Evaluating the Adjuvant Therapeutic Effects of Probiotic Strains Lactococcus cremoris and Lacticaseibacillus paracasei on Canine Atopic Dermatitis and Their Impact on the Gut and Skin Microbiome
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Preparation of Probiotic Capsule
2.3. Clinical Canine Trial
- (1)
- The initial onset of symptoms occurred at or before 3 years of age.
- (2)
- The dog primarily resided indoors.
- (3)
- Itching symptoms significantly improved with steroid treatment.
- (4)
- Skin lesions were primarily located on the ear pinna, interdigital spaces, around the mouth, eyes, dorsal surfaces of joints, and inguinal areas.
- (5)
- The edges of the ears appeared normal.
- (6)
- The skin of the lower back was normal.
2.4. Assessment of the Degree of Atopic Dermatitis and Pruritus
2.5. Cytokine Production from Peripheral Blood Mononuclear Cells (PBMCs)
2.6. IgE Production in Serum
2.7. Analysis of Fecal and Skin Microbiota
2.8. Short-Chain Fatty Acids Analysis
2.9. Statistical Analysis
3. Results
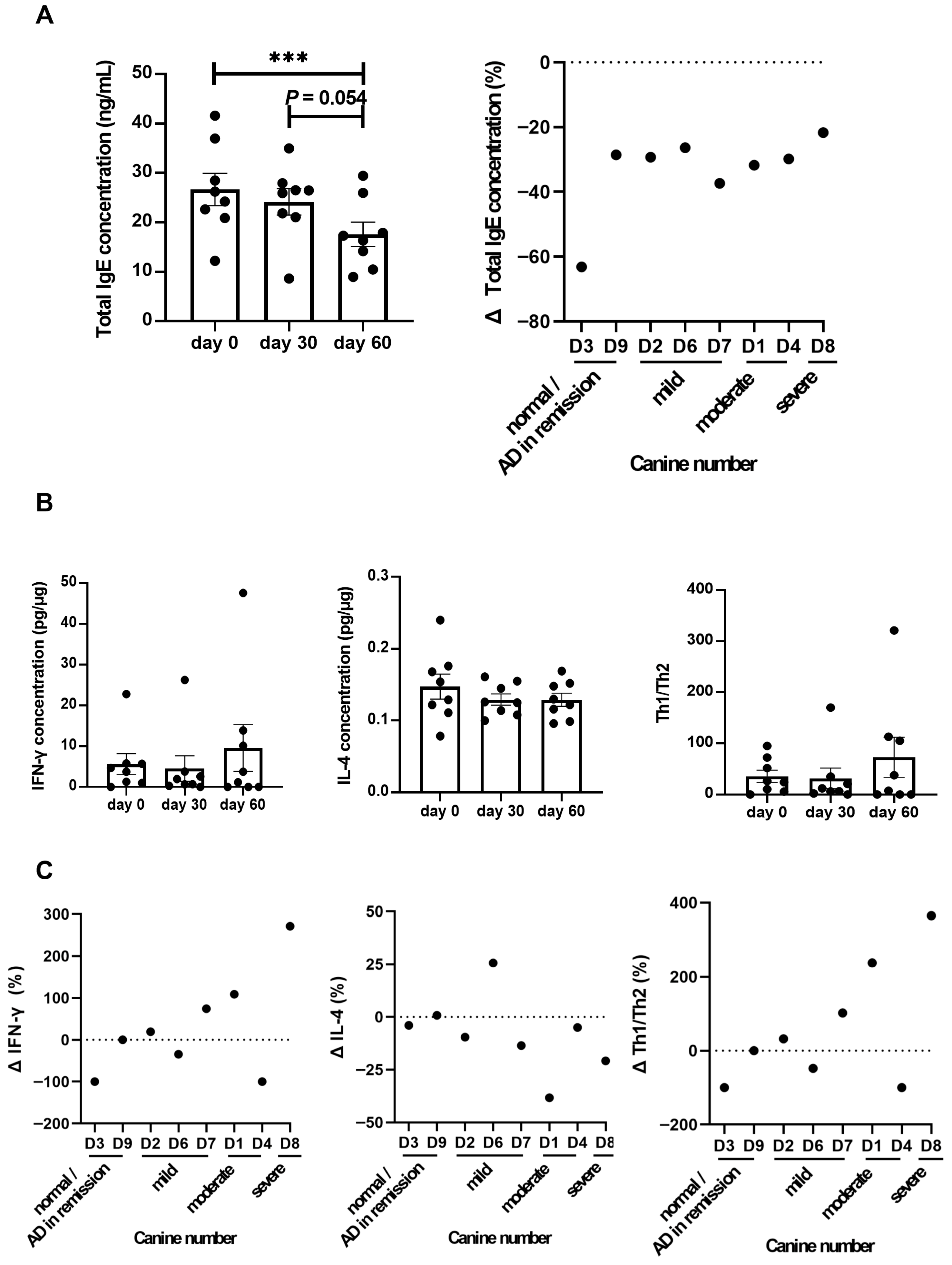
3.1. Clinical and Immunological Responses to LCP Treatment in Dogs with Atopic Dermatitis
3.2. Fecal Microbiota Composition in Dogs with Atopic Dermatitis Before and After LCP Treatment
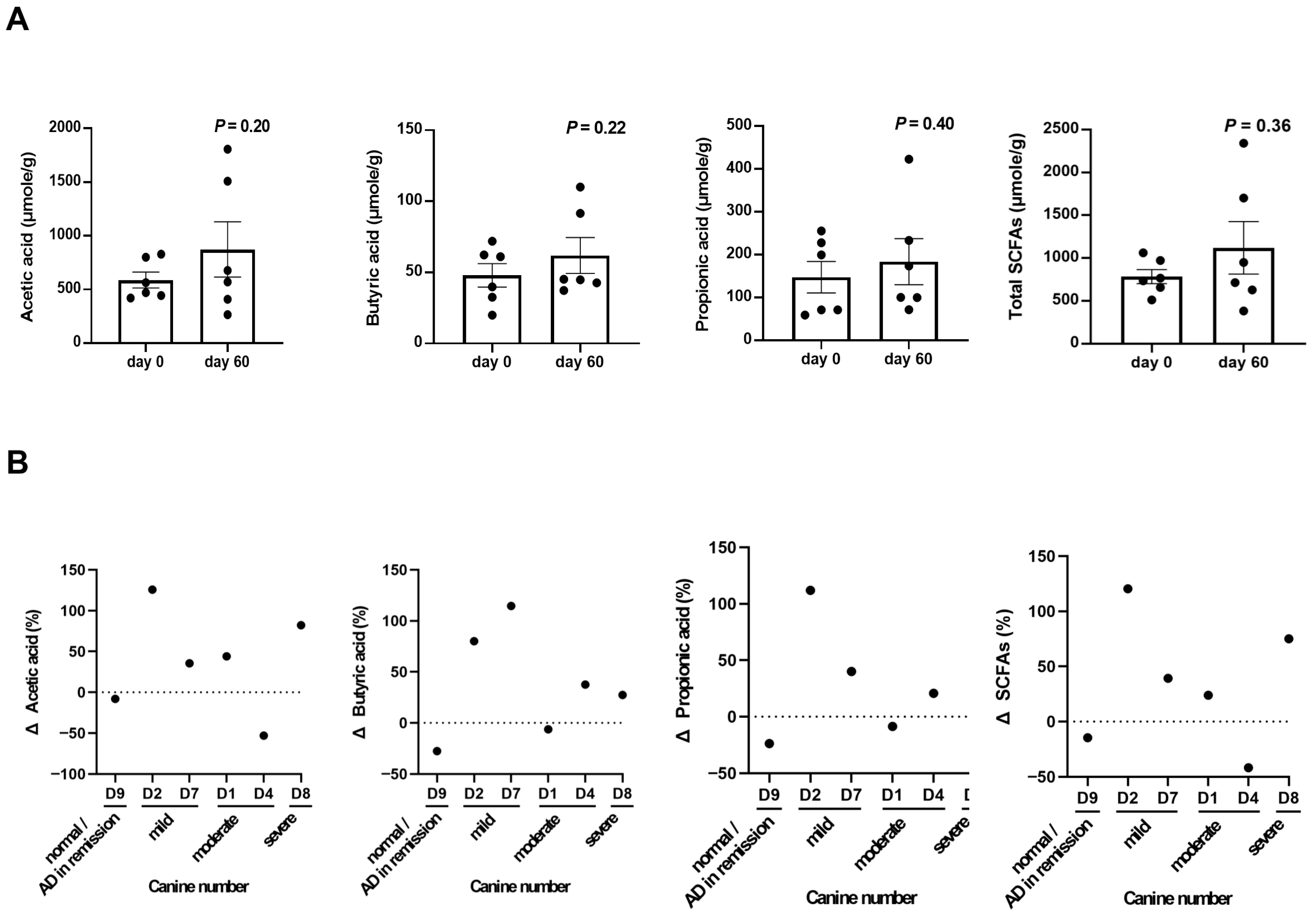
3.3. Predicted Fecal Microbial Functions and SCFA Profiles After LCP Treatment
3.4. Skin Microbiota Composition in Dogs with Atopic Dermatitis Before and After LCP Treatment
3.5. Predicted Functional Pathways of the Skin Microbiota After LCP Treatment
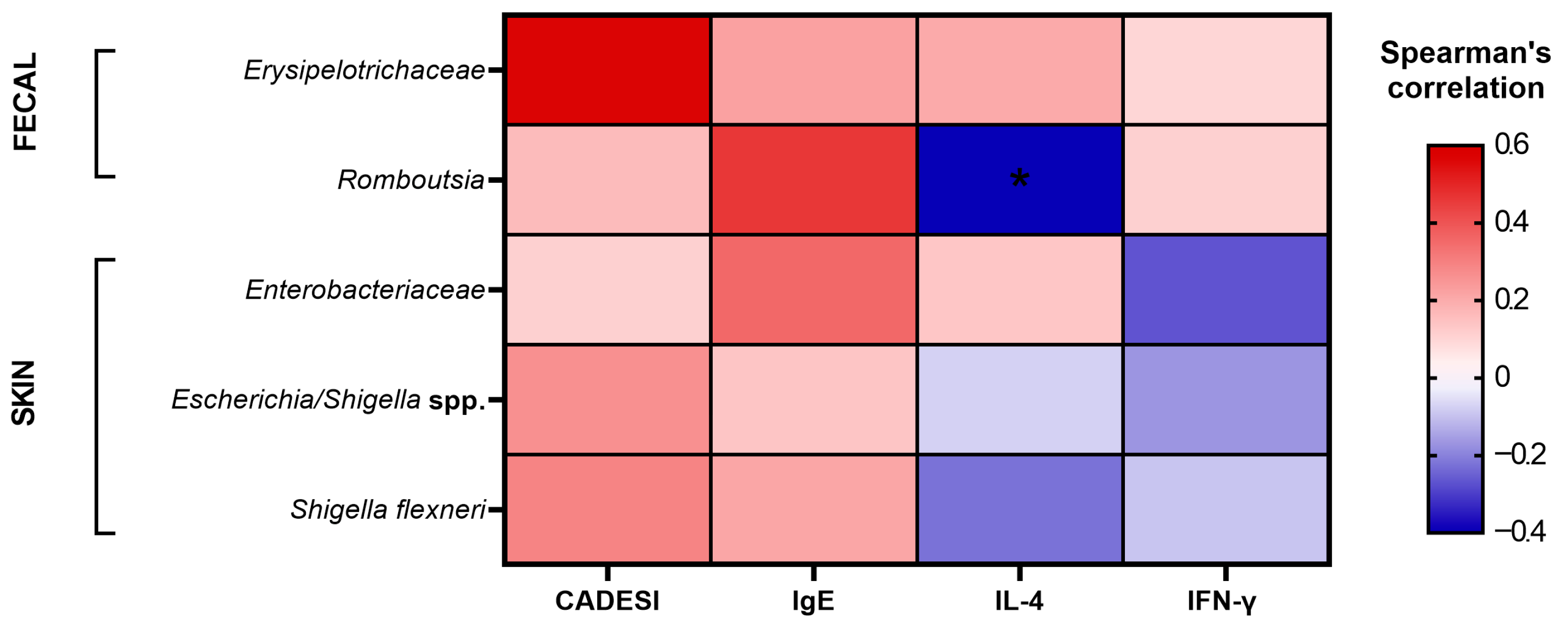
3.6. Correlation Analysis Between Fecal and Skin Microbiota and Atopic Dermatitis Indicators
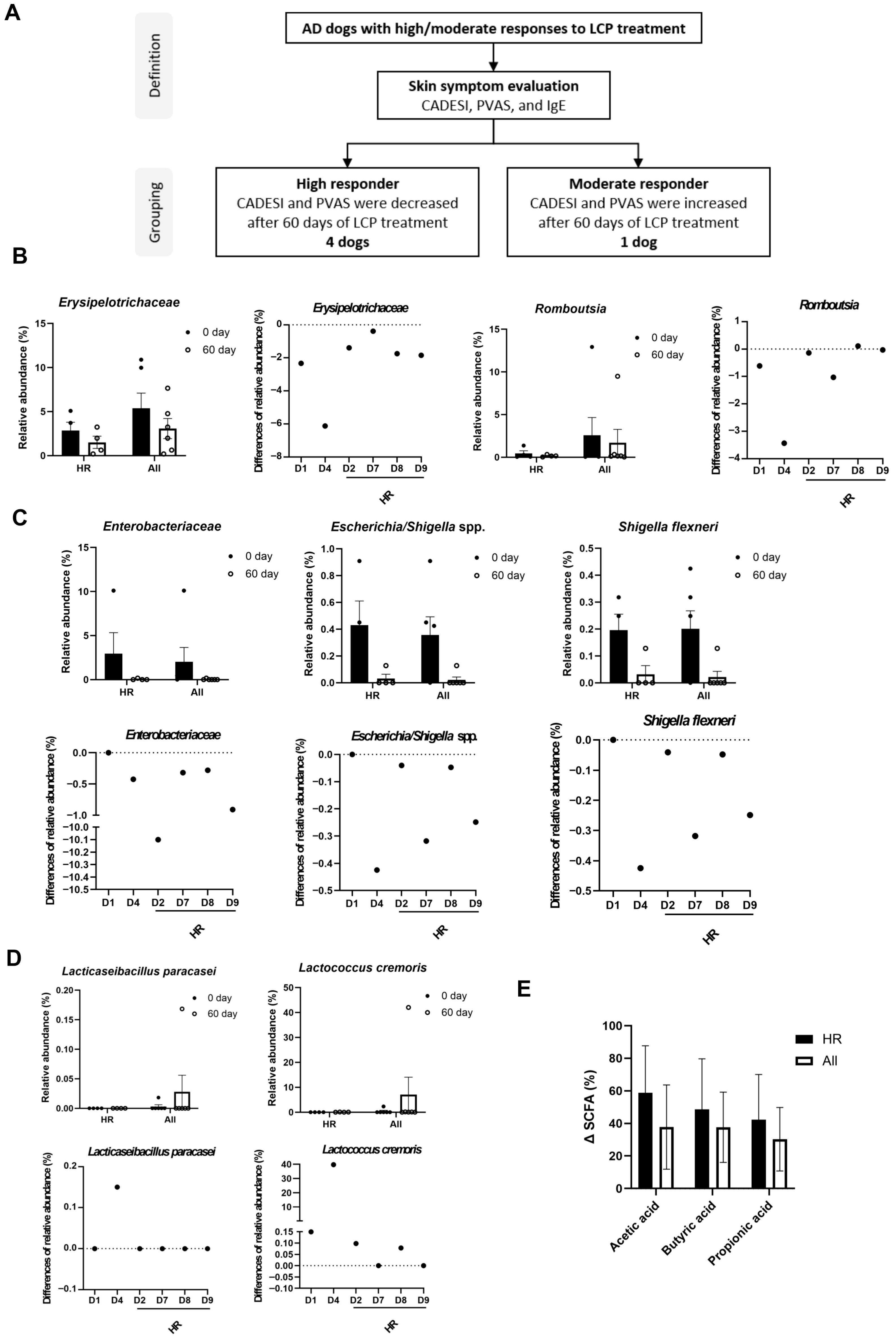
3.7. Microbial and Metabolic Biomarker Changes in High and Moderate Responders to LCP Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Widorn, L.; Zabolotski, Y.; Mueller, R.S. A prospective study evaluating the correlation between local weather conditions, pollen counts and pruritus of dogs with atopic dermatitis. Vet. Dermatol. 2024, 35, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Gedon, N.K.Y.; Mueller, R.S. Atopic dermatitis in cats and dogs: A difficult disease for animals and owners. Clin. Transl. Allergy 2018, 8, 41. [Google Scholar] [CrossRef]
- Outerbridge, C.A.; Jordan, T.J. Current knowledge on canine atopic dermatitis: Pathogenesis and treatment. Adv. Small Anim. Care 2021, 2, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Couceiro, G.A.; Ribeiro, S.M.M.; Monteiro, M.M.; Meneses, A.M.C.; Sousa, S.K.S.; Coutinho, L.N. Prevalence of canine atopic dermatitis at the Veterinary Hospital of the “Universidade Federal Rural da Amazônia” in Belém/Pará, Brazil. Pesqui. Vet. Bras. 2021, 41, e06778. [Google Scholar] [CrossRef]
- Humeniuk, P.; Dubiela, P.; Hoffmann-Sommergruber, K. Dendritic cells and their role in allergy: Uptake, proteolytic processing and presentation of allergens. Int. J. Mol. Sci. 2017, 18, 1491. [Google Scholar] [CrossRef]
