Effects of Preventive Administration of Propylene Glycol or Sucrose in Dairy Cows with Elevated Blood Non-Esterified Fatty Acids During the Close-Up Period
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blood Sampling and Physical Examinations
2.3. Cows Eligible for Preventive Treatment
2.4. Separation of Lipoproteins
2.5. Measurement of Serum Biochemical Parameters and Components of Lipoproteins
2.6. Measurement of Serum Malondialdehyde (MDA) Concentration and Paraoxonase-1 (PON-1) Activity
2.7. Milk Production
2.8. Postpartum Disease Incidence and Culling Rate
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drackley, J.K. Biology of Dairy Cows during the Transition Period: The Final Frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef] [PubMed]
- Grummer, R.R. Impact of Changes in Organic Nutrient Metabolism on Feeding the Transition Dairy Cow. J. Anim. Sci. 1995, 73, 2820–2833. [Google Scholar] [CrossRef] [PubMed]
- Bauman, D.E.; Bruce Currie, W. Partitioning of Nutrients during Pregnancy and Lactation: A Review of Mechanisms Involving Homeostasis and Homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef]
- Baird, G.D. Primary Ketosis in the High-Producing Dairy Cow: Clinical and Subclinical Disorders, Treatment, Prevention, and Outlook. J. Dairy Sci. 1982, 65, 1–10. [Google Scholar] [CrossRef]
- Fetrow, J.; Nordlund, K.V.; Norman, H.D. Invited Review: Culling: Nomenclature, Definitions, and Recommendations. J. Dairy Sci. 2006, 89, 1896–1905. [Google Scholar] [CrossRef]
- Gröhn, Y.T.; Eicker, S.W.; Ducrocq, V.; Hertl, J.A. Effect of Diseases on the Culling of Holstein Dairy Cows in New York State. J. Dairy Sci. 1998, 81, 966–978. [Google Scholar] [CrossRef]
- Herdt, T.H. Ruminant Adaptation to Negative Energy Balance: Influences on the Etiology of Ketosis and Fatty Liver. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 215–230. [Google Scholar] [CrossRef]
- LeBlanc, S. Monitoring Metabolic Health of Dairy Cattle in the Transition Period. J. Reprod. Dev. 2010, 56, S29–S35. [Google Scholar] [CrossRef]
- Chisato, K.; Yamazaki, T.; Kayasaki, S.; Fukumori, R.; Higuchi, H.; Makita, K.; Oikawa, S. Metabolites and Physical Scores as Possible Predictors for Postpartum Culling in Dairy Cows. Res. Vet. Sci. 2024, 179, 105387. [Google Scholar] [CrossRef]
- Oetzel, G.R. Monitoring and Testing Dairy Herds for Metabolic Disease. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 651–674. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Evaluation of Nonesterified Fatty Acids and β-Hydroxybutyrate in Transition Dairy Cattle in the Northeastern United States: Critical Thresholds for Prediction of Clinical Diseases. J. Dairy Sci. 2010, 93, 546–554. [Google Scholar] [CrossRef]
- Mudroň, P.; Rehage, J.; Qualmann, K.; Sallmann, H.-P.; Scholz, H. A Study of Lipid Peroxidation and Vitamin E in Dairy Cows with Hepatic Insufficiency. J. Vet. Med. A 1999, 46, 219–224. [Google Scholar] [CrossRef]
- Senoh, T.; Oikawa, S.; Nakada, K.; Tagami, T.; Iwasaki, T. Increased Serum Malondialdehyde Concentration in Cows with Subclinical Ketosis. J. Vet. Med. Sci. 2019, 81, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. The Importance of the Oxidative Status of Dairy Cattle in the Periparturient Period: Revisiting Antioxidant Supplementation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.B. The Treatment of Ketosis with Glycerol and Propylene Glycol. Cornell Vet. 1954, 44, 6–21. [Google Scholar]
- Nielsen, N.I.; Ingvartsen, K.L. Propylene Glycol for Dairy Cows: A Review of the Metabolism of Propylene Glycol and Its Effects on Physiological Parameters, Feed Intake, Milk Production and Risk of Ketosis. Anim. Feed Sci. Technol. 2004, 115, 191–213. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Raun, B.M.L. Ruminal and Intermediary Metabolism of Propylene Glycol in Lactating Holstein Cows. J. Dairy Sci. 2007, 90, 4707–4717. [Google Scholar] [CrossRef]
- Oba, M.; Mewis, J.L.; Zhining, Z. Effects of Ruminal Doses of Sucrose, Lactose, and Corn Starch on Ruminal Fermentation and Expression of Genes in Ruminal Epithelial Cells. J. Dairy Sci. 2015, 98, 586–594. [Google Scholar] [CrossRef]
- Ordway, R.S.; Ishler, V.A.; Varga, G.A. Effects of Sucrose Supplementation on Dry Matter Intake, Milk Yield, and Blood Metabolites of Periparturient Holstein Dairy Cows. J. Dairy Sci. 2002, 85, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Formigoni, A.; Cornil, M.-C.; Prandi, A.; Mordenti, A.; Rossi, A.; Portetelle, D.; Renaville, R. Effect of Propylene Glycol Supplementation around Parturition on Milk Yield, Reproduction Performance and Some Hormonal and Metabolic Characteristics in Dairy Cows. J. Dairy Res. 1996, 63, 11–24. [Google Scholar] [CrossRef]
- Rukkwamsuk, T.; Rungruang, S.; Choothesa, A.; Wensing, T. Effect of Propylene Glycol on Fatty Liver Development and Hepatic Fructose 1,6 Bisphosphatase Activity in Periparturient Dairy Cows. Livest. Prod. Sci. 2005, 95, 95–102. [Google Scholar] [CrossRef]
- Studer, V.A.; Grummer, R.R.; Bertics, S.J.; Reynolds, C.K. Effect of Prepartum Propylene Glycol Administration on Periparturient Fatty Liver in Dairy Cows. J. Dairy Sci. 1993, 76, 2931–2939. [Google Scholar] [CrossRef]
- Christensen, J.O.; Grummer, R.R.; Rasmussen, F.E.; Bertics, S.J. Effect of Method of Delivery of Propylene Glycol on Plasma Metabolites of Feed-Restricted Cattle. J. Dairy Sci. 1997, 80, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Grummer, R.R.; Winkler, J.C.; Bertics, S.J.; Studer, V.A. Effect of Propylene Glycol Dosage during Feed Restriction on Metabolites in Blood of Prepartum Holstein Heifers. J. Dairy Sci. 1994, 77, 3618–3623. [Google Scholar] [CrossRef]
- National Research Council Nutrient Requirements of Dairy Cattle, 7th ed.; National Academies Press: Washington, DC, USA, 2001.
