miR-200a Targets PITX2 to Mediate Goose Fibroblast Proliferation Through the Wnt Pathway
Simple Summary
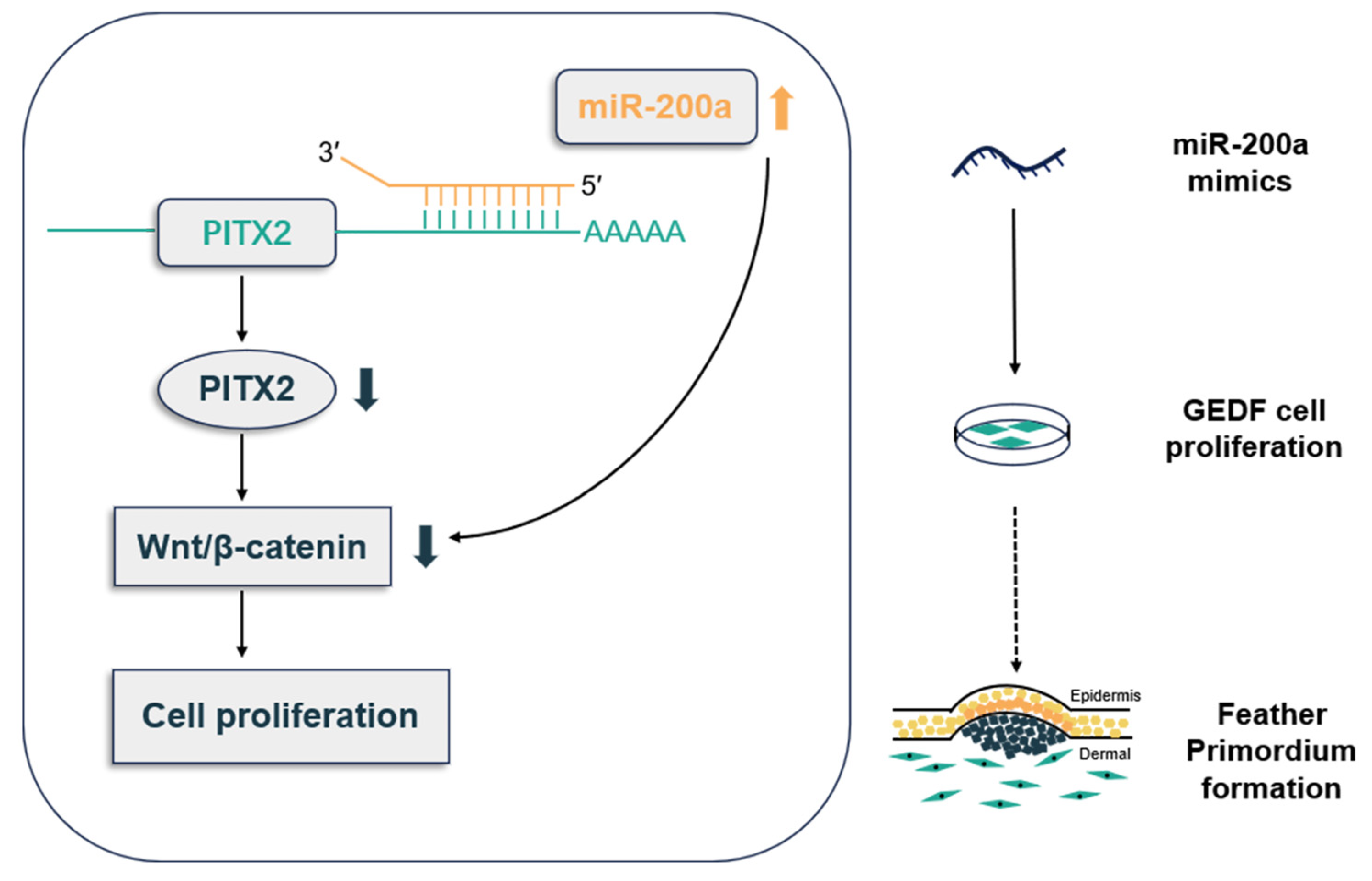
Abstract
1. Introduction
2. Materials and Methods
2.1. Utilization of Experimental Animals and Procedures for Sample Collection
2.2. Primary GEDF Cell Isolation and Culture
2.3. DE miRNAs Analysis and Target Genes Prediction
2.4. GO Annotation and KEGG Pathway Enrichment
2.5. RNA Oligonucleotides and Cell Transfection
2.6. Dual Luciferase Reporter Gene Assay
2.7. Extraction of Total RNA with Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Cell Counting Kit-8 (CCK8) Assay
2.9. 5-Ethynyl-2′-deoxyuridine (EdU) Assay
2.10. Data Analysis
3. Results
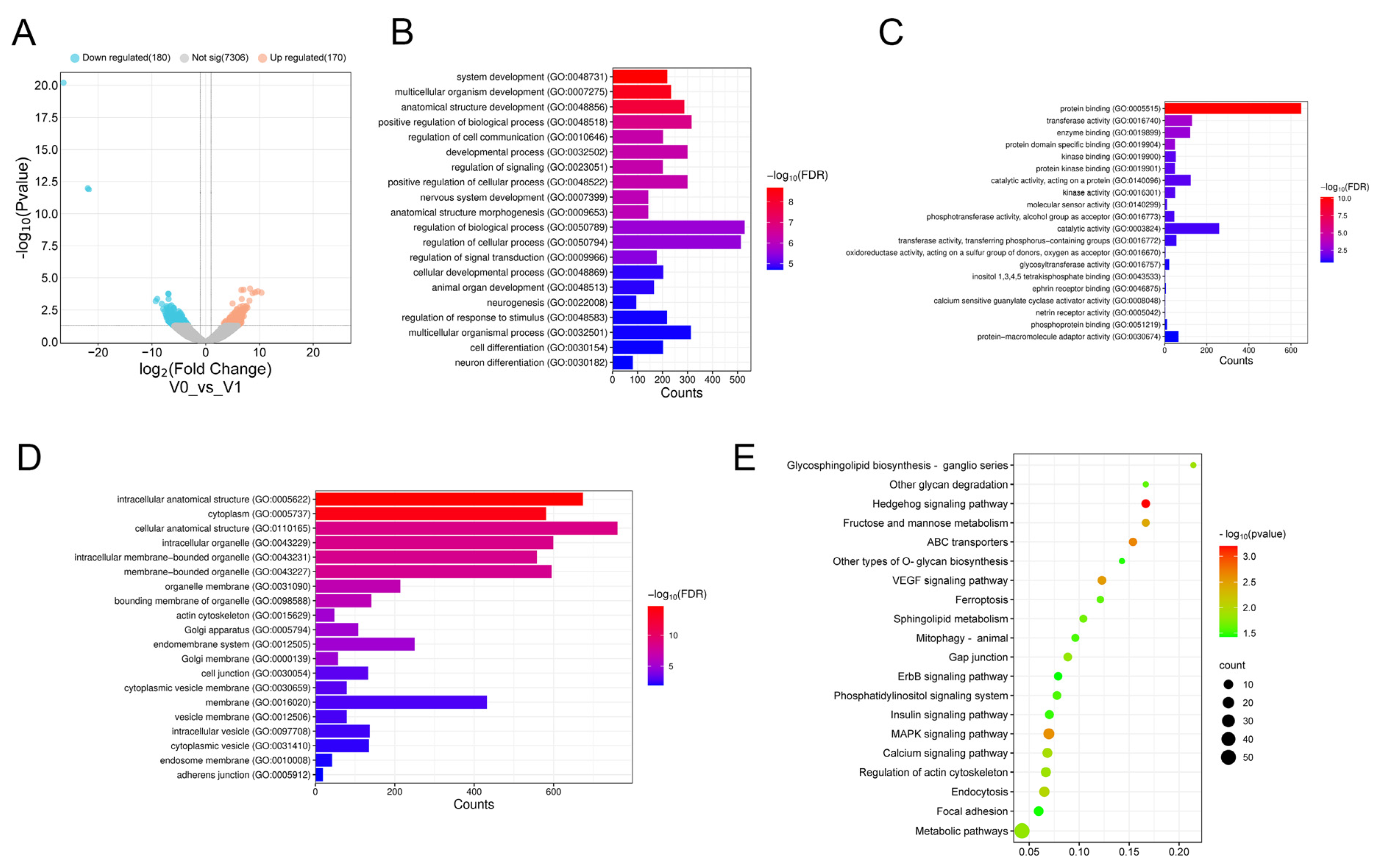
3.1. Differential Expression of miRNAs Before and After the Stage of Primordium Formation
3.2. GO Annotation and KEGG Pathway Analysis of DE miRNAs
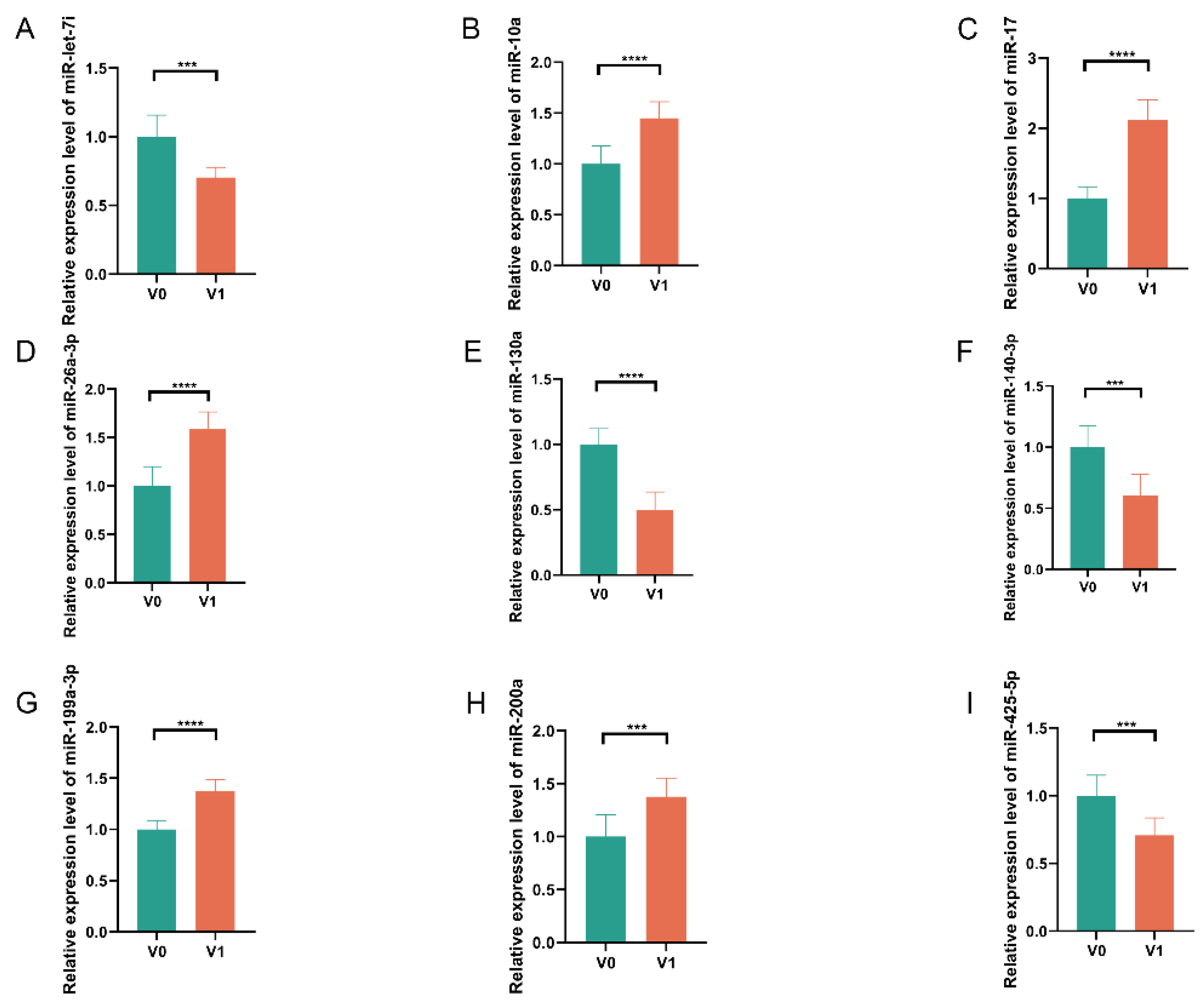
3.3. Expression Detection of DE miRNAs in Embryonic Skin Tissues of Geese
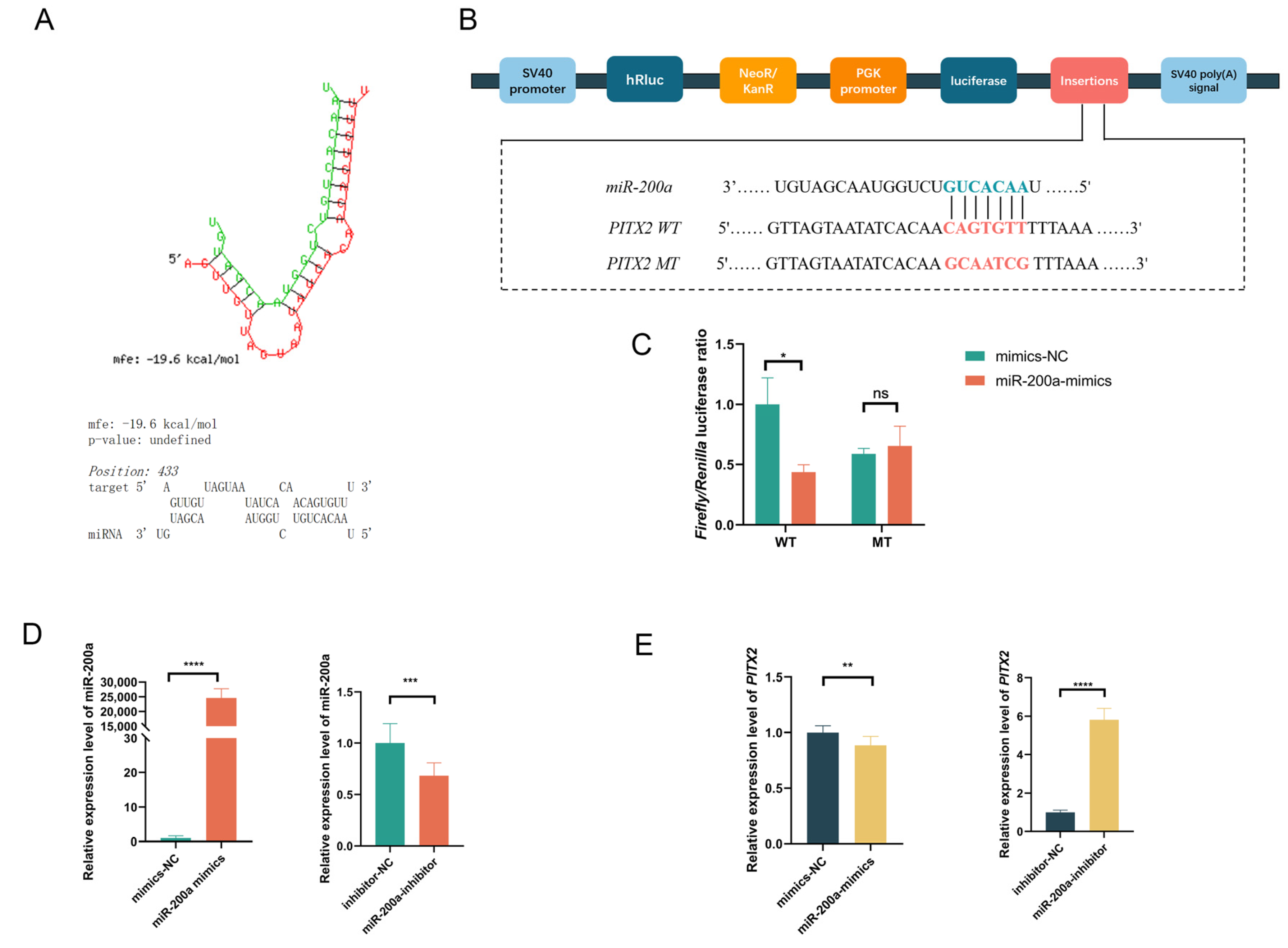
3.4. miR-200a Negatively Regulates PITX2
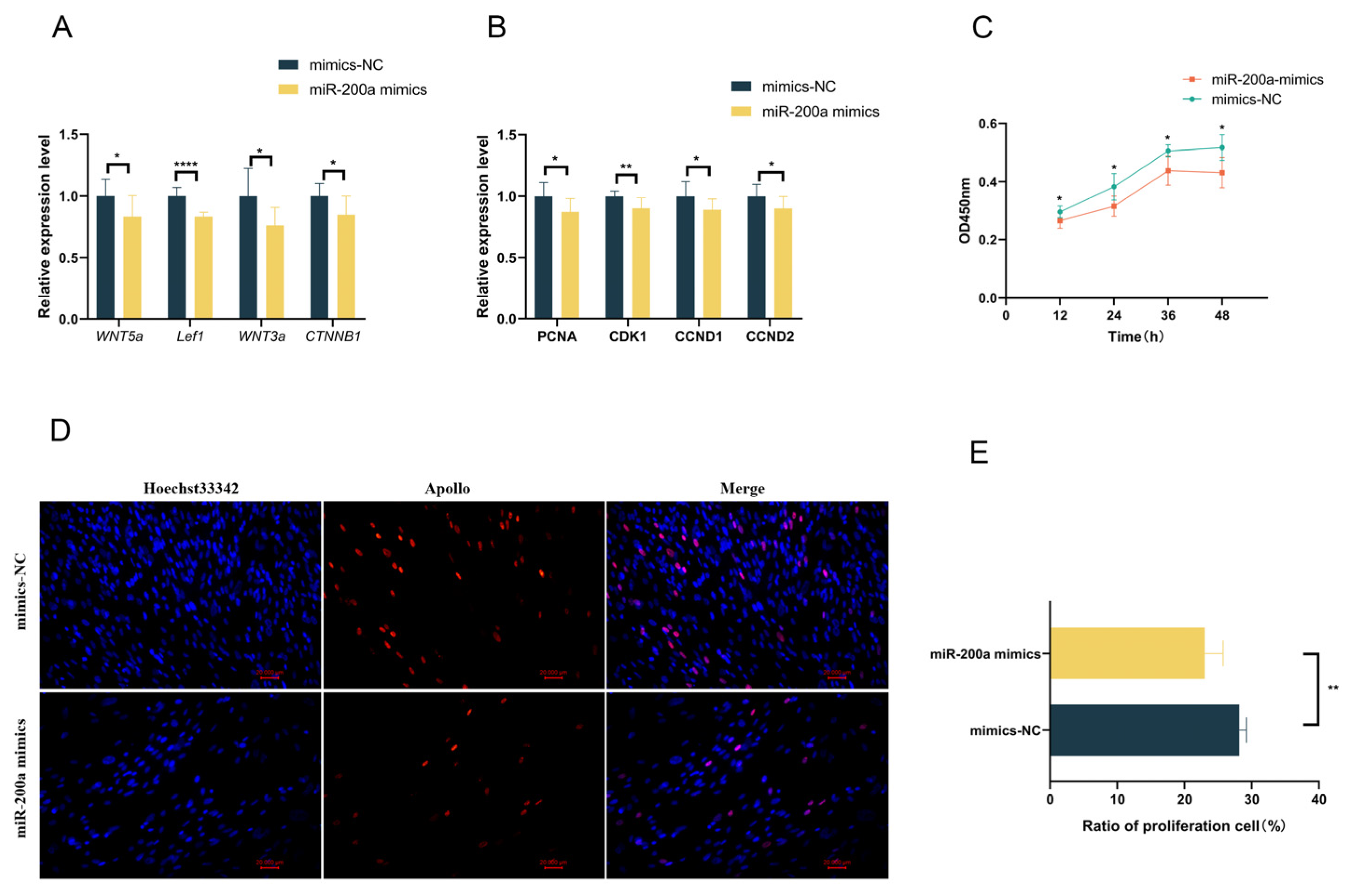
3.5. Overexpression of miR-200a Reduces the Expression of Genes Related to the Wnt Pathway and Inhibits the Proliferation of GEDFs
3.6. Silencing miR-200a Expression Elevated the Expression of Genes Associated with the Wnt Signaling Pathway and Promoted the Proliferation of GEDFs
3.7. Overexpression of PITX2 Increases the Expression of Genes Associated with the Wnt Signaling Pathway and Promotes the Proliferation of GEDFs
3.8. Incorporation of pcDNA3.1-PITX2 Rescued the Phenotypes Caused by Overexpression of miR-200a