- Marsella, R.; Sousa, C.A.; Gonzales, A.J.; Fadok, V.A. Current understanding of the pathophysiologic mechanisms of canine atopic dermatitis. J. Am. Vet. Med. Assoc. 2012, 241, 194–207. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Anderson, K.R.; Tollefson, M.M. New and emerging therapies for pediatric atopic dermatitis. Paediatr. Drugs 2019, 21, 239–260. [Google Scholar] [CrossRef]
- Cabanillas, B.; Novak, N. Atopic dermatitis and filaggrin. Curr. Opin. Immunol. 2016, 42, 1–8. [Google Scholar] [CrossRef]
- McAleer, M.A.; Irvine, A.D. The multifunctional role of filaggrin in allergic skin disease. J. Allergy Clin. Immunol. 2013, 131, 280–291. [Google Scholar] [CrossRef]
- Li, S.; Villarreal, M.; Stewart, S.; Choi, J.; Ganguli-Indra, G.; Babineau, D.C.; Philpot, C.; David, G.; Yoshida, T.; Boguniewicz, M.; et al. Altered composition of epidermal lipids correlates with Staphylococcus aureus colonization status in atopic dermatitis. Br. J. Dermatol. 2017, 177, e125–e127. [Google Scholar] [CrossRef]
- Shimada, K.; Yoon, J.S.; Yoshihara, T.; Iwasaki, T.; Nishifuji, K. Increased transepidermal water loss and decreased ceramide content in lesional and non-lesional skin of dogs with atopic dermatitis. Vet. Dermatol. 2009, 20, 541–546. [Google Scholar] [CrossRef]
- Gonzales, A.J.; Bowman, J.W.; Fici, G.J.; Zhang, M.; Mann, D.W.; Mitton-Fry, M. Oclacitinib (APOQUEL®) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy. J. Vet. Pharmacol. Ther. 2014, 37, 317–324. [Google Scholar] [CrossRef]
- Bağci, I.S.; Ruzicka, T. IL-31: A new key player in dermatology and beyond. J. Allergy Clin. Immunol. 2018, 141, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Chrobak-Chmiel, D.; Golke, A.; Kwiecień, E.; Biegańska, M.J.; Dembele, K.; Dziekiewicz-Mrugasiewicz, M.; Czopowicz, M.; Kizerwetter-Świda, M.; Rzewuska, M. Is vitamin D3 a worthy supplement protecting against secondary infections in dogs with atopic dermatitis? Pathogens 2023, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-skin axis: Current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Pfefferle, P.I.; Keber, C.U.; Cohen, R.M.; Garn, H. The hygiene hypothesis-learning from but not living in the past. Front. Immunol. 2021, 12, 635935. [Google Scholar] [CrossRef]
- Dou, J.; Zeng, J.; Wu, K.; Tan, W.; Gao, L.; Lu, J. Microbiosis in pathogenesis and intervention of atopic dermatitis. Int. Immunopharmacol. 2019, 69, 263–269. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef]
- Fang, Z.; Li, L.; Zhang, H.; Zhao, J.; Lu, W.; Chen, W. Gut microbiota, probiotics, and their interactions in prevention and treatment of atopic dermatitis: A review. Front. Immunol. 2021, 12, 720393. [Google Scholar] [CrossRef] [PubMed]
