Evaluation of Total Eosinophil Counts, Serum Allergen-Specific IgE and Related Cytokines in Dogs with Atopic Dermatitis
Simple Summary
Abstract
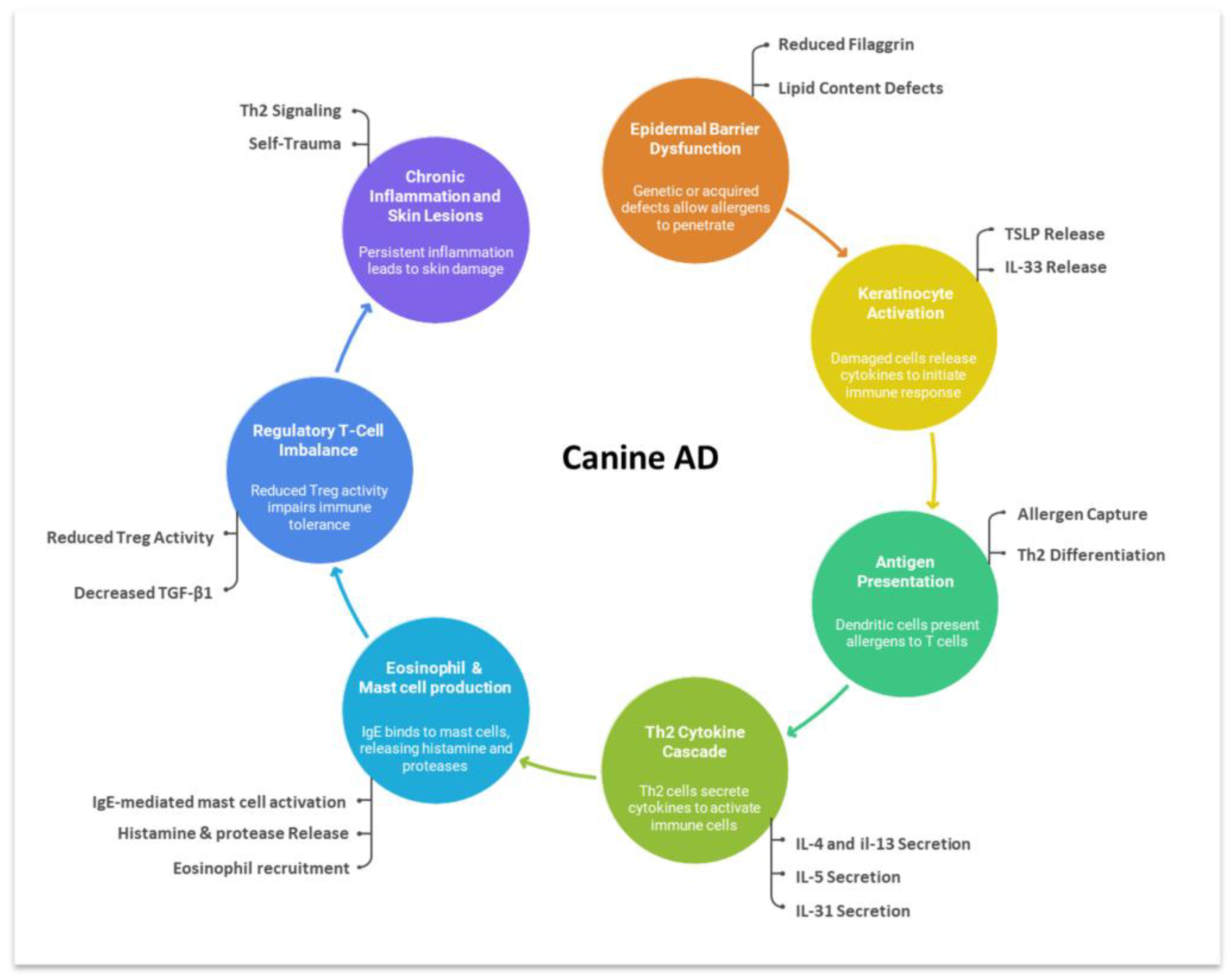
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and Storage
2.3. Eosinophil Counts
2.4. Allergen-Specific IgE Assay
2.5. Cytokine Assays
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Peripheral Eosinophil Counts
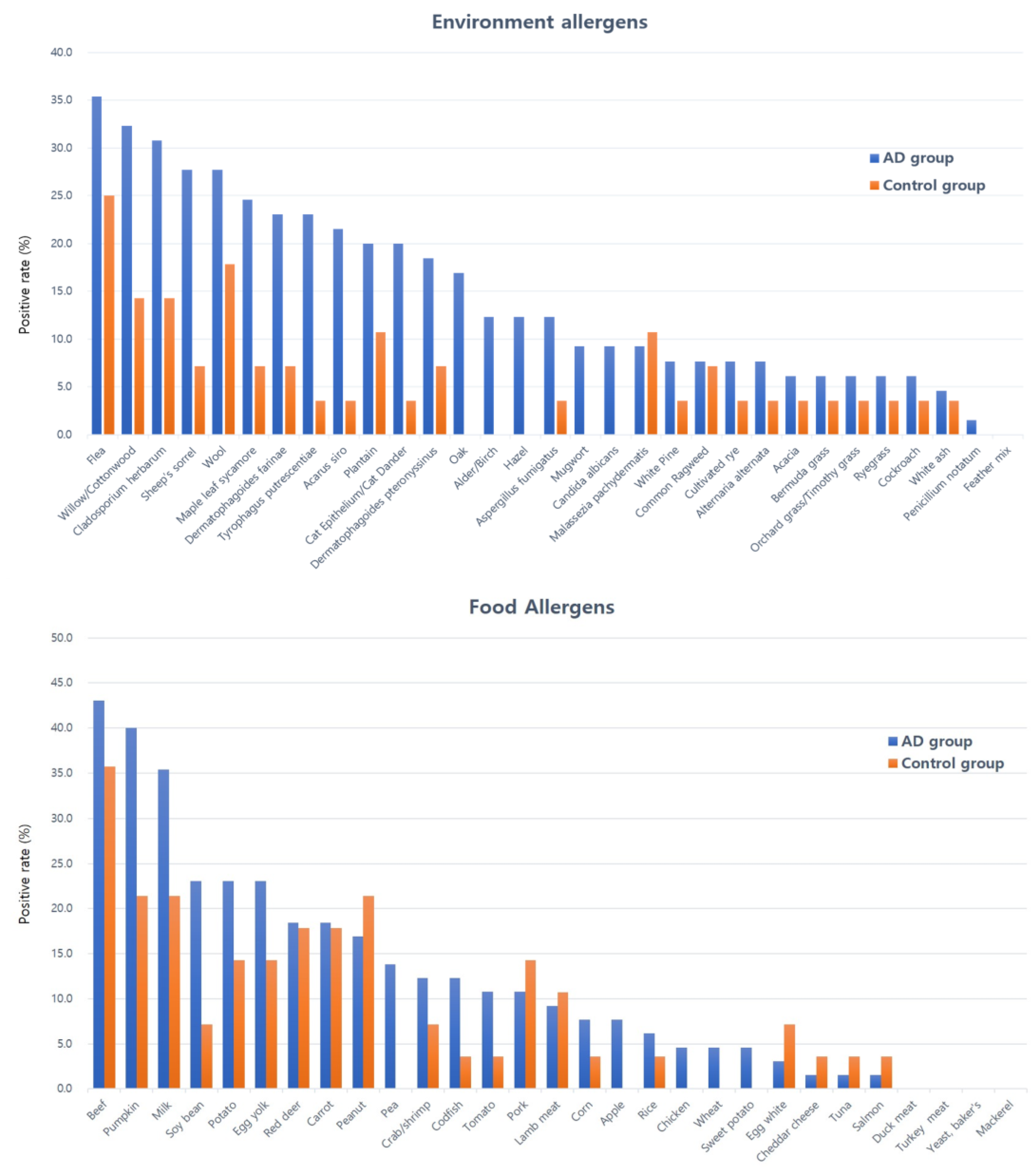
3.3. Serum Allergen-Specific IgE
3.4. Cytokine Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gedon, N.K.Y.; Mueller, R.S. Atopic dermatitis in cats and dogs: A difficult disease for animals and owners. Clin. Transl. Allergy 2018, 8, 41. [Google Scholar] [CrossRef]
- Outerbridge, C.A.; Jordan, T.J.M. Current knowledge on canine atopic dermatitis: Pathogenesis and treatment. Adv. Small Anim. Care 2021, 2, 101–115. [Google Scholar] [CrossRef]
- Im, D.H.; Yang, Y.S.; Choi, H.R.; Choi, S.J.; Nahm, H.J.; Han, K.J.; Hong, S.C.; Kim, J.K.; Cho, J.H. Prevalence of allergic disease in Korean adults: Results from the Korea National Health and Nutrition Examination Survey (2010–2012). Korean J. Otorhinolaryngol. Head Neck Surg. 2017, 60, 504–511. [Google Scholar] [CrossRef]
- Hensel, P.; Santoro, D.; Favrot, C.; Hill, P.; Griffin, C. Canine atopic dermatitis: Detailed guidelines for diagnosis and allergen identification. BMC Vet. Res. 2015, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Santoro, D.; Marsella, R.; Pucheu-Haston, C.M.; Eisenschenk, M.N.; Nuttall, T.; Bizikova, P. Pathogenesis of canine atopic dermatitis: Skin barrier and host–micro-organism interaction. Vet. Dermatol. 2015, 26, 84-e25. [Google Scholar] [CrossRef]
- Favrot, C.; Steffan, J.; Seewald, W.; Picco, F. A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Vet. Dermatol. 2010, 21, 23–31. [Google Scholar] [CrossRef]
- Miller, J.; Simpson, A.; Bloom, P.; Diesel, A.; Friedeck, A.; Paterson, T.; Wisecup, M.; Yu, C.M. 2023 AAHA management of allergic skin diseases in dogs and cats guidelines. J. Am. Anim. Hosp. Assoc. 2023, 59, 255–284. [Google Scholar] [CrossRef]
- Lilliehook, I.; Gunnarsson, L.; Zakrisson, G.; Tvedten, H. Disease associated with pronounced eosinophilia: A study of 105 dogs in Sweden. J. Small Anim. Pract. 2000, 41, 248–253. [Google Scholar] [CrossRef]