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. Beta-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef]
- Qu, R.; Gupta, K.; Dong, D.; Jiang, Y.; Landa, B.; Saez, C.; Strickland, G.; Levinsohn, J.; Weng, P.L.; Taketo, M.M.; et al. Decomposing a deterministic path to mesenchymal niche formation by two intersecting morphogen gradients. Dev. Cell 2022, 57, 1053–1067. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, Y.; Liu, H.; Liu, G.; Li, F. Wnt10b promotes hair follicles growth and dermal papilla cells proliferation via Wnt/β-Catenin signaling pathway in Rex rabbits. Biosci. Rep. 2020, 40, BSR20191248. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, K.; Ye, J.X.; Lian, X.H.; Yang, T. Wnt10b promotes growth of hair follicles via a canonical Wnt signalling pathway. Clin. Exp. Dermatol. 2011, 36, 534–540. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Lv, X.; Cao, X.; Yuan, Z.; Getachew, T.; Li, Y.; Wang, S.; Sun, W. SOX18 Promotes the Proliferation of Dermal Papilla Cells via the Wnt/β-Catenin Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 16672. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Lv, X.; Cao, X.; Yuan, Z.; Quan, K.; Getachew, T.; Mwacharo, J.M.; Haile, A.; Li, Y.; Wang, S.; et al. CRABP2 Promotes the Proliferation of Dermal Papilla Cells via the Wnt/β-Catenin Pathway. Animals 2023, 13, 2033. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, J.; Zhang, X.; Dai, Y.; Yang, N.; Bao, Z.; Chen, Y.; Wu, X. Exosomal miRNA-181a-5p from the cells of the hair follicle dermal papilla promotes the hair follicle growth and development via the Wnt/β-catenin signaling pathway. Int. J. Biol. Macromol. 2022, 207, 110–120. [Google Scholar] [CrossRef]