- Rostaher, A.; Morsy, Y.; Favrot, C.; Unterer, S.; Schnyder, M.; Scharl, M.; Fischer, N.M. Comparison of the gut microbiome between atopic and healthy dogs-preliminary data. Animals 2022, 12, 2377. [Google Scholar] [CrossRef]
- Sinkko, H.; Lehtimäki, J.; Lohi, H.; Ruokolainen, L.; Hielm-Björkman, A. Distinct healthy and atopic canine gut microbiota is influenced by diet and antibiotics. R. Soc. Open Sci. 2023, 10, 221104. [Google Scholar] [CrossRef]
- Ellis, S.R.; Nguyen, M.; Vaughn, A.R.; Notay, M.; Burney, W.A.; Sandhu, S.; Sivamani, R.K. The skin and gut microbiome and its role in common dermatologic conditions. Microorganisms 2019, 7, 550. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.K.; Lee, C.G.; So, J.S.; Chae, C.S.; Hwang, J.S.; Sahoo, A.; Nam, J.H.; Rhee, J.H.; Hwang, K.C.; Im, S.H. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc. Natl. Acad. Sci. USA 2010, 107, 2159–2164. [Google Scholar] [CrossRef]
- Shin, J.H.; Chung, M.J.; Seo, J.G. A multistrain probiotic formulation attenuates skin symptoms of atopic dermatitis in a mouse model through the generation of CD4+ Foxp3+ T cells. Food Nutr. Res. 2016, 60, 32550. [Google Scholar] [CrossRef]
- Kim, W.K.; Jang, Y.J.; Han, D.H.; Seo, B.; Park, S.; Lee, C.H.; Ko, G. Administration of Lactobacillus fermentum KBL375 causes taxonomic and functional changes in gut microbiota leading to improvement of atopic dermatitis. Front. Mol. Biosci. 2019, 6, 92. [Google Scholar] [CrossRef]
- Cauquil, M.; Olivry, T. Immunomodulating effects of heat-killed Lactobacillus rhamnosus and Lactobacillus reuteri on peripheral blood mononuclear cells from healthy dogs. Vet. Sci. 2025, 12, 226. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chen, Y.T.; Li, K.Y.; Huang, H.W.; Lin, Y.C.; Chen, M.J. A heat-killed probiotic mixture regulates immune T cells balance and IgE production in house dust mite extraction-induced atopic dermatitis mice. Microorganisms 2022, 10, 1881. [Google Scholar] [CrossRef]
- Favrot, C.; Steffan, J.; Seewald, W.; Picco, F. A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Vet. Dermatol. 2010, 21, 23–31. [Google Scholar] [CrossRef]
- Terada, Y.; Nagata, M.; Murayama, N.; Nanko, H.; Furue, M. Clinical comparison of human and canine atopic dermatitis using human diagnostic criteria (Japanese Dermatological Association, 2009): Proposal of provisional diagnostic criteria for canine atopic dermatitis. J. Dermatol. 2011, 38, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T.; Saridomichelakis, M.; Nuttall, T.; Bensignor, E.; Griffin, C.E.; Hill, P.B.; International Committee on Allergic Diseases of Animals (ICADA). Validation of the canine atopic dermatitis extent and severity index (CADESI)-4, a simplified severity scale for assessing skin lesions of atopic dermatitis in dogs. Vet. Dermatol. 2014, 25, 77-e25. [Google Scholar] [CrossRef] [PubMed]