- Thomas, P.; Alexander, J.S.; Eliazbeth, A.E.; Scott, C.; Judith, A.W. IgE in the diagnosis and treatment of allergic disease. J. Allergy Clin. Immunol. 2016, 137, 1662–1670. [Google Scholar] [CrossRef]
- Morales-Romero, R.; Gonzalez-Dominguez, M.S.; Sánchez, J.; Correa-Valencia, N.M.; Maldonado-Estrada, J.G. Efficacy of diagnostic testing for allergen sensitization in canine atopic dermatitis: A systematic review. Front. Vet. Sci. 2025, 12, 1551207. [Google Scholar] [CrossRef]
- Klonowska, J.; Glen, J.; Nowicki, R.J.; Trzeciak, M. New cytokines in the pathogenesis of atopic dermatitis—New therapeutic targets. Int. J. Mol. Sci. 2018, 19, 3086. [Google Scholar] [CrossRef]
- McCandless, E.E.; Rugg, C.A.; Fici, G.J.; Messamore, J.E.; Aleo, M.M.; Gonzales, A.J. Allergen-induced production of IL-31 by canine Th2 cells and identification of immune, skin, and neuronal target cells. Vet. Immunol. Immunopathol. 2014, 157, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.J.; Humphrey, W.R.; Messamore, J.E.; Fleck, T.J.; Fici, G.J.; Shelly, J.A.; Teel, J.F.; Bammert, G.F.; Dunham, S.A.; Fuller, T.E.; et al. Interleukin-31: Its role in canine pruritus and naturally occurring canine atopic dermatitis. Vet. Dermatol. 2013, 24, 48-e12. [Google Scholar] [CrossRef]
- Mazrier, H.; Vogelnest, L.J.; Taylor, R.M.; Williamson, P. Altered plasma cytokines in dogs with atopic dermatitis. Vet. Dermatol. 2022, 33, 131-e38. [Google Scholar] [CrossRef]
- Tamamoto-Mochizuki, C.; Santoro, D.; Saridomichelakis, M.N.; Eisenschenk, M.N.C.; Hensel, P.; Pucheu-Haston, C.; International Committee on Allergic Diseases of Animals (ICADA). Update on the role of cytokines and chemokines in canine atopic dermatitis. Vet. Dermatol. 2024, 35, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Wickman, M.; Ahlstedt, S.; Lilja, G.; van Hage Hamsten, M. Quantification of IgE antibodies simplifies the classification of allergic diseases in 4-year-old children: A report from the prospective birth cohort study BAMSE. Pediatr. Allergy. Immunol. 2003, 14, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Yun, T.; Koo, Y.; Lee, D.; Chae, Y.; Park, J.; Choi, D.; Kim, H.; Yang, M.P.; Kang, B.T. Comparison of intradermal skin test and multiple allergen simultaneous test results in canine atopic dermatitis. J. Vet. Clin. 2021, 38, 120–126. [Google Scholar] [CrossRef]
