1. Introduction
Human milk is the optimal infant nutrition [
1], yet the global exclusive breastfeeding rate is only 38%, making infant formula a necessary alternative [
2,
3]. Milk serves as a primary ingredient in most formulas [
4], yet cow’s milk allergy affects 2–3% of infants under one year old, primarily triggered by whey proteins and caseins [
5]. Consequently, alternative milk sources like donkey, mare, and camel milk have been investigated due to their potentially reduced allergenicity [
6,
7]. Comparative studies indicate that immunological cross-reactivity with cow’s milk generally follows the order: cow milk > goat milk > camel milk [
8,
9]. Evidence demonstrates that goat milk is a promising alternative source of oligosaccharides to bovine milk for use in infant formula [
10], and goat milk powder is expected to become a better infant formula than cow milk powder.
Goat milk offers several distinct advantages. Notably, goat milk forms a looser coagula under acidic conditions, facilitating protease penetration and casein breakdown [
11]. Goat milk exhibits distinct advantages over cow milk, such as smaller milk fat globules and more short/medium-chain fatty acids, enhancing protein digestibility [
12,
13,
14]. Xu et al. [
15] demonstrated that GMF improves early growth and immune function in rats after weaning. Under simulated infant gastric conditions, goat milk formula powder demonstrates physicochemical and proteolytic similarities to cow milk formula powder [
16]. These properties position goat milk formula powder as a safer alternative for cow’s milk allergy management, alleviating gastrointestinal symptoms and parental concerns [
17]. Piglets are an adaptable and robust model for pediatric nutrition and metabolism research, with demonstrated physiological parallels to human infants in nutritional physiology, intestinal development, and brain development [
18]. Based on the documented beneficial properties of goat milk and the physiological relevance of the piglet model, we hypothesize that a GMF will enhance immunity, improve amino acid and fatty acid metabolism, and modulate intestinal microbiota in neonatal piglets. This study aims to optimize infant formula design and inform clinical applications.
2. Materials and Methods
2.1. Experimental Design and Diets
The experiment was conducted at the Institute of Subtropical Agriculture, Chinese Academy of Sciences. Piglets were humanely euthanized via intravenous pentobarbital sodium administration under deep anesthesia to ensure painless death. This experiment followed guidelines for animal research approved by the Animal Welfare Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (Changsha, ISA20240017). Sixteen healthy 7-day-old male piglets with similar body weights (BW = 2.23 ± 0.40 kg) were randomly divided into two groups. They were orally administered standard formula milk powder (CON) and goat milk formula powder (GMF) via feeding bottles, respectively. Each group consisted of 8 replicates, with 1 piglet per replicate, and the piglets were individually housed in infant incubators that maintained a constant temperature (30–32 °C). The pre-feeding period lasted for 3 days, followed by a formal experimental period of 14 days. The feeding procedure followed the protocol established by Bai [
19]. The daily feed allowance was adjusted each day based on the piglet’s body weight, calculated at a rate of 42 g DM/kg. Each pig had access to milk every 2 h from 07:30 to 24:00 in each group. The occurrence of bloating and milk spitting in piglets was observed and recorded. The formula milk powder used in this experiment was provided by Ausnutria Dairy Co., Ltd. (Changsha, China), under the brand name Kabrita. The selected piglets were obtained from a commercial pig farm. The nutritional composition of different infant formulas is presented in
Table 1.
2.2. Sample Collection
Before trial completion, piglets were fasted for 12 h, anesthetized, and then euthanized. Blood samples were collected from the anterior vena cava using sterile 10 mL vacuum tubes without anticoagulant. After clotting for 2 h at room temperature, the samples were centrifuged at 3000× g for 10 min at 4 °C using a refrigerated centrifuge (Xiangyi Centrifuge Instrument Co., Ltd., Changsha, China). The resulting serum was carefully separated and stored at −80 °C in a refrigerator (Haier DW86L578J, China). Tissue samples and intestinal content were immediately snap-frozen in liquid nitrogen and stored at −80 °C for subsequent use within six months.
