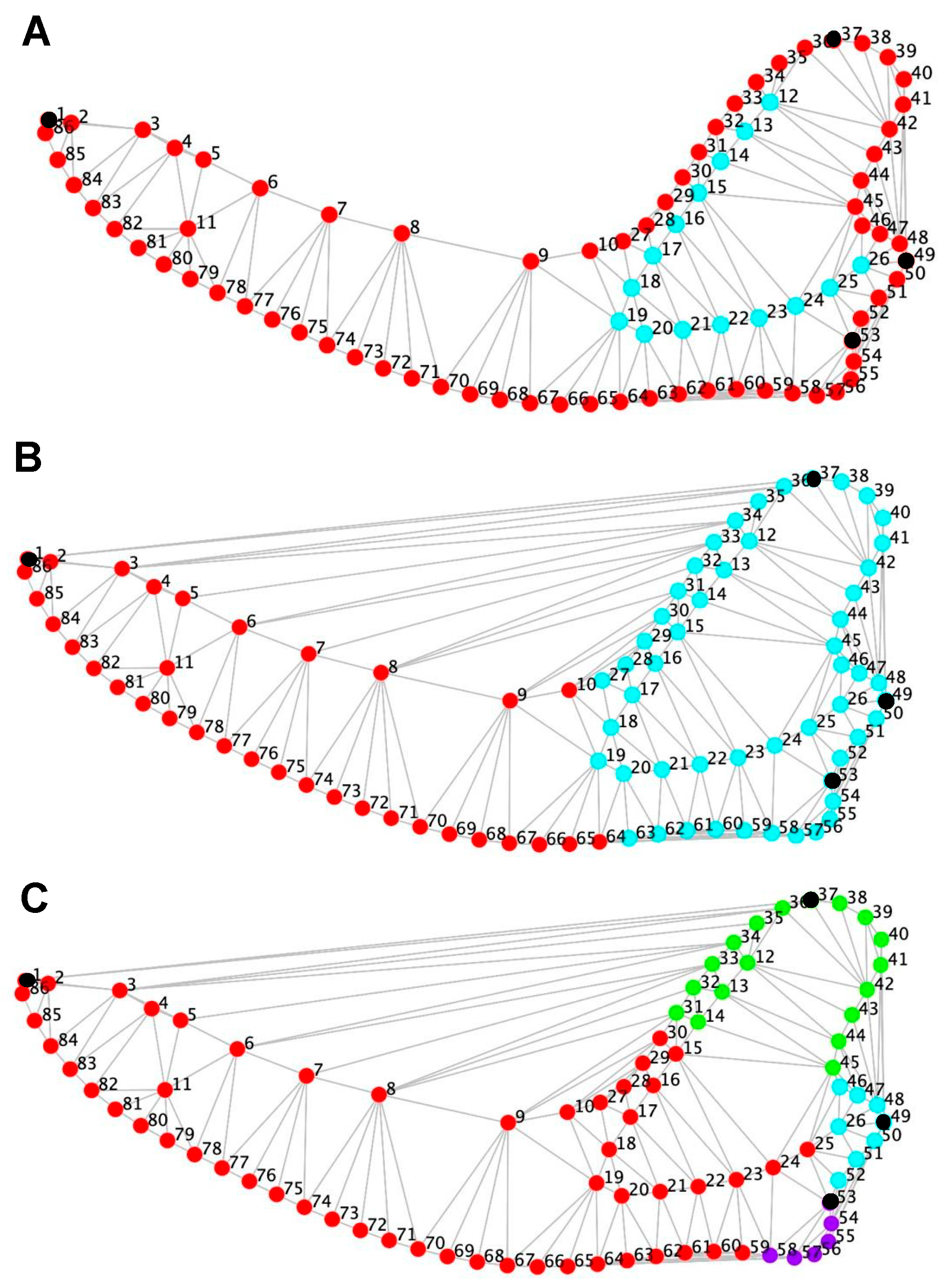
2.4. Landmark Digitization
2.5. Morphometric Processing and Statistical Analyses
2.6. Ethical Considerations
2.7. Methodological Limitations
3. Results
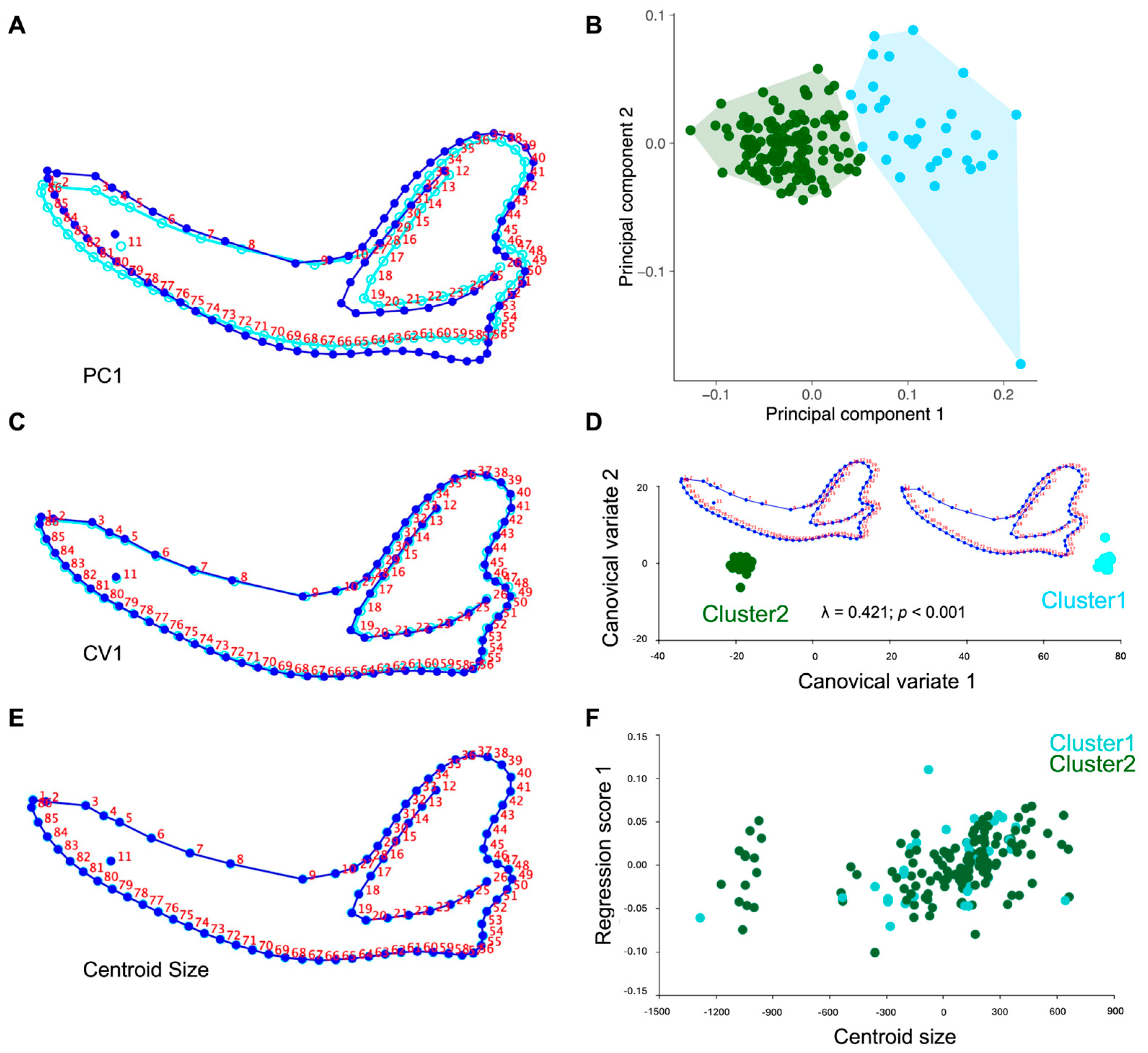
3.1. Principal Component Analysis (PCA)
3.2. Cluster Analysis (K-Means)
3.3. Canonical Variate Analysis (CVA)
3.4. Procrustes Regression (Allometry)
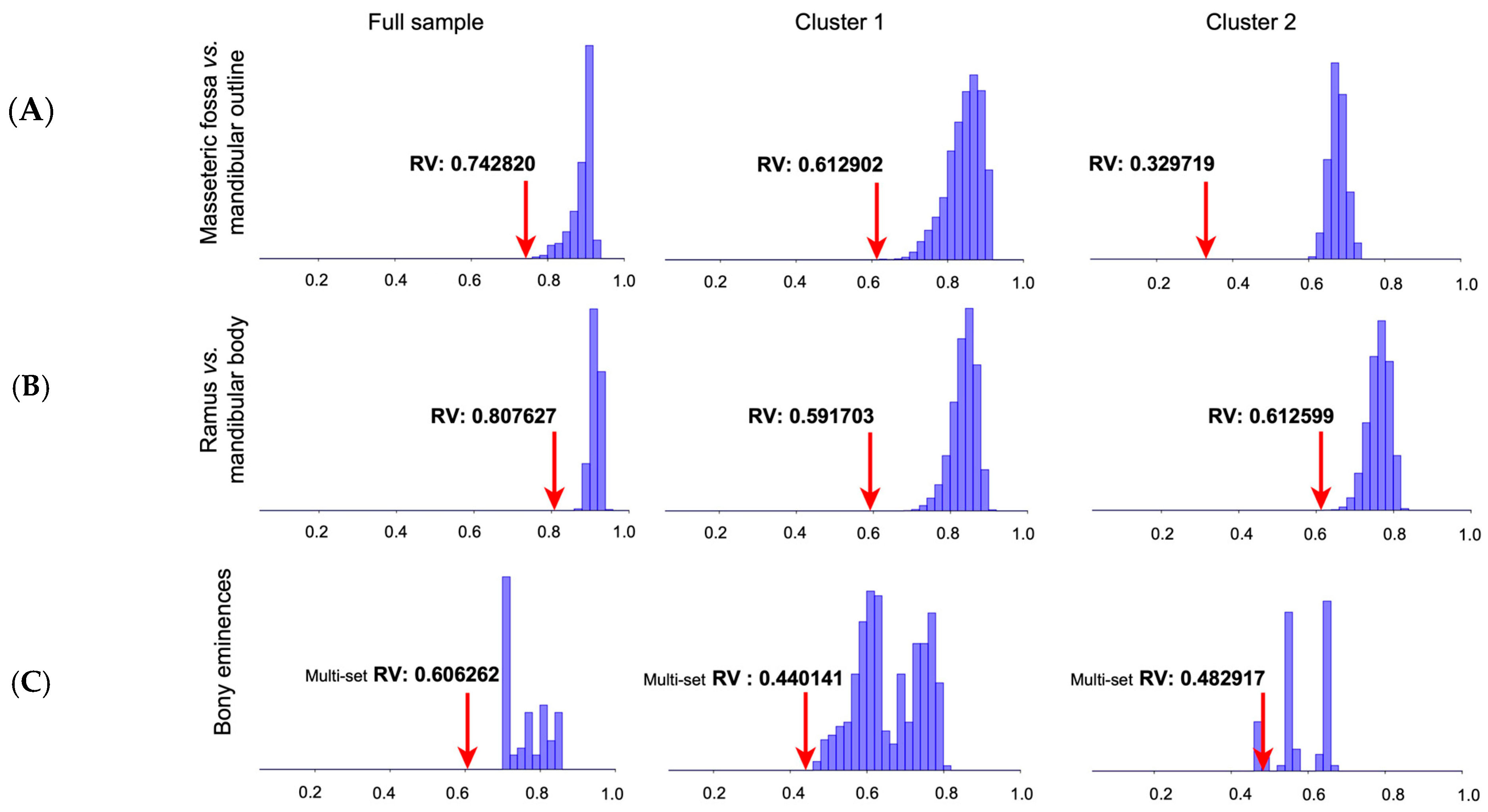
3.5. Modularity Analyses
4. Discussion
4.1. General
4.2. Main Axes of Variation in Mandibular Shape
4.3. Differentiation of Morphological Groups
4.4. Influence of Size on Mandibular Shape
4.5. Modular Organization of the Mandible
4.6. Relevance of the Findings
4.7. Limitations
4.8. Projections
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| BIC | Bayesian Information Criterion |
| CR | Covariance Ratio |
| CVA | Canonical Variate Analysis |
| DFA | Discriminant Function Analysis |
| DAPC | Discriminant Analysis of Principal Components |
| GM | Geometric Morphometrics |
| PCA | Principal Component Analysis |
| RV | Escoufier’s RV coefficient (measure of covariation between modules) |
| TPS | Thin Plate Spline |
| 2D | Two-Dimensional |
References