- Ahmed, M.I.; Alam, M.; Emelianov, V.U.; Poterlowicz, K.; Patel, A.; Sharov, A.A.; Mardaryev, A.N.; Botchkareva, N.V. MicroRNA-214 controls skin and hair follicle development by modulating the activity of the Wnt pathway. J. Cell Biol. 2014, 207, 549–567. [Google Scholar] [CrossRef]
- Zhang, Y.; Tomann, P.; Andl, T.; Gallant, N.M.; Huelsken, J.; Jerchow, B.; Birchmeier, W.; Paus, R.; Piccolo, S.; Mikkola, M.L.; et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev. Cell 2009, 17, 49–61. [Google Scholar] [CrossRef]
- Botchkarev, V.A.; Fessing, M.Y. Edar signaling in the control of hair follicle development. J. Investig. Dermatol. Symp. Proc. 2005, 10, 247–251. [Google Scholar] [CrossRef]
- Mou, C.; Jackson, B.; Schneider, P.; Overbeek, P.A.; Headon, D.J. Generation of the primary hair follicle pattern. Proc. Natl. Acad. Sci. USA 2006, 103, 9075–9080. [Google Scholar] [CrossRef]
- Jung, H.S.; Francis-West, P.H.; Widelitz, R.B.; Jiang, T.X.; Ting-Berreth, S.; Tickle, C.; Wolpert, L.; Chuong, C.M. Local inhibitory action of bmps and their relationships with activators in feather formation: Implications for periodic patterning. Dev. Biol. 1998, 196, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Noramly, S.; Morgan, B.A. Bmps mediate lateral inhibition at successive stages in feather tract development. Development 1998, 125, 3775–3787. [Google Scholar] [CrossRef] [PubMed]