- Ferguson, J.D.; Galligan, D.T.; Thomsen, N. Principal Descriptors of Body Condition Score in Holstein Cows. J. Dairy Sci. 1994, 77, 2695–2703. [Google Scholar] [CrossRef]
- Hulsen, J. Cow Signals; Rood Bont: Zutphen, The Netherlands, 2007. [Google Scholar]
- Silva-del-Río, N.; Fricke, P.M.; Grummer, R.R. Effects of Twin Pregnancy and Dry Period Feeding Strategy on Milk Production, Energy Balance, and Metabolic Profiles in Dairy Cows. J. Anim. Sci. 2010, 88, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, S.; Mizunuma, Y.; Iwasaki, Y.; Tharwat, M. Changes of Very Low-Density Lipoprotein Concentration in Hepatic Blood from Cows with Fasting-Induced Hepatic Lipidosis. Can. J. Vet. Res. 2010, 74, 317–320. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Holtenius, P.; Holtenius, K. A Model to Estimate Insulin Sensitivity in Dairy Cows. Acta Vet. Scand. 2007, 49, 29. [Google Scholar] [CrossRef]
- Antončić-Svetina, M.; Turk, R.; Svetina, A.; Gereš, D.; Rekić, B.; Juretić, D. Lipid Status, Paraoxonase-1 Activity and Metabolic Parameters in Serum of Heifers and Lactating Cows Related to Oxidative Stress. Res. Vet. Sci. 2011, 90, 298–300. [Google Scholar] [CrossRef]
- Senti, M.; Tomas, M.; Fito, M.; Weinbrenner, T.; Covas, M.-I.; Sala, J.; Masiá, R.; Marrugat, J. Antioxidant Paraoxonase 1 Activity in the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 5422–5426. [Google Scholar] [CrossRef] [PubMed]
- Cascone, G.; Licitra, F.; Stamilla, A.; Amore, S.; Dipasquale, M.; Salonia, R.; Antoci, F.; Zecconi, A. Subclinical Ketosis in Dairy Herds: Impact of Early Diagnosis and Treatment. Front. Vet. Sci. 2022, 9, 895468. [Google Scholar] [CrossRef]
- Jeong, J.K.; Choi, I.S.; Moon, S.H.; Lee, S.C.; Kang, H.G.; Jung, Y.H.; Park, S.B.; Kim, I.H. Effect of Two Treatment Protocols for Ketosis on the Resolution, Postpartum Health, Milk Yield, and Reproductive Outcomes of Dairy Cows. Theriogenology 2018, 106, 53–59. [Google Scholar] [CrossRef]
- Burfeind, O.; Sepúlveda, P.; von Keyserlingk, M.A.G.; Weary, D.M.; Veira, D.M.; Heuwieser, W. Technical Note: Evaluation of a Scoring System for Rumen Fill in Dairy Cows. J. Dairy Sci. 2010, 93, 3635–3640. [Google Scholar] [CrossRef] [PubMed]