- Jashari, T.; Kahvecioğlu, O.; Duro, S.; Gündemir, O. Morphometric analysis for the sex determination of the skull of the Deltari Ilir dog (Canis lupus familiaris) of Kosovo. Anat. Histol. Embryol. 2022, 51, 443–451. [Google Scholar] [CrossRef]
- Amores-Ampuero, A. Sexual dimorphism in base of skull. Anthropol. Anz. 2017, 74, 9–14. [Google Scholar] [CrossRef]
- Trouth, C.O.; Winter, S.; Gupta, K.C.; Millis, R.M.; Holloway, J.A. Analysis of the sexual dimorphism in the basioccipital portion of the dog’s skull. Acta Anat. 1977, 98, 469–473. [Google Scholar] [CrossRef]
- The, T.L.; Trouth, C.O. Sexual dimorphism in the basilar part of the occipital bone of the dog (Canis familiaris). Acta Anat. 1976, 95, 565–571. [Google Scholar] [CrossRef]
- Kieser, J.A.; Groeneveld, H.T. Comparative morphology of the mandibulodental complex in wild and domestic canids. J. Anat. 1992, 180, 419–424. [Google Scholar] [PubMed]
- Brassard, C.; Callou, C. Sex determination of archaeological dogs using the skull: Evaluation of morphological and metric traits on various modern breeds. J. Archaeol. Sci. Rep. 2020, 31, 102294. [Google Scholar] [CrossRef]
- Drake, A.G.; Coquerelle, M.; Kosintsev, P.A.; Bachura, O.P.; Sablin, M.; Gusev, A.V.; Fleming, L.S.; Losey, R.J. Three-Dimensional Geometric Morphometric Analysis of Fossil Canid Mandibles and Skulls. Sci. Rep. 2017, 7, 9508. [Google Scholar] [CrossRef]