- Botchkarev, V.A.; Botchkareva, N.V.; Sharov, A.A.; Funa, K.; Huber, O.; Gilchrest, B.A. Modulation of bmp signaling by noggin is required for induction of the secondary (nontylotrich) hair follicles. J. Investig. Dermatol. 2002, 118, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.K.W.; Freem, L.; Zhao, D.; Painter, K.J.; Woolley, T.E.; Gaffney, E.A.; McGrew, M.J.; Tzika, A.; Milinkovitch, M.C.; Schneider, P.; et al. Feather arrays are patterned by interacting signalling and cell density waves. PLoS Biol. 2019, 17, e3000132. [Google Scholar] [CrossRef]
- Huh, S.H.; Narhi, K.; Lindfors, P.H.; Haara, O.; Yang, L.; Ornitz, D.M.; Mikkola, M.L. Fgf20 governs formation of primary and secondary dermal condensations in developing hair follicles. Genes Dev. 2013, 27, 450–458. [Google Scholar] [CrossRef]
- Leybova, L.; Biswas, A.; Sharan, R.; Trejo, B.M.; Kim, K.; Soto-Muniz, Y.; Jones, R.A.; Phillips, B.K.; Devenport, D. Radially patterned morphogenesis of murine hair follicle placodes ensure robust epithelial budding. Dev. Cell 2024, 59, 3272–3289.e5. [Google Scholar] [CrossRef]
- Semina, E.V.; Reiter, R.; Leysens, N.J.; Alward, W.L.; Small, K.W.; Datson, N.A.; Siegel-Bartelt, J.; Bierke-Nelson, D.; Bitoun, P.; Zabel, B.U.; et al. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat. Genet. 1996, 14, 392–399. [Google Scholar] [CrossRef]
- Yu, W.; Sun, Z.; Sweat, Y.; Sweat, M.; Venugopalan, S.R.; Eliason, S.; Cao, H.; Paine, M.L.; Amendt, B.A. Pitx2-Sox2-Lef1 interactions specify progenitor oral/dental epithelial cell signaling centers. Development 2020, 147, dev186023. [Google Scholar] [CrossRef]