- Ruddick, J.A. Toxicology, Metabolism, and Biochemistry of 1,2-Propanediol. Toxicol. Appl. Pharmacol. 1972, 21, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Vernon, R.G. Lipid Metabolism during Lactation: A Review of Adipose Tissue-Liver Interactions and the Development of Fatty Liver. J. Dairy Res. 2005, 72, 460–469. [Google Scholar] [CrossRef]
- Fonseca, L.F.; Rodrigues, P.H.; Santos, M.V.; Lima, A.P.; Lucci, C.S. Supplementation of Dairy Cows with Propylene Glycol during the Periparturient Period: Effects on Body Condition Score, Milk Yield, First Estrus Post-Partum, Beta-Hydroxybutyrate, Non-Esterified Fatty Acids and Glucose Concentrations. Ciência Rural 2004, 34, 897–903. [Google Scholar] [CrossRef]
- Lien, T.F.; Chang, L.B.; Horng, Y.M.; Wu, C.P. Effects of Propylene Glycol on Milk Production, Serum Metabolites and Reproductive Performance during the Transition Period of Dairy Cows. Asian-Australas. J. Anim. Sci. 2010, 23, 372–378. [Google Scholar] [CrossRef]
- Stokes, S.R.; Goff, J.P. Evaluation of Calcium Propionate and Propylene Glycol Administered into the Esophagus of Dairy Cattle at Calving. Prof. Anim. Sci. 2001, 17, 115–122. [Google Scholar] [CrossRef]
- Miyoshi, S.; Pate, J.L.; Palmquist, D.L. Effects of Propylene Glycol Drenching on Energy Balance, Plasma Glucose, Plasma Insulin, Ovarian Function and Conception in Dairy Cows. Anim. Reprod. Sci. 2001, 68, 29–43. [Google Scholar] [CrossRef]
- Weisbjerg, M.R.; Hvelplund, T.; Bibby, B.M. Hydrolysis and Fermentation Rate of Glucose, Sucrose and Lactose in the Rumen. Acta Agric. Scand. A Anim. Sci. 1998, 48, 12–18. [Google Scholar] [CrossRef]
- Clapperton, J.L.; Czerkawski, J.W. Metabolism of Propane-1:2-Diol Infused into the Rumen of Sheep. Br. J. Nutr. 1972, 27, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Emery, R.S.; Burg, N.; Brown, L.D.; Blank, G.N. Detection, Occurrence, and Prophylactic Treatment of Borderline Ketosis with Propylene Glycol Feeding. J. Dairy Sci. 1964, 47, 1074–1079. [Google Scholar] [CrossRef]