- Rybnícek, J.; Lau-Gillard, P.J.; Harvey, R.; Hill, P.B. Further validation of a pruritus severity scale for use in dogs. Vet. Dermatol. 2009, 20, 115–122. [Google Scholar] [CrossRef]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Kado, Y.; Takada, T.; Matsumoto, K.; Tanaka, R. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl. Environ. Microbiol. 2004, 70, 167–173. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quin, C.; Estaki, M.; Vollman, D.M.; Barnett, J.A.; Gill, S.K.; Gibson, D.L. Probiotic supplementation and associated infant gut microbiome and health: A cautionary retrospective clinical comparison. Sci. Rep. 2018, 8, 8283. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Balvočiūtė, M.; Huson, D.H. SILVA, RDP, Greengenes, NCBI and OTT—How do these taxonomies compare? BMC Genom. 2017, 8, 114. [Google Scholar] [CrossRef]
- Gyarmati, P.; Kjellander, C.; Aust, C.; Song, Y.; Öhrmalm, L.; Giske, C.G. Metagenomic analysis of bloodstream infections in patients with acute leukemia and therapy-induced neutropenia. Sci. Rep. 2016, 6, 23532. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Chen, J.; Liu, L.; Wu, H.; Tan, H.; Xie, G.; Xu, Q.; Zou, H.; Yu, W.; Wang, L.; et al. Metagenomic sequencing reveals the relationship between microbiota composition and quality of Chinese rice wine. Sci. Rep. 2016, 6, 26621. [Google Scholar] [CrossRef]
- Triplett, J.; Ellis, D.; Braddock, A.; Roberts, E.; Ingram, K.; Perez, E.; Short, A.; Brown, D.; Hutzley, V.; Webb, C.; et al. Temporal and region-specific effects of sleep fragmentation on gut microbiota and intestinal morphology in Sprague Dawley rats. Gut Microbes 2020, 11, 706–720. [Google Scholar] [CrossRef]
- Shin, J.; Noh, J.R.; Choe, D.; Lee, N.; Song, Y.; Cho, S.; Kang, E.J.; Go, M.J.; Ha, S.K.; Chang, D.H.; et al. Ageing and rejuvenation models reveal changes in key microbial communities associated with healthy ageing. Microbiome 2021, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Roelands, J.; Kuppen, P.J.K.; Ahmed, E.I.; Mall, R.; Masoodi, T.; Singh, P.; Monaco, G.; Raynaud, C.; de Miranda, N.F.; Ferraro, L.; et al. An integrated tumor, immune and microbiome atlas of colon cancer. Nat. Med. 2023, 29, 1273–1286. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Grabrucker, S.; Marizzoni, M.; Silajdžić, E.; Lopizzo, N.; Mombelli, E.; Nicolas, S.; Dohm-Hansen, S.; Scassellati, C.; Moretti, D.V.; Rosa, M.; et al. Microbiota from Alzheimer’s patients induce deficits in cognition and hippocampal neurogenesis. Brain 2023, 146, 4916–4934. [Google Scholar] [CrossRef]
- Wemheuer, F.; Taylor, J.A.; Daniel, R.; Johnston, E.; Meinicke, P.; Thomas, T.; Wemheuer, B. Tax4Fun2: Prediction of habitat-specific functional profiles and functional redundancy based on 16S rRNA gene sequences. Environ. Microbiomes 2020, 15, 11. [Google Scholar] [CrossRef]
- Torii, T.; Kanemitsu, K.; Wada, T.; Itoh, S.; Kinugawa, K.; Hagiwara, A. Measurement of short-chain fatty acids in human faeces using high-performance liquid chromatography: Specimen stability. Ann. Clin. Biochem. 2010, 47, 447–452. [Google Scholar] [CrossRef]