- Sun, Z.; Yu, W.; Sanz, N.M.; Sweat, M.; Eliason, S.; Sharp, T.; Liu, H.; Seidel, K.; Zhang, L.; Moreno, M.; et al. Sox2 and Lef-1 interact with Pitx2 to regulate incisor development and stem cell renewal. Development 2016, 143, 4115–4126. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Florez, S.; Wang, J.; Cao, H.; Amendt, B.A. Dact2 represses PITX2 transcriptional activation and cell proliferation through Wnt/beta-catenin signaling during odontogenesis. PLoS ONE 2013, 8, e54868. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.; Wang, J.; Li, X.; Cao, H.; Gao, S.; Moreno, M.; Amendt, B.A. A pituitary homeobox 2 (Pitx2): microRNA-200a-3p: β-catenin pathway converts mesenchymal cells to amelogenin-expressing dental epithelial cells. J. Biol. Chem. 2014, 289, 27327–27341. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Jheon, A.; Li, X.; Sun, Z.; Wang, J.; Florez, S.; Zhang, Z.; McManus, M.T.; Klein, O.D.; Amendt, B.A. The Pitx2: miR-200c/141: Noggin pathway regulates Bmp signaling and ameloblast differentiation. Development 2013, 140, 3348–3359. [Google Scholar] [CrossRef]
- Sohn, K.C.; Shi, G.; Jang, S.; Choi, D.K.; Lee, Y.; Yoon, T.J.; Park, H.; Hwang, C.; Kim, H.J.; Seo, Y.J.; et al. Pitx2, a beta-catenin-regulated transcription factor, regulates the differentiation of outer root sheath cells cultured in vitro. J. Dermatol. Sci. 2009, 54, 6–11. [Google Scholar] [CrossRef]
- Han, W.; Yang, F.; Wu, Z.; Guo, F.; Zhang, J.; Hai, E.; Shang, F.; Su, R.; Wang, R.; Wang, Z.; et al. Inner Mongolian Cashmere Goat Secondary Follicle Development Regulation Research Based on mRNA-miRNA Co-analysis. Sci. Rep. 2020, 10, 4519. [Google Scholar] [CrossRef]
