Fourier Transform Infrared Spectroscopy to Measure Cholesterol in Goat Spermatozoa
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Semen Collection and Sperm Assessment
2.3. FTIR Analysis
2.4. Experimental Design
2.5. Statistical Analysis
3. Results
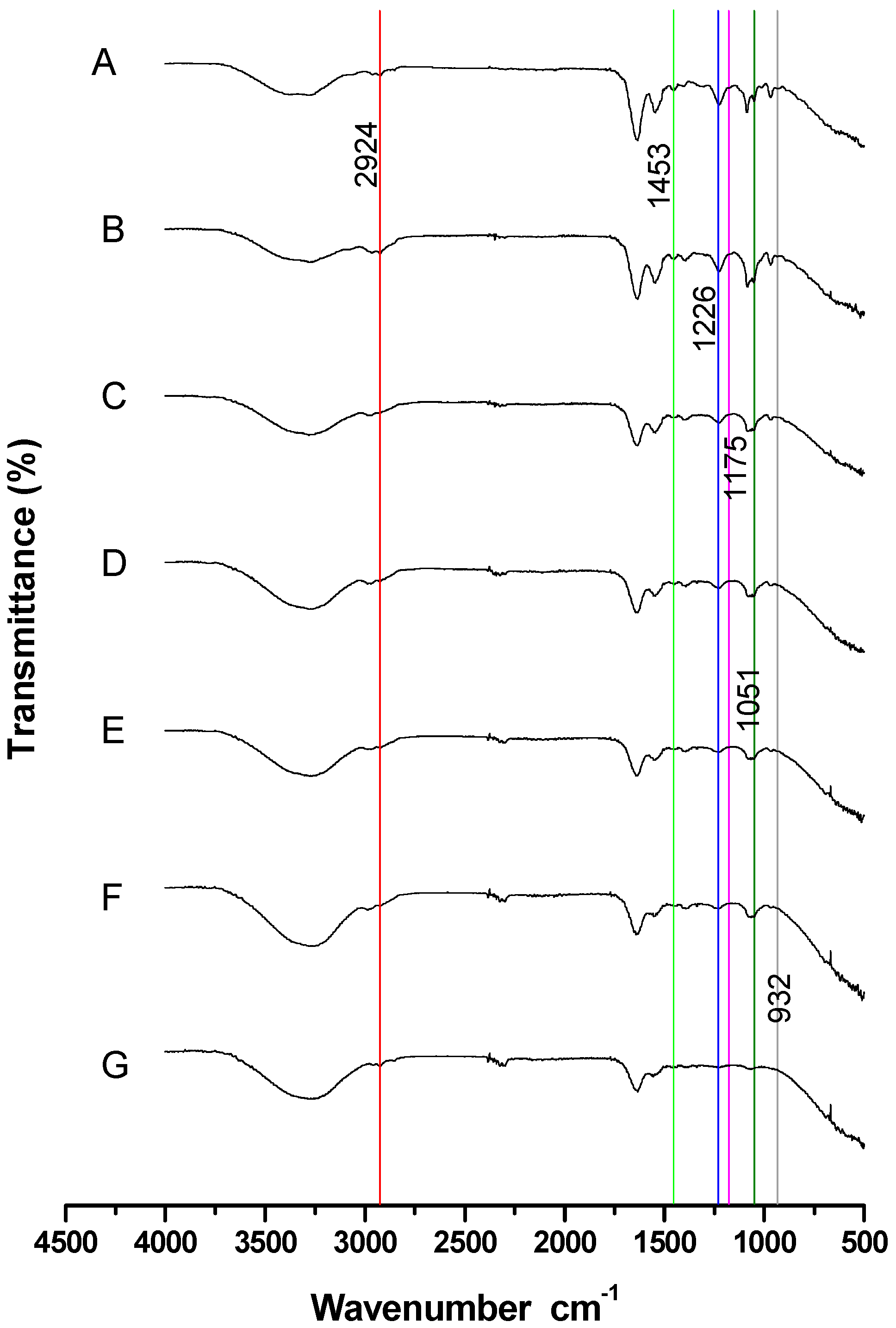
3.1. Stage 1: Determination of the Appropriate Sperm Concentration to Detect Cholesterol in the FTIR Spectrum
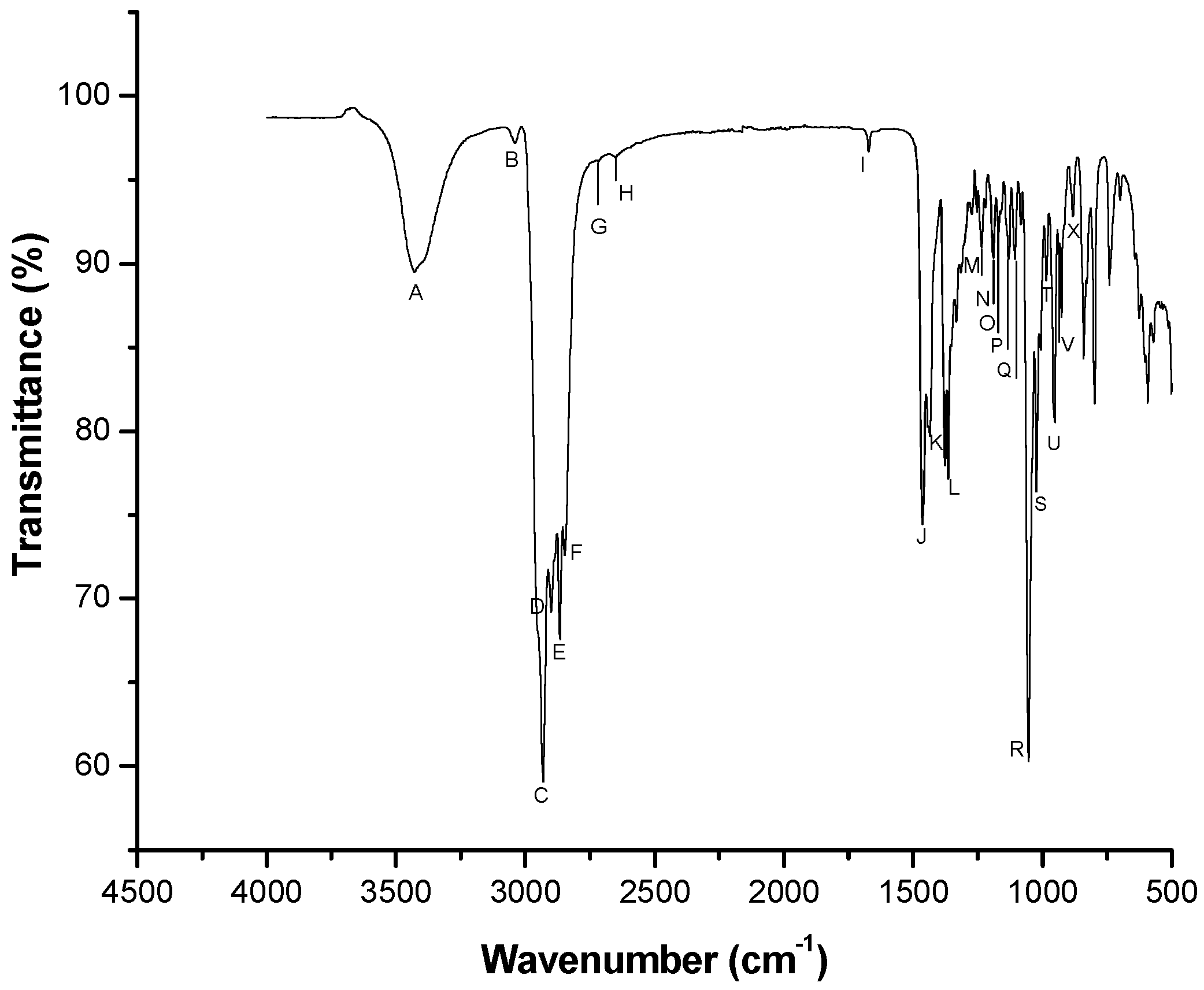
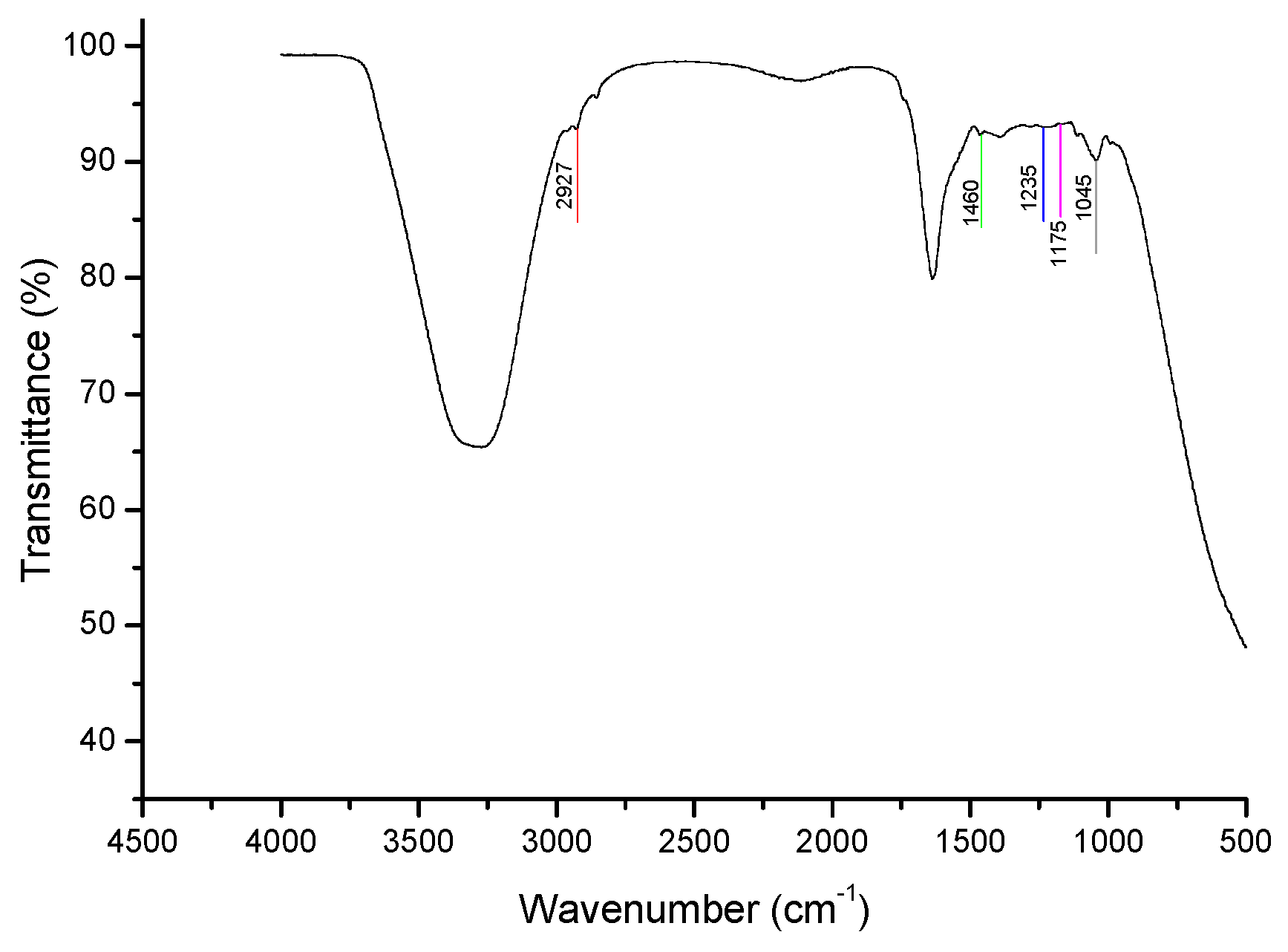
Cholesterol Spectrum
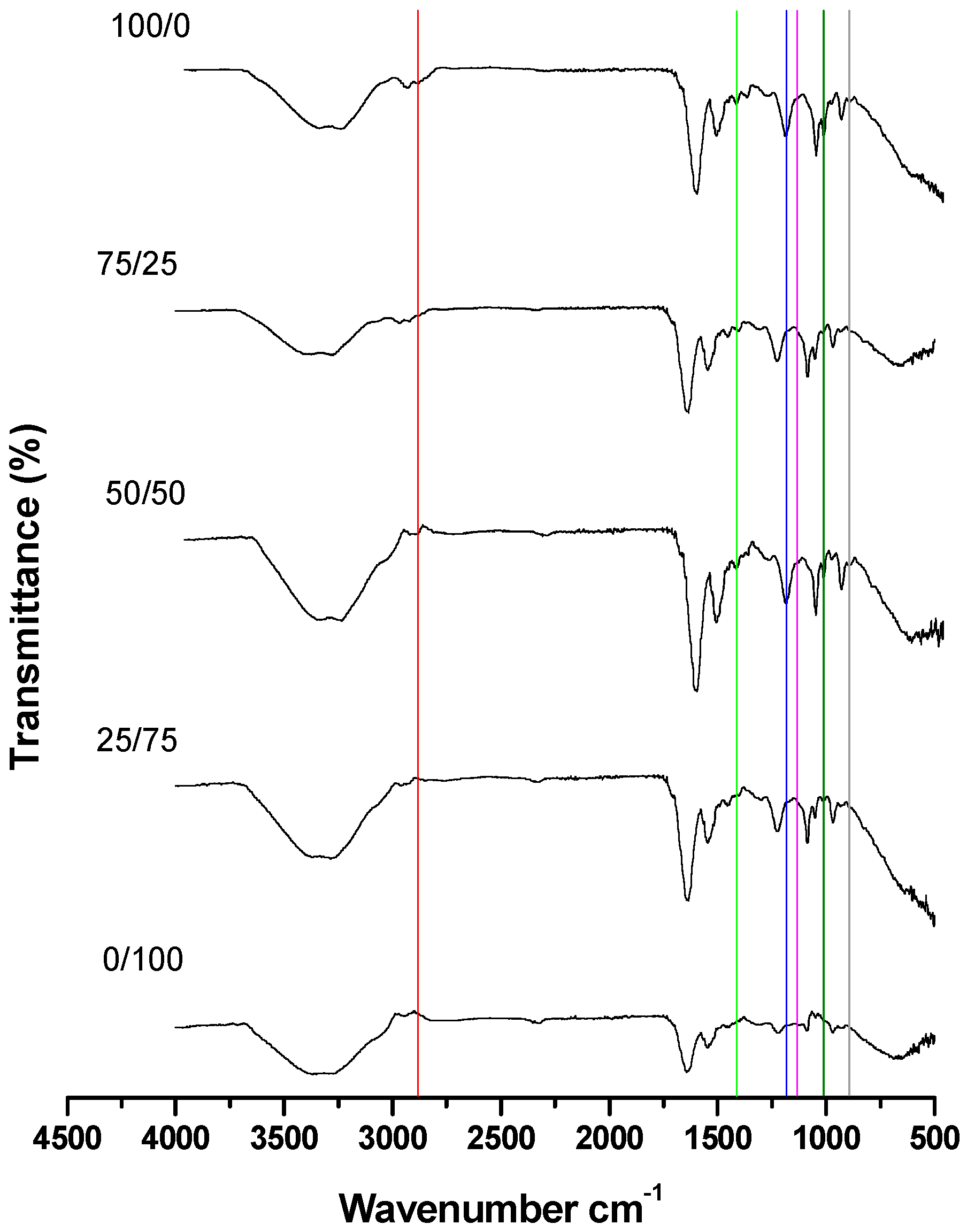
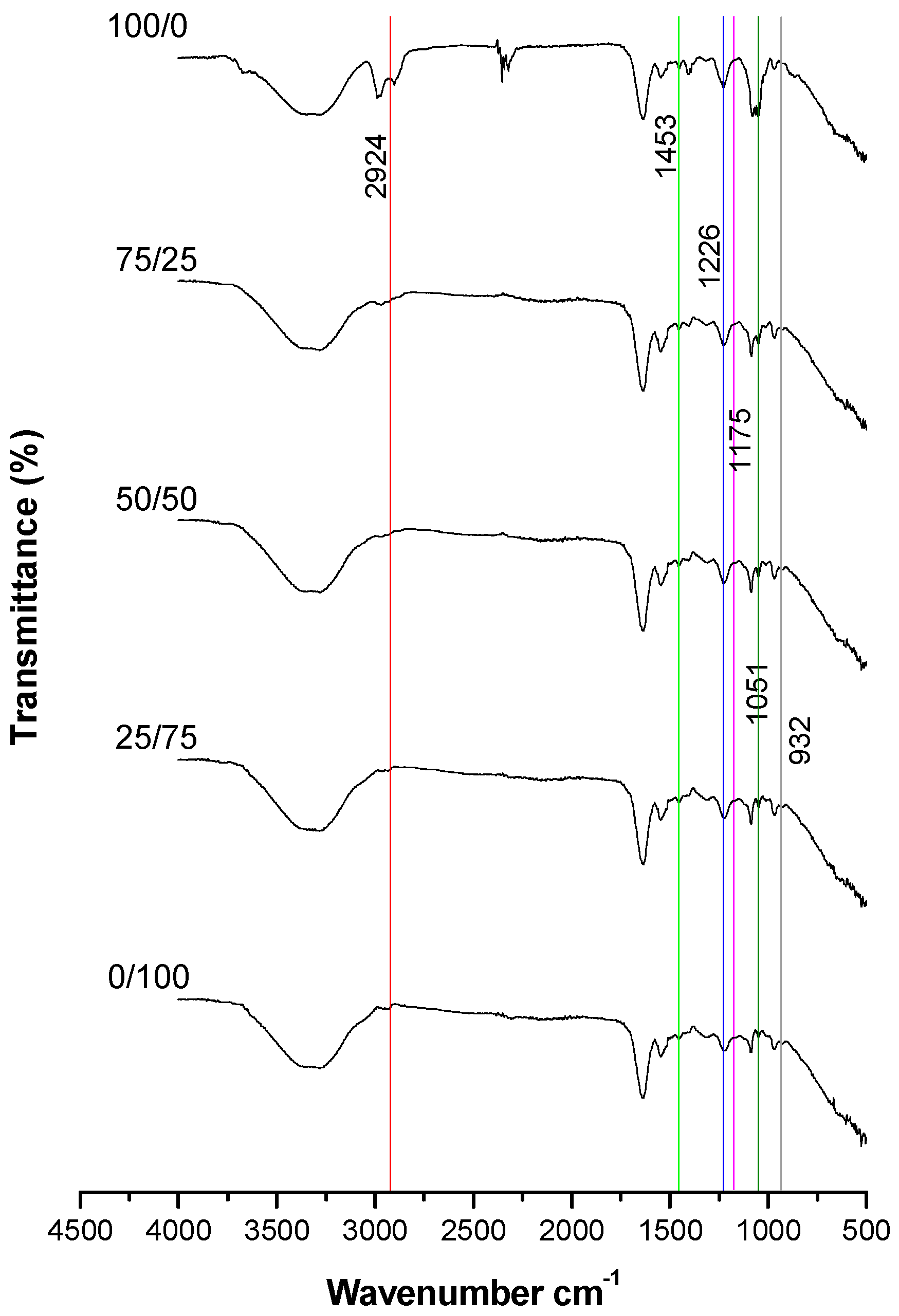
3.2. Stage 2: Determination of the Minimum Percentage of Viable Spermatozoa Required to Observe at Least Five Spectral Bands in Common with Pure Cholesterol
3.3. Stage 3: Buck Sperm Cryopreservation
4. Discussion
4.1. Stage 1: Determination of the Appropriate Sperm Concentration to Detect Cholesterol in the FTIR Spectrum
4.2. Stage 2: Determination of the Minimum Percentage of Viable Spermatozoa Required to Observe at Least Five Spectral Bands in Common with Pure Cholesterol
4.3. Stage 3: Buck Semen Cryopreservation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATRs | Assisted Reproductive Techniques |
| A.U. | Arbitrary units |
| FTIR | Fourier-transform infrared spectroscopy |
| CTC | Chlortetracycline assay |
| MC540 | Merocyanine 540 |
| ROS | Reactive oxygen species |
References
- Ferré, L.B.; Grötter, L.; Cattaneo, L.; Marini, P. Últimos avances en la criopreservación de semen de animales domésticos. Recent advances in domestic animals sperm cryopreservation: Part I: Basic Principles of Sperm Cryopreservation. Taurus 2017, 19, 8–19. [Google Scholar]