- Creevy, K.E.; Grady, J.; Little, S.E.; Moore, G.E.; Strickler, B.G.; Thompson, S.; Webb, J.A. 2019 AAHA canine life stage guidelines. J. Am. Anim. Hosp. Assoc. 2019, 55, 267–290. [Google Scholar] [CrossRef]
- Harvey, N.D.; Craigon, P.J.; Shaw, S.C.; Blott, S.C.; England, G.C. Behavioural differences in dogs with atopic dermatitis suggest stress could be a significant problem associated with chronic pruritus. Animals 2019, 9, 813. [Google Scholar] [CrossRef]
- Linek, M.; Favrot, C. Impact of canine atopic dermatitis on the health-related quality of life of affected dogs and quality of life of their owners. Vet. Dermatol. 2010, 21, 456–462. [Google Scholar] [CrossRef]
- Udoye, C.C.; Rau, C.N.; Freye, S.M.; Almeida, L.N.; Vera-Cruz, S.; Othmer, K.; Korkmaz, R.Ü.; Clauder, A.; Lindemann, T.; Manz, R.A. B-cell receptor physical properties affect relative IgG1 and IgE responses in mouse egg allergy. Mucosal Immunol. 2022, 15, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Carballo, I.; Alonso-Sampedro, M.; Gonzalez-Conde, E.; Sanchez-Castro, J.; Vidal, C.; Gude, F.; Gonzalez-Quintela, A. Factors influencing total serum IgE in adults: The role of obesity and related metabolic disorders. Int. Arch. Allergy Immunol. 2021, 182, 220–228. [Google Scholar] [CrossRef]
- Chaudhary, S.K.; Singh, S.K.; Kumari, P.; Kanwal, S.; Soman, S.P.; Choudhury, S.; Garg, S.K. Alterations in circulating concentrations of IL-17, IL-31 and total IgE in dogs with atopic dermatitis. Vet. Dermatol. 2019, 30, 383-e114. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, E.; Park, Y.M.; Hong, S.J. Microbiome in the Gut-Skin Axis in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 354–362. [Google Scholar] [CrossRef]
- Thomsen, M.; Künstner, A.; Wohlers, I.; Olbrich, M.; Lenfers, T.; Osumi, T.; Shimazaki, Y.; Nishifuji, K.; Ibrahim, S.M.; Watson, A.; et al. A comprehensive analysis of gut and skin microbiota in canine atopic dermatitis in Shiba Inu dogs. Microbiome 2023, 11, 232. [Google Scholar] [CrossRef]
- Song, H.; Mun, S.H.; Han, D.W.; Kang, J.H.; An, J.U.; Hwang, C.Y.; Cho, S. Probiotics ameliorate atopic dermatitis by modulating the dysbiosis of the gut microbiota in dogs. BMC Microbiol. 2025, 25, 228. [Google Scholar] [CrossRef] [PubMed]
- Seite, S.; Bieber, T. Barrier function and microbiotic dysbiosis in atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2015, 8, 479–483. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.S. Microbiome of the Skin and Gut in Atopic Dermatitis (AD): Understanding the pathophysiology and finding novel management strategies. J. Clin. Med. 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Reddel, S.; Del Chierico, F.; Quagliariello, A.; Giancristoforo, S.; Vernocchi, P.; Russo, A.; Fiocchi, A.; Rossi, P.; Putignani, L.; El Hachem, M. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci. Rep. 2019, 9, 4996. [Google Scholar] [CrossRef] [PubMed]