- Selba, M.C.; Oechtering, G.U.; Heng, H.G.; DeLeon, V.B. The Impact of Selection for Facial Reduction in Dogs: Geometric Morphometric Analysis of Canine Cranial Shape. Anat. Rec. 2020, 303, 330–346. [Google Scholar] [CrossRef] [PubMed]
- Brassard, C.; Bălăşescu, A.; Arbogast, R.M.; Forest, V.; Bemilli, C.; Boroneanţ, A.; Convertini, F.; Gandelin, M.; Radu, V.; Fleming, P.A.; et al. Unexpected morphological diversity in ancient dogs compared to modern relatives. Proc. Biol. Sci. 2022, 289, 20220147. [Google Scholar] [CrossRef]
- Schmidt, M.J.; Amort, K.H.; Failing, K.; Klingler, M.; Kramer, M.; Ondreka, N. Comparison of the endocranial- and brain volumes in brachycephalic dogs, mesaticephalic dogs and Cavalier King Charles spaniels in relation to their body weight. Acta Vet. Scand. 2014, 56, 30. [Google Scholar] [CrossRef]
- Curth, S.; Fischer, M.S.; Kupczik, K. Patterns of integration in the canine skull: An inside view into the relationship of the skull modules of domestic dogs and wolves. Zoology 2017, 125, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Drake, A.G.; Klingenberg, C.P. Large-scale diversification of skull shape in domestic dogs: Disparity and modularity. Am. Nat. 2010, 175, 289–301. [Google Scholar] [CrossRef]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- Dryden, I.L.; Mardia, K.V. Size-and-shape space. In Statistical Shape Analysis, with Applications in R, 2nd ed.; Wiley: Hoboken, NJ, USA, 2016; Chapter 5. [Google Scholar] [CrossRef]
- Onar, V. A morphometric study on the skull of the German shepherd dog (Alsatian). Anat. Histol. Embryol. 1999, 28, 253–256. [Google Scholar] [CrossRef]
- Onar, V.; Ozcan, S.; Pazvant, G. Skull typology of adult male Kangal dogs. Anat. Histol. Embryol. 2001, 30, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Onar, V.; Çakirlar, C.; Janeczek, M.; Kiziltan, Z. Skull typology of Byzantine dogs from the Theodosius Harbour at Yenikapi, Istanbul. Anat. Histol. Embryol. 2012, 41, 341–352. [Google Scholar] [CrossRef] [PubMed]
- König, H.E.; Liebich, H.-G. (Eds.) Veterinary Anatomy of Domestic Animals: Textbook and Colour Atlas, 7th ed.; Thieme: Stuttgart, Germany, 2020. [Google Scholar]
- Rohlf, F.J. TPSdig2 (Version 2.31) [Computer Software]. Department of Ecology and Evolution, State University of New York at Stony Brook. 2017. Available online: https://www.sbmorphometrics.org/soft-dataacq.html (accessed on 6 November 2025).