- Holt, W.V.; Medrano, A.; Thurston, L.M.; Watson, P.F. The significance of cooling rates and animal variability for boar sperm cryopreservation: Insights from the cryomicroscope. Theriogenology 2005, 63, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Sieme, H.; Oldenhof, H.; Wolkers, W.F. Sperm Membrane Behaviour during Cooling and Cryopreservation. Reprod. Domest. Anim. 2015, 50, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef]
- Yánez-Ortiz, I.; Catalán, J.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Advances in sperm cryopreservation in farm animals: Cattle, horse, pig and sheep. Anim. Reprod. Sci. 2022, 246, 106904. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Pachetti, M.; Venturin, I.; Mitri, E.; D’amico, F.; Vaccari, L.; Zupin, L.; Boscolo, R.; Ricci, G.; Pascolo, L.; Crovella, S. Ftir spectroscopy to reveal lipid and protein changes induced on sperm by capacitation: Bases for an improvement of sample selection in art. Int. J. Mol. Sci. 2020, 21, 8659. [Google Scholar] [CrossRef] [PubMed]
- Watson, P. Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. J. Reprod. Fertil. Dev. 1995, 7, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, M.M.; Irudayaraj, J. Determination of cholesterol in dairy products using infrared techniques: 1. FTIR spectroscopy. Int. J. Dairy Technol. 2002, 55, 127–132. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Hoekstra, F.A. In situ FTIR Assessment of Desiccation-Tolerant Tissues. Spectrosc. 2003, 17, 297–313. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Oldenhof, H. In situ FTIR studies on mammalian cells. Spectroscopy 2010, 24, 525–534. [Google Scholar] [CrossRef]