- Sugita, K.; Shima, A.; Takahashi, K.; Ishihara, G.; Kawano, K.; Ohmori, K. Pilot evaluation of a single oral fecal microbiota transplantation for canine atopic dermatitis. Sci. Rep. 2023, 13, 8824. [Google Scholar] [CrossRef]
- Ye, S.; Yan, F.; Wang, H.; Mo, X.; Liu, J.; Zhang, Y.; Li, H.; Chen, D. Diversity analysis of gut microbiota between healthy controls and those with atopic dermatitis in a Chinese population. J. Dermatol. 2021, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, J.; Osumi, T.; Mizukami, K.; Fukuyama, T.; Shima, A.; Unno, A.; Takemura-Uchiyama, I.; Une, Y.; Murakami, H.; Sakaguchi, M. Characterization of the oral and faecal microbiota associated with atopic dermatitis in dogs selected from a purebred Shiba Inu colony. Lett. Appl. Microbiol. 2022, 75, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, J.; Tsui, J.C.C.; Wang, L.; Zhou, J.; Chan, U.K.; Lo, C.J.Y.; Siu, P.L.K.; Loo, S.K.F.; Tsui, S.K.W. Unique gut microbiome signatures among adult patients with moderate to severe atopic dermatitis in southern Chinese. Int. J. Mol. Sci. 2023, 24, 12856. [Google Scholar] [CrossRef]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef]
- Swaney, M.H.; Nelsen, A.; Sandstrom, S.; Kalan, L.R. Sweat and Sebum Preferences of the Human Skin Microbiota. Microbiol. Spectr. 2023, 11, e0418022. [Google Scholar] [CrossRef]
- Turpin, W.; Bedrani, L.; Espin-Garcia, O.; Xu, W.; Silverberg, M.S.; Smith, M.I.; Garay, J.A.R.; Lee, S.H.; Guttman, D.S.; Griffiths, A.; et al. Associations of NOD2 polymorphisms with Erysipelotrichaceae in stool of in healthy first degree relatives of Crohn’s disease subjects. BMC Med. Genet. 2020, 21, 204. [Google Scholar] [CrossRef]
- Duan, W.; Mehta, A.K.; Magalhaes, J.G.; Ziegler, S.F.; Dong, C.; Philpott, D.J.; Croft, M. Innate signals from Nod2 block respiratory tolerance and program TH2-driven allergic inflammation. J. Allergy Clin. Immunol. 2010, 126, 1284–1293. [Google Scholar] [CrossRef]
- Girardin, S.E.; Boneca, I.G.; Viala, J.; Chamaillard, M.; Labigne, A.; Thomas, G.; Philpott, D.J.; Sansonetti, P.J. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2003, 278, 8869–8872. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Maeda, S.; Hsu, L.C.; Liu, H.; Bankston, L.A.; Iimura, M.; Kagnoff, M.F.; Eckmann, L.; Karin, M. Nod2 mutation in Crohn’s disease potentiates NF-κB activity and IL-1ß processing. Science 2005, 307, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Corridoni, D.; Rodriguez-Palacios, A.; Di Stefano, G.; Di Martino, L.; Antonopoulos, D.A.; Chang, E.B.; Arseneau, K.O.; Pizarro, T.T.; Cominelli, F. Genetic deletion of the bacterial sensor NOD2 improves murine Crohn’s disease-like ileitis independent of functional dysbiosis. Mucosal Immunol. 2017, 10, 971–982. [Google Scholar] [CrossRef]