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D. Geometric Morphometrics for Biologists: A Primer, 2nd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Klingenberg, C.P. Morphometric integration and modularity in configurations of landmarks: Tools for evaluating a priori hypotheses. Evol. Dev. 2009, 11, 405–421. [Google Scholar] [CrossRef]
- Blackwell, E.A. Sexual Dimorphism in the Cranial Shape of Canids (Honors Thesis, Smith College). Bachelor’s Thesis, Smith ScholarWorks, Smith College, Northampton, MA, USA, 2022. Available online: https://scholarworks.smith.edu/theses/2426 (accessed on 6 November 2025).
- Law, C.J.; Blackwell, E.A.; Curtis, A.A.; Dickinson, E.; Hartstone-Rose, A.; Santana, S.E. Decoupled evolution of the cranium and mandible in carnivoran mammals. Evol. Int. J. Org. Evol. 2022, 76, 2959–2974. [Google Scholar] [CrossRef]
- Brannick, A.L. Shape evolution and Sexual Dimorphism in the Mandible of the Dire Wolf, Canis Dirus, at Rancho La Brea. Master’s Thesis, Marshall University, Huntington, WV, USA, 2014. Marshall Digital Scholar. Available online: https://mds.marshall.edu/etd/804 (accessed on 6 November 2025).
- Gittleman, J.L.; Van Valkenburgh, B. Sexual dimorphism in the canines and skulls of carnivores: Effects of size, phylogeny, and behavioural ecology. J. Zool. 1997, 242, 97–117. [Google Scholar] [CrossRef]
- Blázquez-Orta, R.; Rodríguez, L.; Major González, M.; Estaca-Gómez, V.; De Gaspar, I.; Feranec, R.S.; Carretero, J.M.; Arsuaga, J.L.; García, N. Dogs from the past: Exploring morphology in mandibles from Iberian archaeological sites using 3D geometric morphometrics. J. Archaeol. Sci. Rep. 2024, 57, 104660. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Studying morphological integration and modularity at multiple levels: Concepts and analysis. Philosophical transactions of the Royal Society of London. Ser. B Biol. Sci. 2014, 369, 20130249. [Google Scholar] [CrossRef]
- Wilson, L.A.B.; Balcarcel, A.; Geiger, M.; Heck, L.; Sánchez-Villagra, M.R. Modularity patterns in mammalian domestication: Assessing developmental hypotheses for diversification. Evol. Lett. 2021, 5, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.J.; Georgevsky, D.; Valenzuela, M.; McGreevy, P.D. A pilot study of sexual dimorphism in the head morphology of domestic dogs. J. Vet. Behav. 2014, 9, 43–46. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Kanemaki, N.; Kanai, K. Breed-Specific Skull Morphology Reveals Insights into Canine Optic Chiasm Positioning and Orbital Structure through 3D CT Scan Analysis. Animals 2024, 14, 197. [Google Scholar] [CrossRef]
- Parker, H.G.; Dreger, D.L.; Rimbault, M.; Davis, B.W.; Mullen, A.B.; Carpintero-Ramirez, G.; Ostrander, E.A. Genomic Analyses Reveal the Influence of Geographic Origin, Migration, and Hybridization on Modern Dog Breed Development. Cell Rep. 2017, 19, 697–708. [Google Scholar] [CrossRef]
- Boyko, A.R.; Quignon, P.; Li, L.; Schoenebeck, J.J.; Degenhardt, J.D.; Lohmueller, K.E.; Zhao, K.; Brisbin, A.; Parker, H.G.; vonHoldt, B.M.; et al. A simple genetic architecture underlies morphological variation in dogs. PLoS Biol. 2010, 8, e1000451. [Google Scholar] [CrossRef]
- Georgevsky, D.; Carrasco, J.; Valenzuela, M.; McGreevy, P.D. Domestic dog skull diversity across breeds, breed groupings, and genetic clusters. J. Vet. Behav. Clin. Appl. Res. 2014, 9, 228–234. [Google Scholar] [CrossRef]