- Stenn, K.S.; Paus, R. Controls of Hair Follicle Cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef]
- Olivera-Martinez, I.; Thélu, J.; Dhouailly, D. Molecular mechanisms controlling dorsal dermis generation from the somitic dermomyotome. Int. J. Dev. Biol. 2004, 48, 93–101. [Google Scholar] [CrossRef]
- Ahn, Y.; Sims, C.; Logue, J.M.; Weatherbee, S.D.; Krumlauf, R. Lrp4 and Wise interplay controls the formation and patterning of mammary and other skin appendage placodes by modulating Wnt signaling. Development 2013, 140, 583–593. [Google Scholar] [CrossRef]
- Ma, J.; Song, Y.; Mabrouk, I.; Zhou, Y.; Liu, Q.; Yu, J.; Li, X.; Xue, G.; Wang, J.; Yu, Z.; et al. miR-140-y targets TCF4 to regulate the Wnt signaling pathway and promote embryonic feather follicle development in Hungarian white goose. Poult. Sci. 2024, 103, 103508. [Google Scholar] [CrossRef]
- Mabrouk, I.; Zhou, Y.; Wang, S.; Song, Y.; Fu, X.; Xu, X.; Liu, T.; Wang, Y.; Feng, Z.; Fu, J.; et al. Transcriptional Characteristics Showed That miR-144-y/FOXO3 Participates in Embryonic Skin and Feather Follicle Development in Zhedong White Goose. Animals 2022, 12, 2099. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Y.; Yang, X.; Xu, P.; Jing, J.; Miao, Y.; Mao, M.; Xu, J.; Wu, X.; Lu, Z. An integrative analysis of the lncRNA-miRNA-mRNA competitive endogenous RNA network reveals potential mechanisms in the murine hair follicle cycle. Front. Genet. 2022, 13, 931797. [Google Scholar] [CrossRef]