- Emery, R.S.; Brown, R.E.; Black, A.L. Metabolism of DL-1,2-Propanediol-2-14C in a Lactating Cow. J. Nutr. 1967, 92, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Leal Yepes, F.A.; Wakshlag, J.J.; Behling-Kelly, E.; McArt, J.A.A. The Effect of Different Treatments for Early-Lactation Hyperketonemia on Liver Triglycerides, Glycogen, and Expression of Key Metabolic Enzymes in Dairy Cattle. J. Dairy Sci. 2018, 101, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W.; Bauman, D.E. Adaptations of Glucose Metabolism during Pregnancy and Lactation. J. Mammary Gland. Biol. Neoplasia. 1997, 2, 265–278. [Google Scholar] [CrossRef]
- Hayirli, A. The Role of Exogenous Insulin in the Complex of Hepatic Lipidosis and Ketosis Associated with Insulin Resistance Phenomenon in Postpartum Dairy Cattle. Vet. Res. Commun. 2006, 30, 749–774. [Google Scholar] [CrossRef]
- Lee, H.-H.; Kida, K.; Miura, R.; Inokuma, H.; Miyamoto, A.; Kawashima, C.; Haneda, S.; Miyake, Y.-I.; Matsui, M. Slow Recovery of Blood Glucose in the Insulin Tolerance Test during the Prepartum Transition Period Negatively Impacts the Nutritional Status and Reproductive Performance Postpartum of Dairy Cows. J. Vet. Med. Sci. 2012, 74, 457–464. [Google Scholar] [CrossRef][Green Version]
- Perseghin, G.; Caumo, A.; Caloni, M.; Testolin, G.; Luzi, L. Incorporation of the Fasting Plasma FFA Concentration into QUICKI Improves Its Association with Insulin Sensitivity in Nonobese Individuals. J. Clin. Endocrinol. Metab. 2001, 86, 4776–4781. [Google Scholar] [CrossRef]
- Rabasa-Lhoret, R.; Bastard, J.-P.; Jan, V.; Ducluzeau, P.-H.; Andreelli, F.; Guebre, F.; Bruzeau, J.; Louche-Pellissier, C.; MaÎtrepierre, C.; Peyrat, J.; et al. Modified Quantitative Insulin Sensitivity Check Index Is Better Correlated to Hyperinsulinemic Glucose Clamp than Other Fasting-Based Index of Insulin Sensitivity in Different Insulin-Resistant States. J. Clin. Endocrinol. Metab. 2003, 88, 4917–4923. [Google Scholar] [CrossRef]
- Reid, I.; Roberts, J. Fatty Liver in Dairy Cows. Vet. Rec. 1982, 111, 164–169. [Google Scholar] [CrossRef]