2.3. Determination of Growth Performance Indexes
Piglets were weighed daily before feeding. Based on initial and final body weight, average daily gain (ADG) was calculated. The milk consumption of piglets was recorded every day, and the average daily feed intake (ADFI) and feed to weight FCR (Feed Conversion Ratio) = ADFI/ADG were calculated.
2.4. Determination of Serum Biochemical Indexes
The Beckman CX4 automatic blood biochemistry analyzer [
20] (Beckman Coulter, Inc., USA) was used to detect serum total protein (TP), blood urea nitrogen (BUN), albumin (ALB), alanine aminotransferase (ALT), glucose (GLU), aspartate aminotransferase (AST), alkaline phosphatase (ALP), CRP4, total cholesterol (CHOL), cholinesterase (CHE2), low-density lipoprotein (LDL-C), triglycerides (TG), Hepatic Lipase (LIPC), high-density lipoprotein (HDL-C). The kits were purchased from Beijing Leadman Biochemistry (China).
IgA, IgG, and IgM were measured using ELISA kits, and all experimental procedures were strictly performed according to the manufacturer’s instructions (Nanjing, China). Complement C3 (C3) and Complement C4 (C4) were determined using kits purchased from Beijing Leadman Biochemical Technology Co., Ltd. (Beijing, China), following the provided protocols.
2.5. Measurement of Serum Free Amino Acids
The process of measurement was as follows: Draw 1 mL of serum into a centrifuge tube, and then add 1 mL of 8% sulfosalicylic acid. After thorough mixing, place the tube in a 4 °C refrigerator for 15 min of standing. Subsequently, centrifuge the mixture at 10,000 rpm for 10 min. Carefully aspirate the supernatant, filter it through a 0.22 μm membrane, and transfer the filtered solution into a clean container for storage. For amino acid analysis, use an amino acid analyzer (Hitachi L8900, Tokyo, Japan) with the following parameters: injection volume of 20 μL, analysis cycle of 150 min, and column equilibrium time of 35 min. For detection wavelength, all amino acids were measured at 570 nm, except for proline (Pro), which was quantified at 440 nm [
21].
2.6. Assessment of mRNA Expression Levels of Liver, and Intestinal Related Genes
Total RNA was extracted from approximately 0.2 g of sample using the TRIzol kit (Accurate Biology, Changsha, China) according to the manufacturer’s instructions. RNA purity and concentration were measured with an ultra-microvolume spectrophotometer (IMPLEN, Germany). The RNA was then diluted to a uniform concentration based on the measurements and reverse-transcribed into cDNA in a 20 μL reaction system using the Prime Script TMRT Master Mix kit (Accurate Biology, Changsha, China). Finally, quantitative real-time PCR (qPCR) was performed using the TB Green Premix Ex Taq reagent kit (Accurate Biology, Changsha, China) on a LightCycler 480II system (Roche, Basel, Switzerland) [
22]. Gene and primer sequences are presented in
Table 2.
2.7. Determination of Long-Chain Fatty Acids in Serum
A 1–10 mL serum sample was placed in a centrifuge tube, mixed with 2 mL of 5% acetyl chloride/methanol solution (v/v = 1:19), vortexed, sealed, and incubated overnight at 50 °C in a water bath shaker (Zhchenc ZWF-110X30, Zhengzhou, China). After incubation, 1 mL of n-hexane was added and the mixture was vigorously shaken and then centrifuged at 3000 rpm for 5 min. The supernatant was transferred to a new tube, dried with anhydrous sodium sulfate, and then aspirated, filtered (0.22 μm), and analyzed by gas chromatography (Agilent Technologies Inc., Palo Alto, CA, USA).
2.8. Determination of Intestinal Digestive Enzymes
The contents of digestive enzymes including α-amylase, chymotrypsin, lactase, trypsin, and lipase in jejunal mucosal samples of piglets were measured using ELISA kits, with all experimental procedures strictly following the manufacturer’s protocols (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) [
23].