- Lv, X.; Chen, W.; Sun, W.; Hussain, Z.; Chen, L.; Wang, S.; Wang, J. Expression profile analysis to identify circular RNA expression signatures in hair follicle of Hu sheep lambskin. Genomics 2020, 112, 4454–4462. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, H.; Ni, J.; Zhao, X.; Zhang, K.; Wang, J.; Kong, X.; Wang, Q. Insights into genetic diversity and phenotypic variations in domestic geese through comprehensive population and pan-genome analysis. J. Anim. Sci. Biotechnol. 2023, 14, 150. [Google Scholar] [CrossRef]
- Mikkola, M.L. Genetic basis of skin appendage development. Semin. Cell Dev. Biol. 2007, 18, 225–236. [Google Scholar] [CrossRef]



| Name | Sequence (5′-3′) |
|---|---|
| mimics NC | UUGUACUACACAAAAGUACUG |
| miR-200a mimics | UAACACUGUCUGGUAACGAUGU |
| Inhibitor NC | CAGUACUUUUGUGUAGUACAA |
| miR-200a inhibitor | ACAUCGUUACCAGACAGUGUUA |
| miR-26a-3p | F: CCTATTCTTGGTTACTTGCACTG |
| R: CAGGTCCAGTTTTTTTTTTTTTT | |
| let-7i | F: TGAGGTAGTAGTTTGTGCTGTT |
| R: CAGGTCCAGTTTTTTTTTTTTTT | |
| miR-199a-3p | F: ACAGTAGTCTGCACATTGGTT |
| R: CAGGTCCAGTTTTTTTTTTTTTT | |
| miR-140-3p | F: TACCACAGGGTAGAACCACGG |
| R: CAGGTCCAGTTTTTTTTTTTTTT | |
| miR-130a | F: CAGTGCAATGTTAAAAGGGCAT |
| R: CAGGTCCAGTTTTTTTTTTTTTT | |
| miR-10a | F: TACCCTGTAGATCCGAATTTGTG |
| R: CAGGTCCAGTTTTTTTTTTTTTT | |
| miR-17 | F: ACTGCAGTGAAGGCACTTGTAGCAT |
| R: CAGGTCCAGTTTTTTTTTTTTTT | |
| miR-425-5p | F: AATGACACGATCACTCCCGTTGAG |
| R: CAGGTCCAGTTTTTTTTTTTTTT | |
| miR-200a | F: TAACACTGTCTGGTAACGATGT |
| R: CAGGTCCAGTTTTTTTTTTTTTT | |
| U6 | F: TACAGAGAAGATTAGCATGG |
| R: CAGGTCCAGTTTTTTTTTTTTTT | |
| PITX2-qPCR | F: TGCACACCATCTCCGACACCT |
| R: CGCCGCTGCCTCTTCTTCTT | |