- Pires, J.A.A.; Souza, A.H.; Grummer, R.R. Induction of Hyperlipidemia by Intravenous Infusion of Tallow Emulsion Causes Insulin Resistance in Holstein Cows. J. Dairy Sci. 2007, 90, 2735–2744. [Google Scholar] [CrossRef]
- West, H.J. Effect on Liver Function of Acetonaemia and the Fat Cow Syndrome in Cattle. Res. Vet. Sci. 1990, 48, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.X.; Li, K.; Li, Z.H.; Liu, X.C.; Li, M.M.; Jiang, S.; Fan, R.F.; Yan, Z.G. Serum Macroelements and Microelements Levels in Periparturient Dairy Cows in Relation to Fatty Liver Diseases. BMC Vet. Res. 2024, 20, 295. [Google Scholar] [CrossRef] [PubMed]
- Vlizlo, V.V.; Prystupa, O.I.; Slivinska, L.G.; Lukashchuk, B.O.; Hu, S.; Gutyj, B.V.; Maksymovych, I.A.; Shcherbatyy, A.R.; Lychuk, M.G.; Chernushkin, B.O.; et al. Functional State of the Liver in Cows winth Fatty Liver Disease. Ukr. J. Ecol. 2021, 11, 167–173. [Google Scholar]
- Bergman, E.N. Hyperketonemia-Ketogenesis and Ketone Body Metabolism. J. Dairy Sci. 1971, 54, 936–948. [Google Scholar] [CrossRef]
- Andersen, J.B.; Mashek, D.G.; Larsen, T.; Nielsen, M.O.; Ingvartsen, K.L. Effects of Hyperinsulinaemia under Euglycaemic Condition on Liver Fat Metabolism in Dairy Cows in Early and Mid-lactation. J. Vet. Med. A 2002, 49, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Uchida, E.; Katoh, N.; Takahashi, K. Induction of Fatty Liver in Cows by Ethionine Administration and Concomitant Decreases of Serum Apolipoproteins B-100 and A-I Concentrations. Am. J. Vet. Res. 1992, 53, 2035–2042. [Google Scholar] [CrossRef]
- Oikawa, S.; Katoh, N.; Kawawa, F.; Ono, Y. Decreased Serum Apolipoprotein B-100 and A-I Concentrations in Cows with Ketosis and Left Displacement of the Abomasum. Am. J. Vet. Res. 1997, 58, 121–125. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Li, Y.; Guan, Y.; Song, Y.; Yin, L.; Chen, H.; Lei, L.; Liu, J.; Li, X.; et al. Effects of Nonesterified Fatty Acids on the Synthesis and Assembly of Very Low Density Lipoprotein in Bovine Hepatocytes in Vitro. J. Dairy Sci. 2014, 97, 1328–1335. [Google Scholar] [CrossRef]