- Su, Y.J.; Luo, S.D.; Hsu, C.Y.; Kuo, H.C. Differences in gut microbiota between allergic rhinitis, atopic dermatitis, and skin urticaria: A pilot study. Medicine 2021, 100, e25091. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yao, Y.; Gao, P.; Bu, S. The therapeutic efficacy of curcumin vs. metformin in modulating the gut microbiota in NAFLD rats: A comparative study. Front. Microbiol. 2021, 11, 555293. [Google Scholar] [CrossRef]
- Wang, H.G.; Zhang, M.N.; Wen, X.; He, L.; Zhang, M.H.; Zhang, J.L.; Yang, X.Z. Cepharanthine ameliorates dextran sulphate sodium-induced colitis through modulating gut microbiota. Microb. Biotechnol. 2022, 15, 2208–2222. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef]
- Chang, C.S.; Liao, Y.C.; Huang, C.T.; Lin, C.M.; Cheung, C.H.Y.; Ruan, J.W.; Yu, W.H.; Tsai, Y.T.; Lin, I.J.; Huang, C.H.; et al. Identification of a gut microbiota member that ameliorates DSS-induced colitis in intestinal barrier enhanced Dusp6-deficient mice. Cell Rep. 2021, 37, 110016. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, S.P.; Kämpfer, P. The family Sphingomonadaceae. In The Prokaryotes: Alphaproteobacteria and Betaproteobacteria, 4th ed.; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin, Germany, 2014; Volume 8, pp. 641–707. [Google Scholar]
- Gamage, H.K.; Vuong, D.; Minns, S.A.; Chen, R.; Piggott, A.M.; Lacey, E.; Paulsen, I.T. The composition and natural variation of the skin microbiota in healthy Australian cattle. Res. Sq. 2022; preprint. [Google Scholar]
- Imamura, R.; Wang, Y.; Kinoshita, T.; Suzuki, M.; Noda, T.; Sagara, J.; Taniguchi, S.; Okamoto, H.; Suda, T. Anti-inflammatory activity of PYNOD and its mechanism in humans and mice. J. Immunol. 2010, 184, 5874–5884. [Google Scholar] [CrossRef]
- Lautz, K.; Damm, A.; Menning, M.; Wenger, J.; Adam, A.C.; Zigrino, P.; Kremmer, E.; Kufer, T.A. NLRP 10 enhances Shigella-induced pro-inflammatory responses. Cell. Microbiol. 2012, 14, 1568–1583. [Google Scholar] [CrossRef]
- Mirza, N.; Sowa, A.S.; Lautz, K.; Kufer, T.A. NLRP10 affects the stability of abin-1 to control inflammatory responses. J. Immunol. 2019, 202, 218–227. [Google Scholar] [CrossRef]
- Fritz, J.H.; Le Bourhis, L.; Sellge, G.; Magalhaes, J.G.; Fsihi, H.; Kufer, T.A.; Collins, C.; Viala, J.; Ferrero, R.L.; Girardin, S.E.; et al. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity 2007, 26, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Higueras, C.; Rey, A.I.; Escudero, R.; Díaz-Regañón, D.; Rodríguez-Franco, F.; García-Sancho, M.; Agulla, B.; Sainz, A. Short-chain and total fatty acid profile of faeces or plasma as predictors of food-responsive enteropathy in dogs: A preliminary study. Animals 2021, 12, 89. [Google Scholar] [CrossRef]
- Trompette, A.; Pernot, J.; Perdijk, O.; Alqahtani, R.A.A.; Santo Domingo, J.; Camacho-Muñoz, D.; Wong, N.C.; Kendall, A.C.; Wiederkehr, A.; Nicod, L.P.; et al. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal Immunol. 2022, 15, 908–926. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, M.; Kotani, J.; Usami, M. Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 2010, 26, 653–661. [Google Scholar] [CrossRef]