- Sánchez, V.; Redmann, K.; Wistuba, J.; Wübbeling, F.; Burger, M.; Oldenhof, H.; Wolkers, W.F.; Kliesch, S.; Schlatt, S.; Mallidis, C. Oxidative DNA damage in human sperm can be detected by Raman microspectroscopy. Fertil. Steril. 2012, 98, 1124–1129.e1123. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Singh, V.; Kumar, V.; Khajuria, Y. Spectroscopic Studies of Cholesterol: Fourier Transform Infra-Red and Vibrational Frequency Analysis. Mater. Focus 2014, 3, 1–7. [Google Scholar] [CrossRef]
- Oldenhof, H.; Schütze, S.; Sieme, H.; Wolkers, W.F. Fourier transform infrared spectroscopic analysis of sperm chromatin structure and DNA stability. Andrology 2016, 4, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Bernecic, N.C.; de Graaf, S.P.; Leahy, T.; Zhang, M.; Gadella, B.M.; Brouwers, J.F.H.M.; Jansen, J.W.A.; Arkesteijn, G.J.A. BODIPY-cholesterol can be reliably used to monitor cholesterol efflux from capacitating mammalian spermatozoa. Sci. Rep. 2019, 9, 9804. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Padilla, A.J.; Jimenez-Trejo, F.; Cerbon, M.; Chavez-Garcia, A.; Cruz-Cano, N.B.; Martinez-Torres, M.; Alcantar-Rodriguez, A.; Medrano, A. Sperm melatonin receptors, seminal plasma melatonin and semen freezability in goats. Theriogenology 2024, 225, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Baril, G. Manuel de Formation Pour L’insémination Artificielle Chez les Ovins et les Caprins; Etude FAO Production et Sante´ Animale; FAO: Rome, Italy, 1993; Volume 83. [Google Scholar]