- Uehara, M.; Izukura, R.; Sawai, T. Blood eosinophilia in atopic dermatitis. Clin. Exp. Dermatol. 1990, 15, 264–266. [Google Scholar] [CrossRef]
- Lee, H.K.; Pyung, B.Y.; Lee, S.J. Etiologic allergens, blood eosinophil count and total IgE level according to age and site of skin lesion in atopic dermatitis. J. Asthma Allergy Clin. Immunol. 1992, 12, 70–77. [Google Scholar]
- Koh, H.S.; Lee, K.S.; Han, D.H.; Rha, Y.H.; Choi, S.H. Relationship between serum total IgE, specific IgE, and peripheral blood eosinophil count according to specific allergic diseases. Allergy Asthma Respir. Dis. 2013, 1, 123–128. [Google Scholar] [CrossRef][Green Version]
- Kang, M.H.; Kim, H.J.; Jang, H.J.; Park, H.M. Sensitization rates of causative allergens for dogs with atopic dermatitis: Detection of canine allergen-specific IgE. J. Vet. Sci. 2014, 15, 545–550. [Google Scholar] [CrossRef]
- Saridomichelakis, M.N.; Marsella, R.; Lee, K.W.; Esch, R.E.; Farmaki, R.; Koutinas, A.F. Assessment of cross-reactivity among five species of house dust and storage mites. Vet. Dermatol. 2008, 19, 67–76. [Google Scholar] [CrossRef]
- Hsiao, Y.; Chen, C.; Willemse, T. Allergen sensitization patterns of allergic dogs: IgE-microarray analysis. Thai J. Vet. Med. 2016, 46, 235–242. [Google Scholar] [CrossRef]
- Masuda, K.; Sakaguchi, M.; Fujiwara, S.; Kurata, K.; Yamashita, K.; Odagiri, T.; Nakao, Y.; Matsuki, N.; Ono, K.; Watari, T.; et al. Positive reactions to common allergens in 42 atopic dogs in Japan. Vet. Immunol. Immunopathol. 2000, 73, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, T.J.; Hill, P.B.; Bensignor, E.; Willemse, T.; Members of the International Task Force on Canine Atopic Dermatitis. House dust and forage mite allergens and their role in human and canine atopic dermatitis. Vet. Dermatol. 2006, 17, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Hobi, J.I.; Hauck, A.; Linek, M.; Chi, Y.M.; Verbrugge, M.; Favrot, C.; Mueller, R.S. Food-specific serum IgE and IgG reactivity in dogs with and without skin disease: Lack of correlation between laboratories. Vet. Dermatol. 2014, 25, 447-e70. [Google Scholar]
- Kennis, R.A. Food allergies: Update of pathogenesis, diagnoses, and management. Vet. Clin. N. Am. Small Anim. Pract. 2006, 36, 175–184. [Google Scholar] [CrossRef]