- Carrion, S.L.; Sutter, C.H.; Sutter, T.R. Combined treatment with sodium butyrate and PD153035 enhances keratinocyte differentiation. Exp. Dermatol. 2014, 23, 211–214. [Google Scholar] [CrossRef]
- Wang, Y.; Kao, M.S.; Yu, J.; Huang, S.; Marito, S.; Gallo, R.L.; Huang, C.M. A precision microbiome approach using sucrose for selective augmentation of Staphylococcus epidermidis fermentation against Propionibacterium acnes. Int. J. Mol. Sci. 2016, 17, 1870. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, L.A.; Travassos, L.H. The Interplay between NLRs and Autophagy in Immunity and Inflammation. Front. Immunol. 2013, 4, 361. [Google Scholar] [CrossRef] [PubMed]
- Platnich, J.M.; Muruve, D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef]
- Negishi, H.; Yanai, H.; Nakajima, A.; Koshiba, R.; Atarashi, K.; Matsuda, A.; Matsuki, K.; Miki, S.; Doi, T.; Aderem, A.; et al. Cross-interference of RLR and TLR signaling pathways modulates antibacterial T cell responses. Nat. Immunol. 2012, 13, 659–666. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Elinav, E. Probiotics in the next-generation sequencing era. Gut Microbes 2020, 11, 77–93. [Google Scholar] [CrossRef]
- Evans, S.R. Clinical trial structures. J. Exp. Stroke Transl. Med. 2010, 3, 8–18. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-W.; Yeh, T.-C.; Hsieh, J.-C.; Tsai, C.-W.; Lee, Y.-J.; Chen, M.-J. Evaluating the Adjuvant Therapeutic Effects of Probiotic Strains Lactococcus cremoris and Lacticaseibacillus paracasei on Canine Atopic Dermatitis and Their Impact on the Gut and Skin Microbiome. Animals 2025, 15, 3098. https://doi.org/10.3390/ani15213098
Huang H-W, Yeh T-C, Hsieh J-C, Tsai C-W, Lee Y-J, Chen M-J. Evaluating the Adjuvant Therapeutic Effects of Probiotic Strains Lactococcus cremoris and Lacticaseibacillus paracasei on Canine Atopic Dermatitis and Their Impact on the Gut and Skin Microbiome. Animals. 2025; 15(21):3098. https://doi.org/10.3390/ani15213098
Chicago/Turabian StyleHuang, Hsiao-Wen, Ting-Chen Yeh, Jui-Chun Hsieh, Ching-Wen Tsai, Ya-Jane Lee, and Ming-Ju Chen. 2025. "Evaluating the Adjuvant Therapeutic Effects of Probiotic Strains Lactococcus cremoris and Lacticaseibacillus paracasei on Canine Atopic Dermatitis and Their Impact on the Gut and Skin Microbiome" Animals 15, no. 21: 3098. https://doi.org/10.3390/ani15213098
APA StyleHuang, H.-W., Yeh, T.-C., Hsieh, J.-C., Tsai, C.-W., Lee, Y.-J., & Chen, M.-J. (2025). Evaluating the Adjuvant Therapeutic Effects of Probiotic Strains Lactococcus cremoris and Lacticaseibacillus paracasei on Canine Atopic Dermatitis and Their Impact on the Gut and Skin Microbiome. Animals, 15(21), 3098. https://doi.org/10.3390/ani15213098