- Turk, R.; Podpečan, O.; Mrkun, J.; Flegar-Meštrić, Z.; Perkov, S.; Zrimšek, P. The Effect of Seasonal Thermal Stress on Lipid Mobilisation, Antioxidant Status and Reproductive Performance in Dairy Cows. Reprod. Domest. Anim. 2015, 50, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Turk, R.; Podpečan, O.; Mrkun, J.; Kosec, M.; Flegar-Meštrić, Z.; Perkov, S.; Starič, J.; Robić, M.; Belić, M.; Zrimšek, P. Lipid Mobilisation and Oxidative Stress as Metabolic Adaptation Processes in Dairy Heifers during Transition Period. Anim. Reprod. Sci. 2013, 141, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: From Basic Research to Clinical Application. Am. J. Med. 1991, 91, S31–S38. [Google Scholar] [CrossRef] [PubMed]
- De Zwart, L.L.; Meerman, J.H.N.; Commandeur, J.N.M.; Vermeulen, N.P.E. Biomarkers of Free Radical Damage: Applications in Experimental Animals and in Humans. Free Radic. Biol. Med. 1999, 26, 202–226. [Google Scholar] [CrossRef]
- Hassett, C.; Richter, R.J.; Humbert, R.; Chapline, C.; Crabb, J.W.; Omiecinski, C.J.; Furlong, C.E. Characterization of CDNA Clones Encoding Rabbit and Human Serum Paraoxonase: The Mature Protein Retains Its Signal Sequence. Biochemistry 1991, 30, 10141–10149. [Google Scholar] [CrossRef]
- Miyamoto, T.; Takahashi, Y.; Oohashi, T.; Sato, K.; Oikawa, S. Bovine Paraoxonase 1 Activities in Serum and Distribution in Lipoproteins. J. Vet. Med. Sci. 2005, 67, 243–248. [Google Scholar] [CrossRef]
- Mackness, M.I.; Arrol, S.; Durrington, P.N. Paraoxonase Prevents Accumulation of Lipoperoxides in Low-density Lipoprotein. FEBS Lett. 1991, 286, 152–154. [Google Scholar] [CrossRef]
- Mackness, M.I.; Arrol, S.; Abbott, C.; Durrington, P.N. Protection of Low-Density Lipoprotein against Oxidative Modification by High-Density Lipoprotein Associated Paraoxonase. Atherosclerosis 1993, 104, 129–135. [Google Scholar] [CrossRef]
- Farid, A.S.; Honkawa, K.; Fath, E.M.; Nonaka, N.; Horii, Y. Serum Paraoxonase-1 as Biomarker for Improved Diagnosis of Fatty Liver in Dairy Cows. BMC Vet. Res. 2013, 9, 73. [Google Scholar] [CrossRef]