- Olivry, T.; Mayhew, D.; Paps, J.S.; Linder, K.E.; Peredo, C.; Rajpal, D.; Hofland, H.; Cote-Sierra, J. Early activation of Th2/Th22 inflammatory and pruritogenic pathways in acute canine atopic dermatitis skin lesions. J. Investig. Dermatol. 2016, 136, 1961–1970. [Google Scholar] [CrossRef]
- Sokołowska, M.; Wilkołek, P.; Glinski, M.; Szweda, W.; Targońska, I.; Twardowska, W. The influence of treatment with lokivetmab on transepidermal water loss (TEWL) in dogs with spontaneously occurring atopic dermatitis. Vet. Dermatol. 2019, 30, 330-e93. [Google Scholar]
- Gonzales, A.J.; Bowman, J.W.; Fici, G.J.; Zhang, M.; Mann, D.W.; Mitton-Fry, M. Oclacitinib (APOQUEL) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy. J. Vet. Pharmacol. Ther. 2014, 37, 317–332. [Google Scholar] [CrossRef]
| ASM Class | IgE Levels (AU/mL) | Evaluation | Clinical Interpretation |
|---|---|---|---|
| 0 | <0.35 | Negative | Allergic reaction excluded |
| 1 | ≥0.35–<0.7 | Weak positive | Possible risk of allergic reaction |
| 2 | ≥0.7–<3.5 | ||
| 3 | ≥3.5–<17.5 | Strong positive | Clinically relevant level |
| 4 | ≥17.5–<50 | ||
| 5 | ≥50–<100 | Very strong positive | Highly clinically relevant level |
| 6 | ≥100 |
| Characteristics | AD Group | Control Group | ||||||
|---|---|---|---|---|---|---|---|---|
| N | % | Mean ± SD | N | % | Mean ± SD | |||
| Age (overall) | 65 | 100 | 6.34 ± 3.99 | 28 | 100 | 3.59 ± 4.12 | ||
| <1 year | 3 | 5 | 12 | 43 | ||||
| 1–6 year | 34 | 52 | 10 | 36 | ||||
| >6 years | 28 | 43 | 6 | 21 | ||||
| Sex | ||||||||
| Male | 6 | 9 | 8 | 29 | ||||
| Castrated male | 33 | 51 | 10 | 36 | ||||
| Female | 8 | 12 | 4 | 14 | ||||
| Spayed female | 18 | 28 | 6 | 21 | ||||
| Breeds | ||||||||
| Bichon Frise (3), Boston Terrier (4), Chihuahua (1), Chow Chow (1), Cocker Spaniel (1), French Bulldog (1), Japanese Spitz (1), Labrador Retriever (2), Maltese (20), Mixed (5), Pomeranian (4), Poodle (7), Shih Tzu (8), Spitz (1), Welsh Corgi (1), Yorkshire Terrier (5) | Bichon Frise (3), Chihuahua (1), Golden Retriever (3), Maltese (6), Miniature Pinscher (1), Mixed (2), Pomeranian (5), Poodle (5), Yorkshire Terrier (2) | |||||||
| Classification | Code | Allergens | AD Group Median [IQR] | Control Group Median [IQR] | p | |
|---|---|---|---|---|---|---|
| Environmental allergens | Mite | d1 | Dermatophagoides pteronyssinus | 0.15 [0.15–0.17] | 0.15 [0.15–0.15] | 0.023 * |