- Barth, A.D.; Oko, R.J. Abnormal Morphology of Bovine Spermatozoa; Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Vyas, P.M.; Joshi, M.J. Surface Micro Topographical and Dielectric Studies of Cholesterol Crystals. Adv. Mater. Res. 2013, 665, 289–296. [Google Scholar] [CrossRef]
- Rajoriya, J.S.; Prasad, J.K.; Ramteke, S.S.; Perumal, P.; Ghosh, S.K.; Singh, M.; Pande, M.; Srivastava, N. Enriching membrane cholesterol improves stability and cryosurvival of buffalo spermatozoa. Anim. Reprod. Sci. 2016, 164, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hazel, J.R. Thermal adaptation in biological membranes: Is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 1995, 57, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.-H.; Xu, B.-B.; Yan, X.-C.; Zhang, J.-X.; Su, R. Sperm Membrane Stability: In-Depth Analysis from Structural Basis to Functional Regulation. Vet. Sci. 2025, 12, 658. [Google Scholar] [CrossRef] [PubMed]

| Dilution | Concentration (×106 sperm/mL) |
|---|---|
| 1 | 109 |
| 2 | 54 |
| 3 | 27 |
| 4 | 13 |
| 5 | 6 |
| 6 | 3 |
| 7 | 1 |
| Band | Wavenumber (cm−1) | Area (A.U.) | Associated Functional Group |
|---|---|---|---|
| A | X = 3428, Y = 89.52 | 1545.09 | OH |
| B | X = 3039, Y = 97.17 | 25.37 | CH2, CH3 |
| C | X = 2930, Y = 59.06 | 738.78 | CH2, CH3 |
| D | X = 2900, Y = 69.19 | 42.96 | CH2 |
| E | X = 2866, Y = 67.58 | 63.64 | CH2, CH3 |