2.9. Gut Microbial Community Analysis
Genomic DNA was extracted from colonic content samples using a fecal genomic DNA extraction kit, with the DNA purity and concentration assessed by agarose gel electrophoresis. PCR amplification was conducted using a high-fidelity PCR enzyme system (GC Buffer, New England Biolabs) with uniquely barcoded primers. The amplification products were verified by 2% agarose gel electrophoresis. Sequencing libraries were constructed using the Ion Plus Fragment Library Kit (48 reactions, Beijing Novogene Technology Co., Ltd., Beijing, China) [
22]. After quantification with Qubit and quality control verification, sequencing was performed on the Ion S5 XL system (Thermo Fisher Scientific, Waltham, MA, USA). The 16S rRNA gene sequences have been deposited in the NCBI Sequence Read Archive under accession number PRJNA1255375.
2.10. Colonic Short-Chain Fatty Acids Content Detection
Fresh colonic contents (1 g) were homogenized with 5 mL ultrapure water by vortexing for 30 min, followed by 4 °C overnight incubation. After centrifugation (10,000 rpm, 10 min), the supernatant was collected and the pellet was re-extracted with 4 mL ultrapure water for 30 min. The supernatant was centrifuged at 12,000 rpm for 15 min and transferred to a new supernatant. The mixture was then added to a centrifuge tube at a ratio of
v:
v = 9:1 (900 μL of new supernatant + 100 μL of 25% phosphoric acid). After mixing the liquid, it was allowed to stand at room temperature for 3 h. It was then filtered through a 45 μm micropore into an upper sample bottle and tested by gas chromatography–mass spectrometry (Agilent 7890A, Agilent Technologies Inc., Palo Alto, CA, USA). Methods were carried out as described by [
22].
2.11. Statistical Analysis
All the results were expressed as means ± SEM. This experiment utilized GraphPad Prism 10.4 (GraphPad Software, Inc., Boston, MA, USA) for data processing. After assessing normality (Shapiro–Wilk test) and homogeneity of variances (F-test), an unpaired t-test was used for data meeting both assumptions; otherwise, the Mann–Whitney U test was employed. p ≤ 0.05 was considered a significant difference. 0.05 < p < 0.1 denoted a statistical trend.
4. Discussion
Growth performance directly reflects the health status of piglets. Studies confirm that GMF supports infant growth and safety [
24]. Roy et al. [
25] compared gastric emptying rates of cow milk, goat milk and sheep milk, revealing faster clearance for goat milk forms gastric curds that modulate nutrient release. Goat milk curds release proteins and lipids more efficiently than cow milk, enhancing small intestine absorption. In this study, GMF group piglets exhibited marginally greater weight gain and feed intake than CON group, likely linked to improved nutrient digestion. Maximino et al. [
26] demonstrated that GMF was well-tolerated and safe in human infants, supporting adequate growth with a low incidence of gastrointestinal symptoms. Serum biomarkers reflect systemic health and organ function [
27]. Colostrum provides passive immunity to neonatal piglets [
28], with 70–80% of immunoglobulins absorbed into circulation [
29,
30]. The GMF group piglets showed elevated IgA, IgG, IgM, and CRP4 levels, indicating immune enhancement. The results of Xu et al. [
15] were consistent with those of the present study. van Lee et al. [
31] found that GMF, similar to human milk, supported longer daytime sleep duration compared to CMF, supporting its use as a valuable alternative when human milk is unavailable. Rutherfurd et al. [
32] reported that GMF promoted better absorption of certain minerals than CMF in 3-week-old piglets.