| d2 | Dermatophagoides farinae | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.051 | ||
| d70 | Acarus siro | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.038 * | ||
| d72 | Tyrophagus putrescentiae | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.016 * | ||
| Pollen | t2/t3 | Alder/Birch | 0.15 [0.15–0.20] | 0.15 [0.15–0.15] | 0.003 ** | |
| t4 | Hazel | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.016 * | ||
| t11 | Maple leaf sycamore | 0.26 [0.15–0.34] | 0.15 [0.15–0.21] | 0.003 ** | ||
| t12/t14 | Willow/Cottonwood | 0.34 [0.15–0.44] | 0.15 [0.15–0.27] | 0.014 * | ||
| t7 | Oak | 0.15 [0.15–0.20] | 0.15 [0.15–0.15] | 0.005 ** | ||
| t16 | White pine | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.108 | ||
| t19 | Acacia | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.585 | ||
| t15 | White ash | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.793 | ||
| w1 | Common ragweed | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.718 | ||
| w9 | Plantain | 0.20 [0.15–0.34] | 0.15 [0.15–0.20] | 0.005 ** | ||
| w6 | Mugwort | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.238 | ||
| w18 | Sheep’s sorrel | 0.15 [0.15–0.51] | 0.15 [0.15–0.15] | 0.007 ** | ||
| g2 | Bermuda grass | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.065 | ||
| g3/g6 | Orchard grass/ Timothy grass | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.443 | ||
| g5 | Ryegrass | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.281 | ||
| g12 | Cultivated rye | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.546 | ||
| Molds | m1 | Penicillium notatum | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.512 | |
| m3 | Cladosporium herbarum | 0.19 [0.15–0.38] | 0.15 [0.15–0.29] | 0.031 * | ||
| m2 | Aspergillus fumigatus | 0.15 [0.15–0.34] | 0.15 [0.15–0.15] | 0.111 | ||
| m6 | Candida albicans | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.098 | ||
| m5 | Alternaria alternata | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.455 | ||
| m227 | Malassezia pachydermatis | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.922 | ||
| Insect | B22 | Flea | 0.15 [0.15–0.93] | 0.15 [0.15–0.21] | 0.187 | |
| i6 | Cockroach | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.443 | ||