| F | X = 2847, Y = 72.59 | 93.31 | Nd |
| G | X = 2718, Y = 96.08 | 1.53 | Nd |
| H | X = 2653, Y = 96.35 | 5.82 | Nd |
| I | X = 1671, Y = 96.68 | 15.08 | C=C |
| J | X = 1464, Y = 74.75 | 184.61 | CH2, CH3 |
| K | X = 1436, Y = 79.70 | 81.45 | CH2, CH3 |
| L | X = 1365, Y = 77.17 | 349.46 | CH2, CH3 |
| M | X = 1235, Y = 90.95 | 38.21 | CH2 |
| N | X = 1191, Y = 90.32 | 53.74 | C-C |
| O | X = 1170, Y = 91.81 | 11.93 | CH |
| P | X = 1131, Y = 90.24 | 63.7 | CH |
| Q | X = 1108, Y = 90.26 | 45.38 | CH |
| R | X = 1054, Y = 60.28 | 571.54 | Deformed ring |
| S | X = 1022, Y = 7639 | 72.57 | CH |
| T | X = 986, Y = 89.01 | 26.32 | CH |
| U | X = 953, Y = 80.50 | 175.83 | CH=CH2 Y CH2 |
| V | X = 926, Y = 90.59 | 86.8 | CH |
| X | X = 882, Y = 92.78 | 45.63 | CH |
| Band | Sperm Concentration (×106 Cells/mL) | F | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 109 | 54 | 27 | 13 | 6 | 3 | 1 | |||
| C | 6.28 ± 0.25 a | 4.67 ± 0.02 b | 4.10 ± 0.11 c | 0.99 ± 0.02 d | 0.84 ± 0.06 d | 0.57 ± 0.06 d | 0.56 ± 0.031 d | 437.46 | 0.00 |
| J | 9.7 ± 0.1 a | 9.1 ± 0.3 a | 3.8 ± 0.1 b | 3.2 ± 0.07 bc | 2.94 ± 0.07 c | 2.13 ± 0.07 d | 0.76 ± 0.06 e | 483.88 | 0.00 |
| M | 61.92 ± 0.2 a | 58.3 ± 1.32 b | 24.8 ± 0.8 c | 17.19 ± 0.4 d | 11.75 ± 0.54 e | 7.97 ± 0.36 e | 3.12 ± 0.13 g | 1340.49 | 0.00 |
| O | 0.89 ± 0.05 a | 0.51 ± 0.02 b | 0.21 ± 0.02 c | 0.11 ± 0.02 d | 0.05 ± 0.01 d | 0.03 ± 0.00 d | 0.02 ± 0.00 d | 270.30 | 0.00 |
| R | 8.6 ± 0.24 a | 6.6 ± 1.20 a | 3.44 ± 0.51 b | 3.2 ± 0.42 b | 2.51 ± 0.05 bc | 2.17 ± 0.19 bc | 0.11 ± 0.01 c | 29.34 | 0.00 |