- Baker, L.D.; Ferguson, J.D.; Chalupa, W. Responses in Urea and True Protein of Milk to Different Protein Feeding Schemes for Dairy Cows. J. Dairy Sci. 1995, 78, 2424–2434. [Google Scholar] [CrossRef]
- Jonker, J.S.; Kohn, R.A.; High, J. Use of Milk Urea Nitrogen to Improve Dairy Cow Diets. J. Dairy Sci. 2002, 85, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Nicola, I.; Chupin, H.; Roy, J.P.; Buczinski, S.; Fauteux, V.; Picard-Hagen, N.; Cue, R.; Dubuc, J. Association between Prepartum Nonesterified Fatty Acid Serum Concentrations and Postpartum Diseases in Dairy Cows. J. Dairy Sci. 2022, 105, 9098–9106. [Google Scholar] [CrossRef] [PubMed]


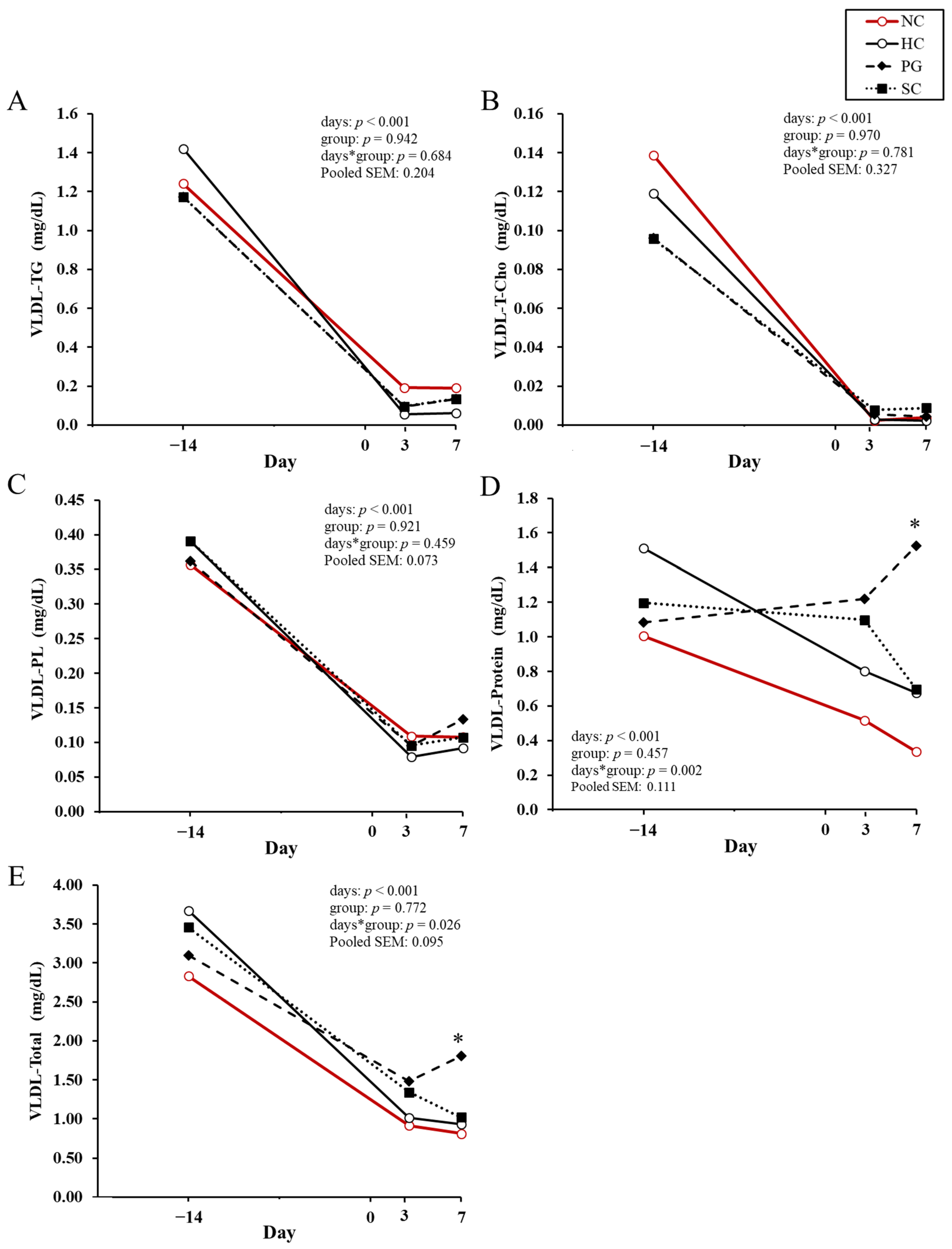
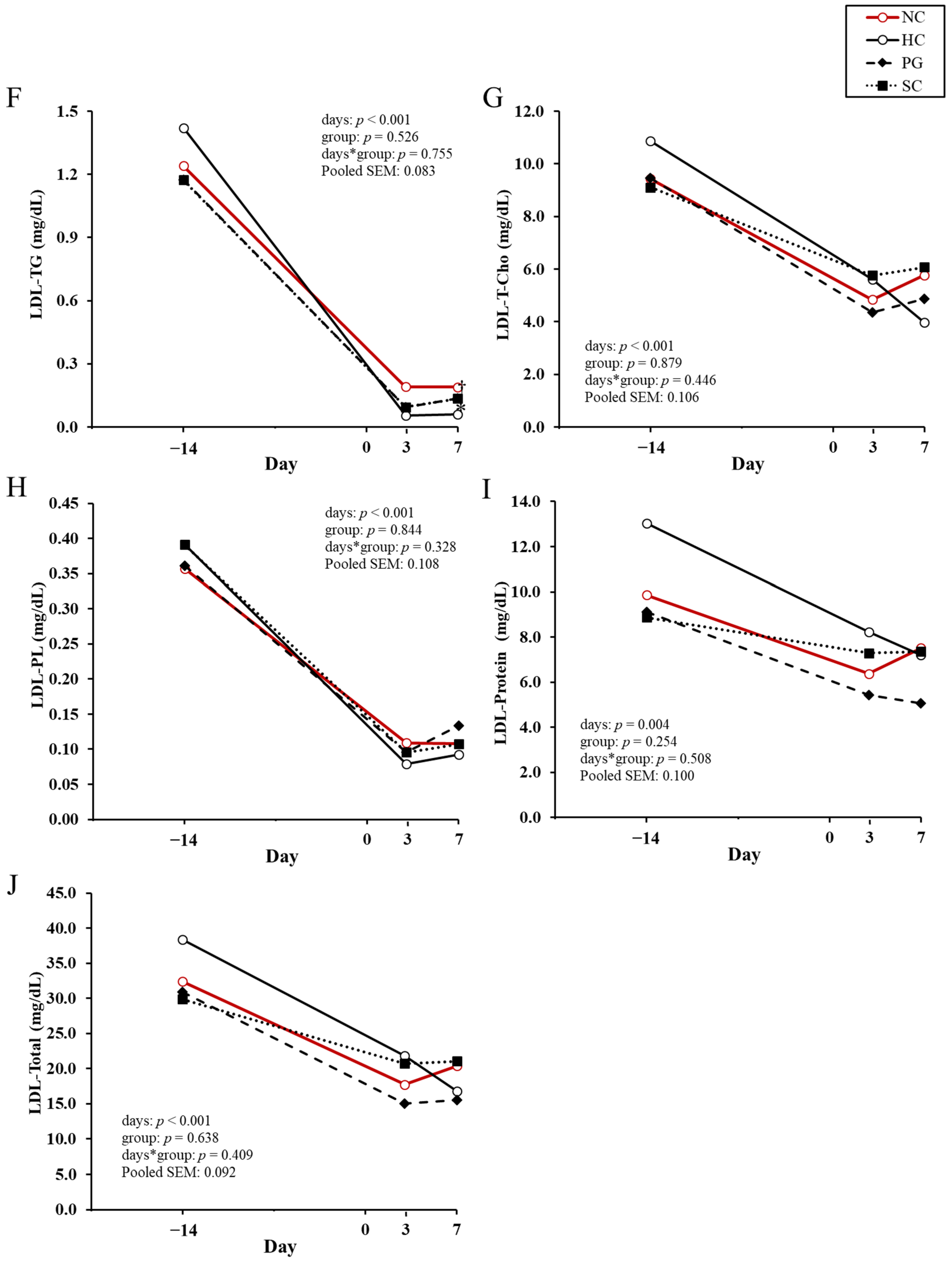
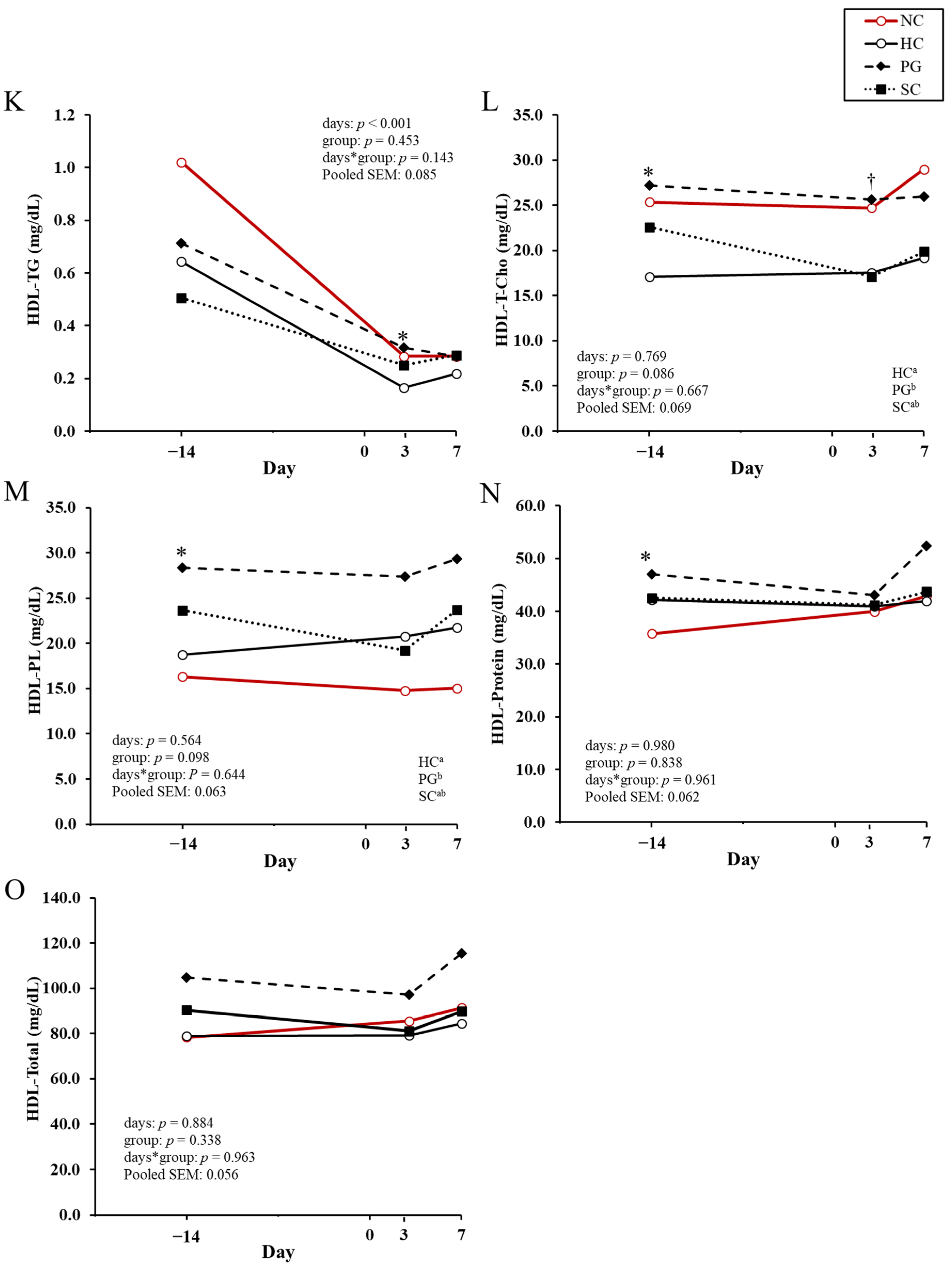
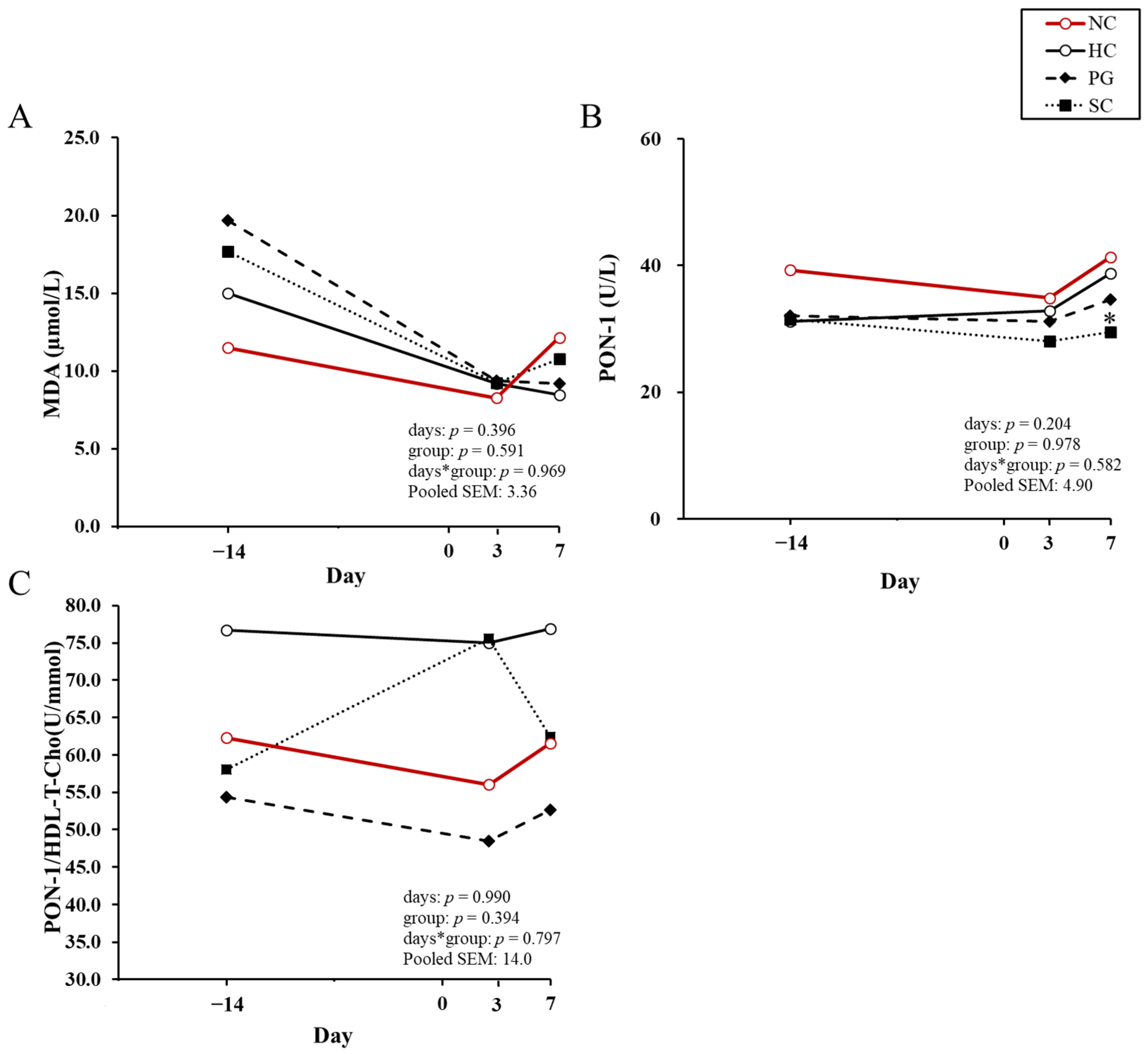
| Variable | NC 1 | HC 1 | PG 1 | SC 1 | p-Value |
|---|---|---|---|---|---|
| n = 15 | n = 13 | n = 11 | n = 11 | ||
| Parity 2 | 2.2 ± 0.3 | 2.8 ± 0.4 | 2.2 ± 0.5 | 2.7 ± 0.5 | 0.603 |
| (1.6–2.8) | (1.9–3.6) | (1.2–3.2) | (1.8–3.7) | ||
| Actual days in milk at the time of initial blood sampling 2 (day) | −12.1 ± 0.7 | −9.4 ± 0.9 | −8.9 ± 1.0 | −10.3 ± 1.0 | 0.636 |
| (−13.7–−10.6) | (−11.3–−7.5) | (−11.0–−6.8) | (−12.4–−8.2) | ||
| Duration of administration 2 (day) | – | – | 4.8 ± 0.1 | 4.7 ± 0.2 | 1.000 |
| Variable | NC 1 | HC 1 | PG 1 | SC 1 | p-Value |
|---|---|---|---|---|---|
| n = 15 | n = 10 | n = 10 | n = 11 | ||
| Days in milk at the first DHI test 2 (day) | 26.3 ± 4.5 | 27.2 ± 5.7 | 23.7 ± 5.7 | 35.3 ± 5.5 | 0.302 |
| Milk yield 2 (kg) | 36.2 ± 2.6 | 32.0 ± 5.0 | 38.4 ± 5.0 | 35.2 ± 4.9 | 0.432 |
| Milk fat 2 (%) | 4.3 ± 0.2 | 4.4 ± 0.2 | 4.4 ± 0.2 | 3.9 ± 0.2 | 0.214 |
| Milk protein 2 (%) | 3.3 ± 0.1 | 3.0 ± 0.1 | 3.2 ± 0.1 | 3.1 ± 0.1 | 0.303 |
| De novo fatty acid 2 (g/100 g fat) | 26.2 ± 0.9 | 22.6 ± 1.4 | 23.4 ± 1.4 | 26.4 ± 1.3 | 0.145 |
| Preformed fatty acid 2 (g/100 g fat) | 43.2 ± 1.7 | 49.0 ± 2.2 | 47.9 ± 2.2 | 42.6 ± 2.1 | 0.107 |
| MUN 2 (mg/dL) | 9.6 ± 0.4 | 7.8 ± 0.9 A | 9.5 ± 0.9 AB | 11.4 ± 0.9 B | 0.012 |