| Others (Epi/indoor) | e1/e2 | Cat epithelium/ Cat dander | 0.18 [0.15–0.34] | 0.15 [0.15–0.15] | 0.001 ** | |
| e81 | Wool | 0.15 [0.15–0.43] | 0.15 [0.15–0.15] | 0.242 | ||
| ex1 | Feather mix | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 1.000 | ||
| Food allergens | Meats | f26 | Pork | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.914 |
| f27 | Beef | 0.19 [0.15–1.90] | 0.16 [0.15–1.32] | 0.817 | ||
| f581 | Duck meat | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.512 | ||
| f83 | Chicken | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.906 | ||
| f88 | Lamb meat | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.881 | ||
| f284 | Turkey meat | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 1.000 | ||
| f867 | Red deer | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.572 | ||
| Grains, Beans | f4 | Wheat | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.182 | |
| f8 | Corn | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.415 | ||
| f9 | Rice | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.906 | ||
| f12 | Pea | 0.16 [0.15–0.25] | 0.15 [0.15–0.16] | 0.017 * | ||
| f14 | Soy bean | 0.15 [0.15–0.24] | 0.15 [0.15–0.15] | 0.026 * | ||
| Fruit, Vegetable | f31 | Carrot | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.901 | |
| f35 | Potato | 0.15 [0.15–0.20] | 0.15 [0.15–0.16] | 0.141 | ||
| f54 | Sweet potato | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.250 | ||
| f225 | Pumpkin | 0.27 [0.15–0.52] | 0.15 [0.15–0.27] | 0.021 * | ||
| f25 | Tomato | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.095 | ||
| f49 | Apple | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.016 * | ||
| Yeast | f45 | Yeast, baker’s | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.537 | |
| Dairy products | f1 | Egg white | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.637 | |
| f75 | Egg yolk | 0.16 [0.15–0.28] | 0.16 [0.15–0.28] | 0.329 | ||
| f2 | Milk | 0.15 [0.15–0.87] | 0.15 [0.15–0.15] | 0.261 | ||
| f81 | Cheddar cheese | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.372 | ||
| Seafood | f23/f24 | Crab/shrimp | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.793 | |
| f3 | Codfish | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.214 | ||
| f40 | Tuna | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.549 | ||