| V | 18.43 ± 0.30 a | 16.04 ± 0.13 b | 6.02 ± 0.18 c | 3.02 ± 0.09 d | 0.21 ± 0.00 e | 0.11 ± 0.02 e | 0.03 ± 0.00 e | 2864.87 | 0.00 |
| Band | Sperm Concentration (×106 Cells/mL) | ||||||
|---|---|---|---|---|---|---|---|
| 109 | 54 | 27 | 13 | 6 | 3 | 1 | |
| C | 100 | 74.4 | 65.3 | 15.8 | 13.4 | 9.1 | 8.9 |
| J | 100 | 93.8 | 39.2 | 33.0 | 30.3 | 22.0 | 7.8 |
| M | 100 | 94.2 | 40.1 | 27.8 | 19.0 | 12.9 | 5.0 |
| O | 100 | 57.3 | 23.6 | 12.4 | 5.6 | 3.4 | 2.2 |
| R | 100 | 76.7 | 40.0 | 37.2 | 29.2 | 25.2 | 1.3 |
| V | 100 | 87.0 | 32.7 | 16.4 | 1.1 | 0.6 | 0.2 |
| Band | Proportions of Live/Dead Spermatozoa | Heat-Killed Spermatozoa (Mean + Sem) | Cold-Killed Spermatozoa (Mean + Sem) | F | p |
|---|---|---|---|---|---|
| C | 100/0 | 4.86 ± 0.23 a | 0.31 ± 0.02 b | 369.98 | 0.00 |
| 75/25 | 3.74 ± 0.03 a | 0.27 ± 0.05 b | 2916.49 | 0.00 | |
| 50/50 | 1.67 ± 0.32 a | 0.19 ± 0.03 b | 21.31 | 0.01 | |
| 25/75 | 0.61 ± 0.16 a | 0.15 ± 0.02 a | 7.36 | 0.05 | |
| 0/100 | 0.00 a | 0.11 ± 0.01 b | 64.47 | 0.00 | |
| J | 100/0 | 11.55 ± 0.72 a | 7.22 ± 0.08 b | 35.38 | 0.00 |
| 75/25 | 9.85 ± 0.71 a | 6.43 ± 0.44 b | 16.55 | 0.01 | |
| 50/50 | 9.13 ± 0.03 a | 5.21 ± 0.25 b | 226.18 | 0.00 | |
| 25/75 | 7.82 ± 0.12 a | 4.90 ± 0.10 b | 329.26 | 0.00 | |
| 0/100 | 0.30 ± 0.08 a | 4.85 ± 0.14 b | 743.21 | 0.00 | |
| M | 100/0 | 105.33 ± 0.9 a | 97.37 ± 0.29 b | 69.59 | 0.00 |
| 75/25 | 97.54 ± 0.00 a | 75.02 ± 0.06 b | 12,6124.44 | 0.00 | |
| 50/50 | 69.42 ± 1.20 a | 73.82 ± 0.59 b | 10.72 | 0.03 | |
| 25/75 | 65.68 ± 1.06 a | 64.54 ± 0.70 a | 0.79 | 0.42 | |