| WNT5a-qPCR | F: CCAGCTCTTGGTGGTCTTTAG |
| R: TCCTTGGGAAAGCCCTGCTA | |
| LEF-1-qPCR | F: AGCCTTCTCATGCGGTTCACC |
| R: AGGAGCTGGAGGATGTCTGGAC | |
| WNT3a-qPCR | F: TGAACAGGCACAACAACGAAGC |
| R: ACCACCAGCAGGTCTTCACTTC | |
| CTNNB1-qPCR | F: CGTGAAGGCTTGTTGGCAATCT |
| R: TGGCATAGAACAGCACGGAGTC | |
| PCNA-qPCR | F: CAGCCATATTGGTGATGCAG |
| R: GGTCAGTTGGACTGGCTCAT | |
| CDK1-qPCR | F: GAAGTCGTGACGCTGTGGTA |
| R: TTGTTGGGTGTCCCTAAAGC | |
| Cyclin D1-qPCR | F: AGACCATCCGACGAGCCTAC |
| R: TTCTGCTCCTCGCAAACCTCC | |
| Cyclin D2-qPCR | F: ACCCCAAGAGCTGCTGGAATG |
| R: TGGCACAAAGGGCGATGAAC | |
| GAPDH-qPCR | F: AAGGGCATCCTGGCATACAC |
| R: CATCAAGTCCACCACACGGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, S.; Yang, H.; Ge, H.; Li, S.; Yang, S.; Mou, C. miR-200a Targets PITX2 to Mediate Goose Fibroblast Proliferation Through the Wnt Pathway. Animals 2025, 15, 3171. https://doi.org/10.3390/ani15213171
Jiao S, Yang H, Ge H, Li S, Yang S, Mou C. miR-200a Targets PITX2 to Mediate Goose Fibroblast Proliferation Through the Wnt Pathway. Animals. 2025; 15(21):3171. https://doi.org/10.3390/ani15213171
Chicago/Turabian StyleJiao, Shuyu, Hongyuan Yang, Heng Ge, Shaomei Li, Suozhou Yang, and Chunyan Mou. 2025. "miR-200a Targets PITX2 to Mediate Goose Fibroblast Proliferation Through the Wnt Pathway" Animals 15, no. 21: 3171. https://doi.org/10.3390/ani15213171
APA StyleJiao, S., Yang, H., Ge, H., Li, S., Yang, S., & Mou, C. (2025). miR-200a Targets PITX2 to Mediate Goose Fibroblast Proliferation Through the Wnt Pathway. Animals, 15(21), 3171. https://doi.org/10.3390/ani15213171