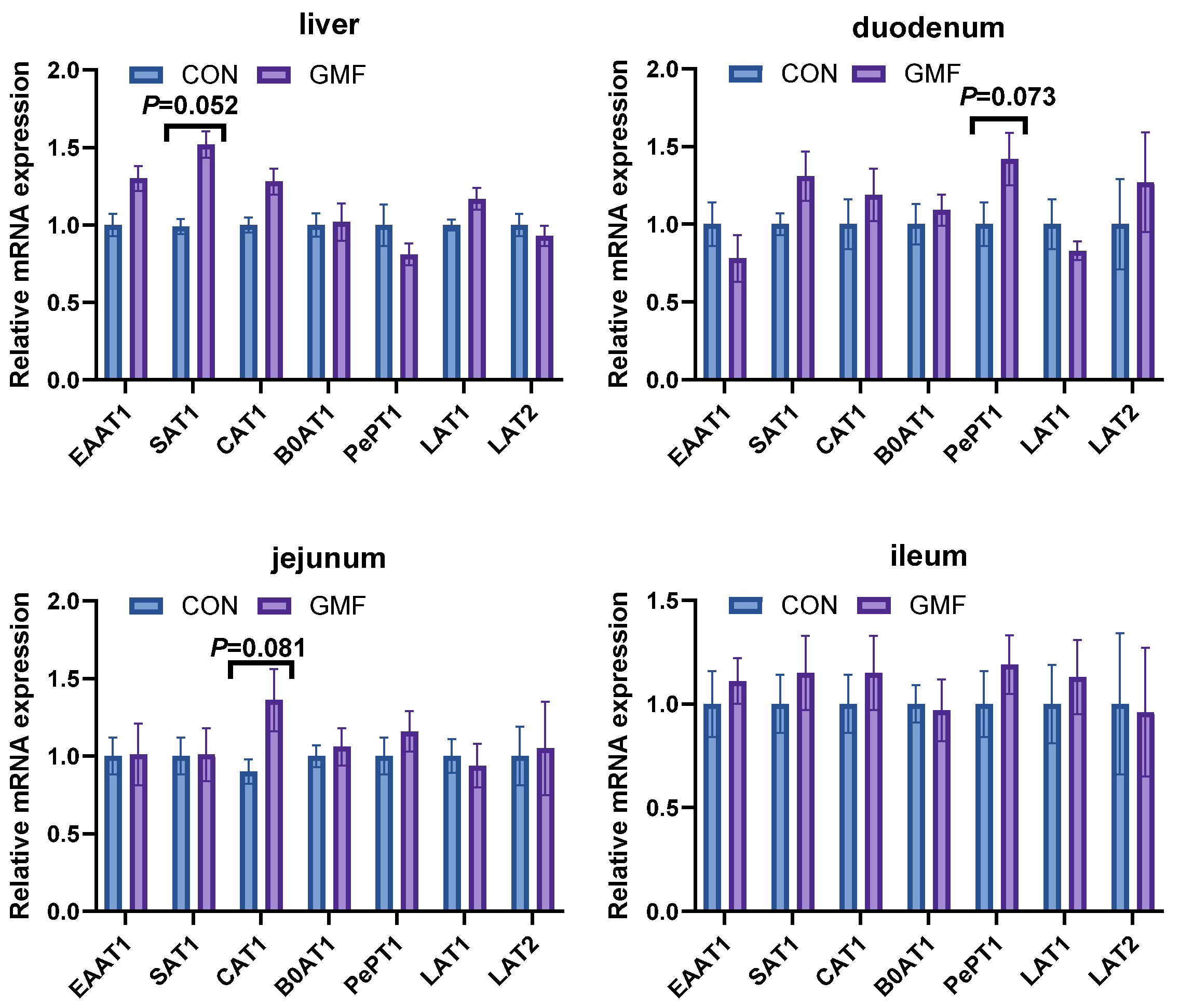
Serum free amino acids reflect nutritional status and are influenced by intestinal absorption, cellular metabolism, and transmembrane transport activity [
33]. Amino acid transporters (AATs) mediate uptake and regulate energy balance, protein synthesis and redox [
34,
35]. Notably, peptide transporter
PEPT1 facilitates di/tripeptide absorption [
21]. Circulating amino acids serve dual roles as protein precursors and immune modulators [
36,
37]. Hodgkinson et al. [
38] found distinct sets of peptides between cow milk and goat milk, which may explain the differences in their digestibility. Maathuiset et al. [
39] reported slower initial protein digestion in GMP under infant conditions; elevated amino acid levels suggest enhanced metabolism, promoting protein deposition, immune function, and energy utilization. Lipid metabolism encompasses the synthesis and degradation of fatty acids, triglycerides, and cholesterol [
40]. Free fatty acids (FFAs) are derived primarily from dietary sources and serve as key energy substrates [
41]. Saturated fatty acids (SFAs) stabilize cell membranes against oxidation [
42], while polyunsaturated fatty acids (PUFAs, e.g., C20:4n6 and C22:6n3) are essential for membrane integrity [