| 0/100 | 13.93 ± 2.29 a | 45.08 ± 2.15 b | 157.26 | 0.00 | |
| O | 100/0 | 0.38 ± 0.00 a | 0.41 ± 0.03 a | 1.29 | 0.32 |
| 75/25 | 0.36 ± 0.00 a | 0.35 ± 0.07 a | 0.03 | 0.87 | |
| 50/50 | 0.21 ± 0.02 a | 0.28 ± 0.04 a | 1.66 | 0.26 | |
| 25/75 | 0.15 ± 0.03 a | 0.10 ± 0.01 a | 1.80 | 0.25 | |
| 0/100 | 0.11 ± 0.01 a | 0.03 ± 0.00 b | 30.25 | 0.00 | |
| R | 100/0 | 18.68 ± 0.97 a | 195.76 ± 1.09 b | 14,667.64 | 0.00 |
| 75/25 | 15.79 ± 0.36 a | 12.41 ± 0.34 b | 45.51 | 0.00 | |
| 50/50 | 9.63 ± 0.1 a | 10.92 ± 0.49 a | 6.47 | 0.06 | |
| 25/75 | 9.44 ± 0.11 a | 9.4 ± 0.14 a | 0.04 | 0.85 | |
| 0/100 | 1.74 ± 0.37 a | 6.70 ± 0.39 b | 82 | 0.00 | |
| V | 100/0 | 4.64 ± 0.31 a | 1.46 ± 0.09 b | 96.16 | 0.00 |
| 75/25 | 3.77 ± 0.2 a | 1.27 ± 0.12 b | 110.25 | 0.00 | |
| 50/50 | 3.69 ± 0.2 a | 0.93 ± 0.06 b | 169.43 | 0.00 | |
| 25/75 | 3.66 ± 0.23 a | 0.64 ± 0.01 b | 172.08 | 0.00 | |
| 0/100 | 0.43 ± 0.03 a | 0.41 ± 0.03 a | 0.09 | 0.77 |
| Band | Before Freezing | After Freezing | F | p | Proportional Decrease in Cholesterol (%) |
|---|---|---|---|---|---|
| C | 0.99 ± 0.02 | 0.67 ± 0.06 | 3.49 | 0.07 | 32.3 |
| J | 3.2 ± 0.07 a | 0.18 ± 0.03 b | 1291.46 | 0.00 | 94.4 |
| M | 17.19 ± 0.4 a | 0.59 ± 0.13 b | 2080.98 | 0.00 | 96.6 |
| O | 0.11 ± 0.02 | 0.23 ± 0.06 | 0.46 | 0.50 | 209.1 * |
| R | 3.2 ± 0.42 a | 1.84 ± 0.2 b | 6.57 | 0.02 | 42.5 |
| V | 3.02 ± 0.09 | 0 | 100 |
| Variable (%) | Fresh Spermatozoa | Frozen–Thawed Spermatozoa | Recovery Rate |
|---|---|---|---|
| Progressive motility | 70.0 ± 7.36 a | 36.2 ± 2.39 b | 51.7 |