| BHBA in milk 2 (mM) | 0.03 ± 0.01 | 0.15 ± 0.04 | 0.07 ± 0.04 | 0.05 ± 0.03 | 0.123 |
| 305-day predicted milk yield 2 (kg) | 12,310.1 ± 371.8 | 11,286.6 ± 518.6 ab | 12,110.0 ± 494.0 a | 10,531.0 ± 518.6 b | 0.067 |
| 4% fat corrected milk 2,3 (kg) | 11,094.9 ± 278.9 | 10,620.3 ± 648.2 | 11,654.5 ± 642.0 | 10,105.6 ± 619.2 | 0.153 |
| Variable | Group 1 | % 2 | OR | 95%CI | p-Value |
|---|---|---|---|---|---|
| Culling rate 3,4 | NC | 13.3 (2/15) | – | – | – |
| HC | 69.2 (9/13) | Referent | – | – | |
| PG | 22.2 (2/11) | 10.1 | 1.47–69.94 | 0.012 | |
| SC | 27.7 (3/11) | 6.0 | 1.02–35.38 | 0.041 | |
| Clinical disease incidence within 60 DIM 3,5 | NC | 30.0 (5/15) | – | – | – |
| HC | 61.5 (8/13) | Referent | – | – | |
| PG | 27.2 (3/11) | 4.3 | 0.75–24.18 | 0.093 | |
| SC | 54.5 (6/11) | 1.3 | 0.26–6.81 | 0.527 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chisato, K.; Ishizaka, M.; Honjo, T.; Watanabe, Y.; Fukumori, R.; Oikawa, S. Effects of Preventive Administration of Propylene Glycol or Sucrose in Dairy Cows with Elevated Blood Non-Esterified Fatty Acids During the Close-Up Period. Animals 2025, 15, 3211. https://doi.org/10.3390/ani15213211
Chisato K, Ishizaka M, Honjo T, Watanabe Y, Fukumori R, Oikawa S. Effects of Preventive Administration of Propylene Glycol or Sucrose in Dairy Cows with Elevated Blood Non-Esterified Fatty Acids During the Close-Up Period. Animals. 2025; 15(21):3211. https://doi.org/10.3390/ani15213211
Chicago/Turabian StyleChisato, Kyoko, Miki Ishizaka, Takumi Honjo, Yuta Watanabe, Rika Fukumori, and Shin Oikawa. 2025. "Effects of Preventive Administration of Propylene Glycol or Sucrose in Dairy Cows with Elevated Blood Non-Esterified Fatty Acids During the Close-Up Period" Animals 15, no. 21: 3211. https://doi.org/10.3390/ani15213211
APA StyleChisato, K., Ishizaka, M., Honjo, T., Watanabe, Y., Fukumori, R., & Oikawa, S. (2025). Effects of Preventive Administration of Propylene Glycol or Sucrose in Dairy Cows with Elevated Blood Non-Esterified Fatty Acids During the Close-Up Period. Animals, 15(21), 3211. https://doi.org/10.3390/ani15213211