43]. Goat milk contains abundant phospholipids, including hexosylceramide, dihexosylceramide, sphingomyelin, ceramide, and phosphatidylcholine [
44]. Wang et al. [
45] demonstrated that GMF diets elevate serum triglyceride levels and modulate lipid metabolism-related gene expression in piglets, aligning with our findings. Protein digestion relies on enzymatic hydrolysis. Digestive enzymes, synthesized as inactive zymogens (e.g., trypsinogen), are activated in the intestine to cleave proteins into absorbable peptides [
46,
47,
48]. In this study, GMF feeding significantly increased intestinal trypsin levels, which enhanced proteolytic efficiency and accelerated the cleavage of goat milk proteins into small-molecule peptides and free amino acids. These degradation products were efficiently absorbed through
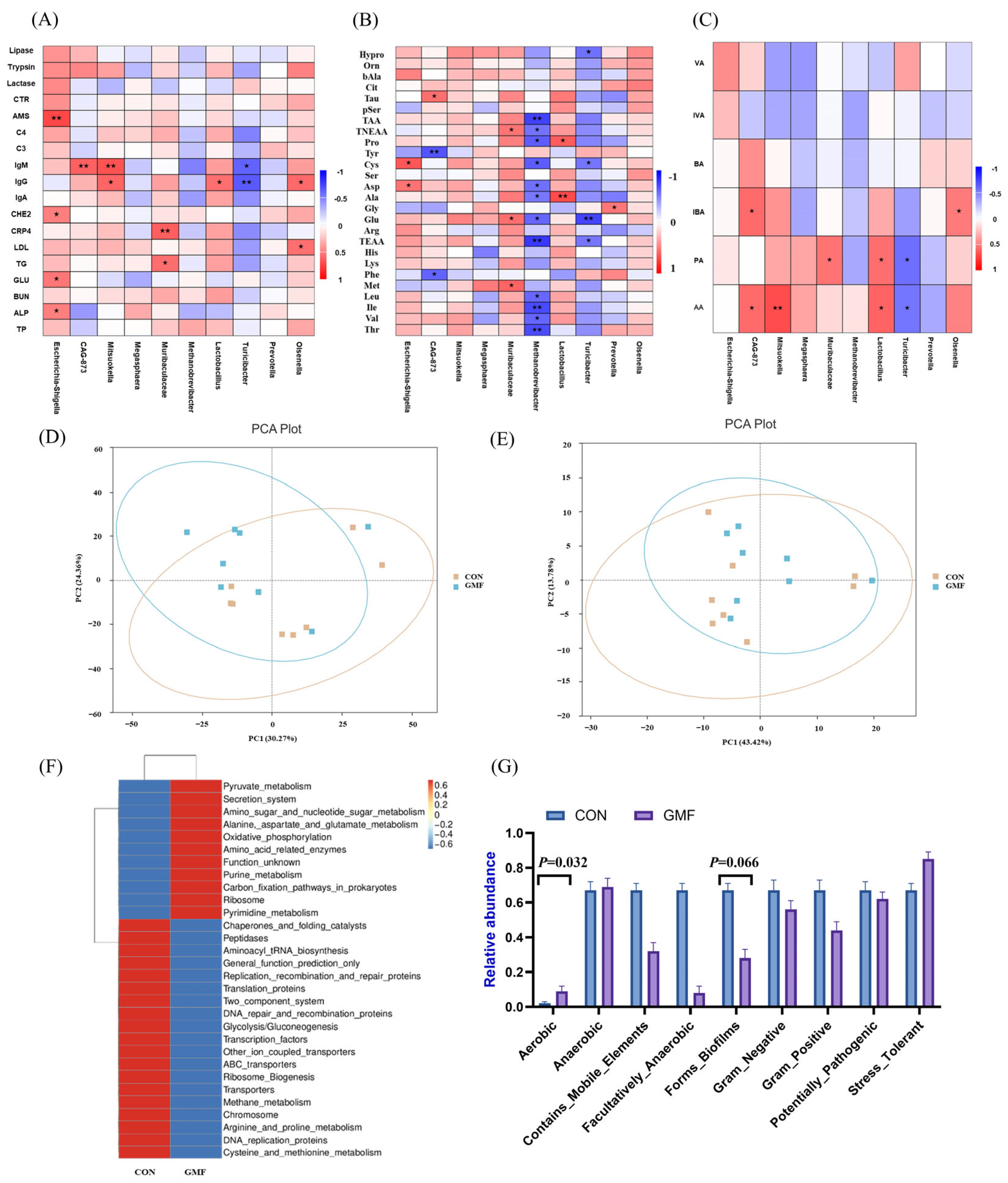
PePT1 and amino acid transporters (AATs) in the small intestinal epithelial cells, ultimately leading to elevated levels of certain free amino acids in the serum. This process improved amino acid utilization in piglets; without increasing total serum protein levels, it provided sufficient precursors for immune cell synthesis, thereby mediating the increase in immune indicators such as IgA, IgG and IgM in the GMF group piglets.