| f41 | Salmon | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 0.549 | ||
| f206 | Mackerel | 0.15 [0.15–0.15] | 0.15 [0.15–0.15] | 1.000 | ||
| Nut | f13 | Peanut | 0.15 [0.15–0.27] | 0.15 [0.15–0.27] | 0.454 | |
| Environmental | Code | Allergens | Odds Ratio | 95% CI | Relative Risk | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||
| Mites | d70 | Acarus siro | 7.412 | 0.924 | 59.428 | 1.427 | 1.156 | 1.762 |
| d72 | Tyrophagus putrescentiae | 8.100 | 1.014 | 64.685 | 1.444 | 1.174 | 1.776 | |
| Pollens | t7 | Oak | 1.519 | 1.299 | 1.775 | |||
| w18 | Sheep’s sorrel | 4.979 | 1.070 | 23.164 | 1.398 | 1.117 | 1.750 | |
| Environmental | Code | Allergens | Odds Ratio | 95% CI | p | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Mites | d70 | Acarus siro | 3.45 | 1.08 | 10.98 | 0.037 * |
| d72 | Tyrophagus putrescentiae | 2.86 | 0.95 | 8.55 | 0.061 | |
| Pollens | t7 | Oak | 1.21 | 0.42 | 3.45 | 0.718 |
| w18 | Sheep’s sorrel | 0.91 | 0.33 | 2.52 | 0.856 | |
| Cytokines | AD Group (Median [IQR]) | Control Group (Median [IQR]) | p |
|---|---|---|---|
| IL-4 (pg/mL) | 215.20 [156.51–341.72] | 212.94 [146.48–250.38] | 0.436 |
| IL-13 (pg/mL) | 139.05 [91.43–267.98] | 122.61 [91.84–165.84] | 0.247 |
| IL-31(pg/mL) | 109.70 [70.79–223.02] | 103.32 [82.52–137.05] | 0.960 |
| TGF-β1(pg/mL) | 406.28 [283.75–598.93] | 519.68 [334.39–628.41] | 0.128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, M.-J.; Kang, M.-H.; Park, H.-M. Evaluation of Total Eosinophil Counts, Serum Allergen-Specific IgE and Related Cytokines in Dogs with Atopic Dermatitis. Animals 2025, 15, 3219. https://doi.org/10.3390/ani15213219
Chae M-J, Kang M-H, Park H-M. Evaluation of Total Eosinophil Counts, Serum Allergen-Specific IgE and Related Cytokines in Dogs with Atopic Dermatitis. Animals. 2025; 15(21):3219. https://doi.org/10.3390/ani15213219
Chicago/Turabian StyleChae, Min-Joo, Min-Hee Kang, and Hee-Myung Park. 2025. "Evaluation of Total Eosinophil Counts, Serum Allergen-Specific IgE and Related Cytokines in Dogs with Atopic Dermatitis" Animals 15, no. 21: 3219. https://doi.org/10.3390/ani15213219
APA StyleChae, M.-J., Kang, M.-H., & Park, H.-M. (2025). Evaluation of Total Eosinophil Counts, Serum Allergen-Specific IgE and Related Cytokines in Dogs with Atopic Dermatitis. Animals, 15(21), 3219. https://doi.org/10.3390/ani15213219