| Viability | 69.1 ± 4.18 a | 47.4 ± 3.54 b | 68.6 |
| Acrosome integrity | 78.0 ± 5.12 a | 32.06 ± 2.16 b | 41.1 |
| CTC-F pattern | 72.4 ± 5.74 a | 8.2 ± 1.30 b | 11.3 |
| Proportional Increase | |||
| CTC-B pattern | 20.2 ± 6.54 | 23.0 ± 3.26 | 113.9 |
| CTC-AR pattern | 7.4 ± 2.01 a | 68.7 ± 3.37 b | 928.4 |
| Hyper-fluid membranes | 3.5 ± 0.79 a | 55.5 ± 1.94 b | 1585.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Fernández-de-Arcipreste, N.; Cardenas-Padilla, A.J.; Alcantar-Rodriguez, A.; Vázquez-Durán, A.; Méndez-Albores, A.; Medrano, A. Fourier Transform Infrared Spectroscopy to Measure Cholesterol in Goat Spermatozoa. Animals 2025, 15, 3107. https://doi.org/10.3390/ani15213107
Cortés-Fernández-de-Arcipreste N, Cardenas-Padilla AJ, Alcantar-Rodriguez A, Vázquez-Durán A, Méndez-Albores A, Medrano A. Fourier Transform Infrared Spectroscopy to Measure Cholesterol in Goat Spermatozoa. Animals. 2025; 15(21):3107. https://doi.org/10.3390/ani15213107
Chicago/Turabian StyleCortés-Fernández-de-Arcipreste, N., A. J. Cardenas-Padilla, A. Alcantar-Rodriguez, A. Vázquez-Durán, A. Méndez-Albores, and A. Medrano. 2025. "Fourier Transform Infrared Spectroscopy to Measure Cholesterol in Goat Spermatozoa" Animals 15, no. 21: 3107. https://doi.org/10.3390/ani15213107
APA StyleCortés-Fernández-de-Arcipreste, N., Cardenas-Padilla, A. J., Alcantar-Rodriguez, A., Vázquez-Durán, A., Méndez-Albores, A., & Medrano, A. (2025). Fourier Transform Infrared Spectroscopy to Measure Cholesterol in Goat Spermatozoa. Animals, 15(21), 3107. https://doi.org/10.3390/ani15213107