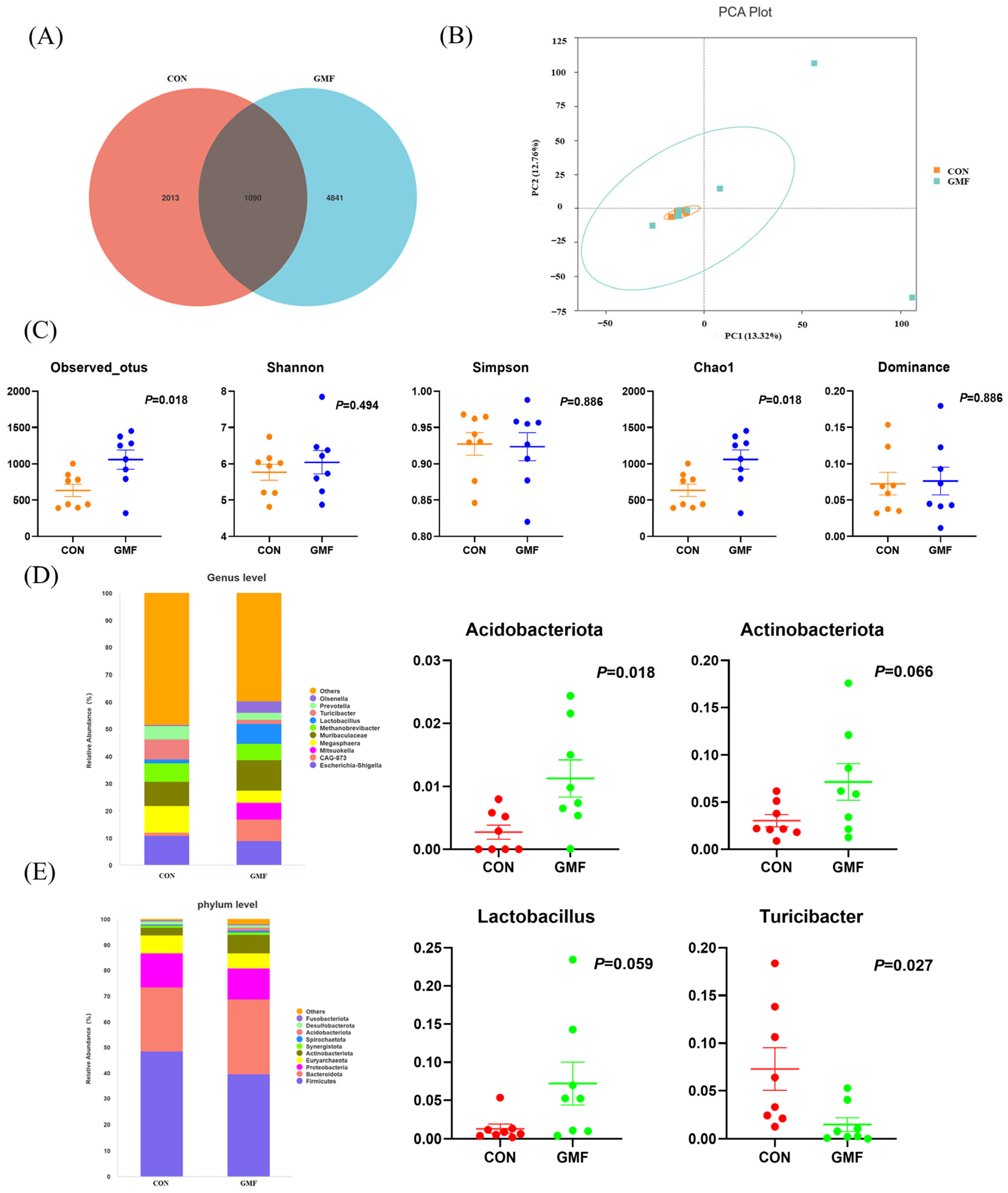
Gut microbiota analysis identified 5764 OTUs, with 2013 and 4841 unique to the CON and the GMF groups, respectively. Greater Chao1 diversity of GMF piglets indicated greater microbial richness—a hallmark of gut health linked to reduced inflammation and metabolic dysfunction [
49]. The gut microbiota critically regulates host glucose and lipid metabolism [
40].