- MacLeod, N.; Kolska Horwitz, L. Machine-learning strategies for testing patterns of morphological variation in small samples: Sexual dimorphism in gray wolf (Canis lupus) crania. BMC Biol. 2020, 18, 113. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, M.A.A.; Mohamed, W.F. Comparative craniometric measurements of two Canid species in Egypt: The Egyptian red fox and the Egyptian Baladi dog. BMC Vet. Res. 2022, 18, 173. [Google Scholar] [CrossRef]
- Toledo González, V.; Ortega Ojeda, F.; Fonseca, G.M.; García-Ruiz, C.; Navarro Cáceres, P.; Pérez-Lloret, P.; Marín García, M.D.P. A Morphological and Morphometric Dental Analysis as a Forensic Tool to Identify the Iberian Wolf (Canis lupus Signatus). Animals 2020, 10, 975. [Google Scholar] [CrossRef]
- Goswami, A.; Polly, P.D. The influence of modularity on cranial morphological disparity in Carnivora and Primates (Mammalia). PLoS ONE 2010, 5, e9517. [Google Scholar] [CrossRef]
- Zelditch, M.L.; Wood, A.R.; Bonett, R.M.; Swiderski, D.L. Modularity of the rodent mandible: Integrating bones, muscles, and teeth. Evol. Dev. 2008, 10, 756–768. [Google Scholar] [CrossRef]
- Tanaka, E.; Tanaka, M.; Miyawaki, Y.; Tanne, K. Viscoelastic properties of canine temporomandibular joint disc in compressive load-relaxation. Arch. Oral Biol. 1999, 44, 1021–1026. [Google Scholar] [CrossRef]
- Arzi, B.; Cissell, D.D.; Pollard, R.E.; Verstraete, F.J. Regenerative Approach to Bilateral Rostral Mandibular Reconstruction in a Case Series of Dogs. Front. Vet. Sci. 2015, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Senol, G.B.; Tuncer, M.K.; Nalcaci, N.; Aydin, K.C. Role of mandibular anatomical structures in sexual dimorphism in Turkish population: A radiomorphometric CBCT study. J. Forensic Odonto-Stomatol. 2022, 40, 53–64. [Google Scholar]
- Efron, B. Estimating the Error Rate of a Prediction Rule: Improvement on Cross-Validation. J. Am. Stat. Assoc. 1983, 78, 316–331. [Google Scholar] [CrossRef]
- Witten, D.M.; Tibshirani, R. A framework for feature selection in clustering. J. Am. Stat. Assoc. 2010, 105, 713–726. [Google Scholar] [CrossRef]
- Hennig, C. Cluster-wise assessment of cluster stability. Comput. Stat. Data Anal. 2007, 52, 258–271. [Google Scholar] [CrossRef]
- Tibshirani, R.; Walther, G.; Hastie, T. Estimating the number of clusters in a data set via the gap statistic. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2001, 63, 411–423. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras, R.; Salinas, P. Mandibular Shape Variation, Allometry and Modularity in Adult Mesocephalic Dogs (Canis lupus familiaris): Insights into Morphological Integration and Animal Anatomy. Animals 2025, 15, 3244. https://doi.org/10.3390/ani15223244
Contreras R, Salinas P. Mandibular Shape Variation, Allometry and Modularity in Adult Mesocephalic Dogs (Canis lupus familiaris): Insights into Morphological Integration and Animal Anatomy. Animals. 2025; 15(22):3244. https://doi.org/10.3390/ani15223244
Chicago/Turabian StyleContreras, Resef, and Paulo Salinas. 2025. "Mandibular Shape Variation, Allometry and Modularity in Adult Mesocephalic Dogs (Canis lupus familiaris): Insights into Morphological Integration and Animal Anatomy" Animals 15, no. 22: 3244. https://doi.org/10.3390/ani15223244
APA StyleContreras, R., & Salinas, P. (2025). Mandibular Shape Variation, Allometry and Modularity in Adult Mesocephalic Dogs (Canis lupus familiaris): Insights into Morphological Integration and Animal Anatomy. Animals, 15(22), 3244. https://doi.org/10.3390/ani15223244