Acidobacteriota, which is capable of degrading plant-derived chitin, xylan, cellulose, and hemicellulose while participating in sulfur cycling [
50].
Actinobacteriota (Gram-positive bacteria), which exhibit filamentous morphology and possess exceptionally high genomic guanine plus cytosine (G+C) content, play a crucial role in maintaining intestinal homeostasis [
51,
52]. Wang et al. [
53] demonstrated that goat milk exerts a more profound influence on gut microbiota than cow milk, with
Akkermansia showing significant enrichment exclusively in the GMF group—a phenomenon potentially attributable to the distinctive functional properties of goat milk, using murine models. Comparative analysis revealed that, relative to breastfed cubs, Siberian tiger cubs fed with goat milk exhibited distinct gut microbial metabolic shifts—reduced carbohydrate metabolism, translation, and DNA replication processes—concomitant with enhanced amino acid metabolism, membrane transport, as well as cofactor and vitamin metabolism [
54].
Turicibacter (
Firmicutes phylum,
Erysipelotrichaceae family) modulates host bile acid and lipid metabolism [
55,
56]. GMF enriched beneficial genera (e.g.,
Blautia,
Roseburia, and
Muribaculum), mirroring patterns observed in breast milk-fed infants [
57]. Clinical trials in Brazil and Mexico demonstrated that GMF reduces gastrointestinal symptoms, likely mediated by gut microbiota modulation [
58].
Lactobacillus, a prominent probiotic, regulates host immunity and gut balance via bacteriocin secretion and acid environment maintenance [
53,
54,
55,
59,
60,
61]. GMF feeding promotes
Lactobacillus proliferation while suppressing pathogens, as shown in murine models [
62].
Olsenella (
Atopobiaceae family) correlates with acetate levels, suggesting metabolic synergy with
Lactobacillus [
63,
64].
SCFAs, produced by microbial fiber fermentation, enhance energy metabolism, intestinal health and immune function [
65]. As end products of microbial breakdown of dietary fibers in the gut, SCFAs can be absorbed by the intestines and utilized by host cells as energy sources. Propionate supports gluconeogenesis and mitigates obesity [
66], while butyrate fuels colonocyte integrity and suppresses inflammation [
67]. The results indicated that acetate and propionate levels in the feces of piglets in the GMF group were significantly increased, enhancing the body’s anti-inflammatory capacity, maintenance of intestinal epithelial barrier function and energy stability. This study demonstrates that GMF modulates the gut microbiota in piglets, promoting the production of SCFAs, specifically acetate and propionate acid. These SCFAs potentially enhance immune-related gene expression and regulate energy metabolism, respectively, collectively mediating the observed improvements in immune markers (CRP4, IgA, IgG, IgM) and lipid metabolism. A key limitation of this study is its underpowered nature due to the constrained sample size and the trial duration. Therefore, these findings should be interpreted with caution. In the future, we will increase the sample size and extend the study period in subsequent trials to further investigate the underlying mechanisms of GMF on immunity.