Comparative Chromosome Painting Clarifies the Intraspecific Chromosomal Variation in Two Ctenomys Species (Rodentia: Ctenomyidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Chromosomal Preparations and Karyotype
2.3. Fluorescence In Situ Hybridization (FISH)
3. Results
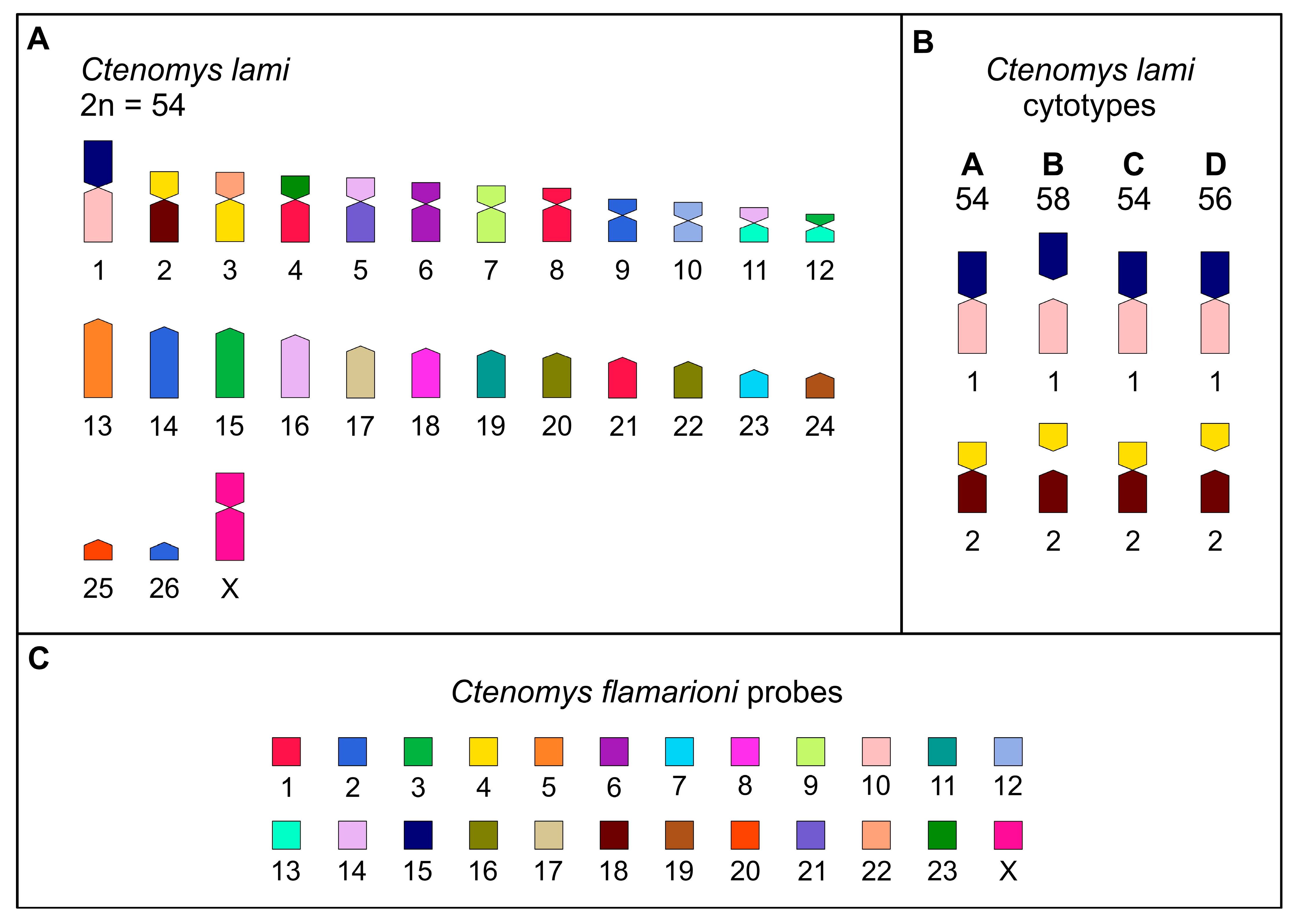
3.1. Karyotypes
3.2. Comparative Chromosome Painting
4. Discussion
4.1. Comparative Chromosome Painting
4.2. Comparative C. minutus x C. lami
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakloushinskaya, I.; Lyapunova, E.A.; Saidov, A.S.; Romanenko, S.A.; O’Brien, P.C.M.; Serdyukova, N.A.; Ferguson-Smith, M.A.; Matveevsky, S.N.; Bogdanov, A.S. Rapid Chromosomal Evolution in Enigmatic Mammal with XX in Both Sexes, the Alay Mole Vole Ellobius alaicus Vorontsov et al., 1969 (Mammalia, Rodentia). Comp. Cytogenet. 2019, 13, 147–177. [Google Scholar] [CrossRef] [PubMed]
- Beklemisheva, V.R.; Romanenko, S.A.; Biltueva, L.S.; Trifonov, V.A.; Vorobieva, N.V.; Serdukova, N.A.; Rubtsova, N.V.; Brandler, O.V.; O’Brien, P.C.M.; Yang, F.; et al. Reconstruction of Karyotype Evolution in Core Glires. I. The Genome Homology Revealed by Comparative Chromosome Painting. Chromosome Res. 2011, 19, 549–565. [Google Scholar] [CrossRef]
- Graphodatsky, A.S.; Yang, F.; Dobigny, G.; Romanenko, S.A.; Biltueva, L.S.; Perelman, P.L.; Beklemisheva, V.R.; Alkalaeva, E.Z.; Serdukova, N.A.; Ferguson-Smith, M.A.; et al. Tracking Genome Organization in Rodents by Zoo-FISH. Chromosome Res. 2008, 16, 261–274. [Google Scholar] [CrossRef]
- Sitnikova, N.A.; Romanenko, S.A.; O’Brien, P.C.M.; Perelman, P.L.; Fu, B.; Rubtsova, N.V.; Serdukova, N.A.; Golenishchev, F.N.; Trifonov, V.A.; Ferguson-Smith, M.A.; et al. Chromosomal Evolution of Arvicolinae (Cricetidae, Rodentia). I. The Genome Homology of Tundra Vole, Field Vole, Mouse and Golden Hamster Revealed by Comparative Chromosome Painting. Chromosome Res. 2007, 15, 447–456. [Google Scholar] [CrossRef]
- Lemskaya, N.A.; Romanenko, S.A.; Golenishchev, F.N.; Rubtsova, N.V.; Sablina, O.V.; Serdukova, N.A.; O’Brien, P.C.M.; Fu, B.; Yiğit, N.; Ferguson-Smith, M.A.; et al. Chromosomal Evolution of Arvicolinae (Cricetidae, Rodentia). III. Karyotype Relationships of Ten Microtus Species. Chromosome Res. 2010, 18, 459–471. [Google Scholar] [CrossRef]
- Pavlova, S.V.; Lebedev, V.S.; Yakushov, V.D.; Zhu, Y.; Fang, Y.; Sun, Y.-H.; Sheftel, B.I. High Diversity of Small Insectivorous Mammals on Qinghai–Tibet Plateau and First Description of Karyotype for Four Endemics of China. Sci. Rep. 2021, 11, 24496. [Google Scholar] [CrossRef]
- Rieseberg, L.H. Chromosomal Rearrangements and Speciation. Trends Ecol. Evol. 2001, 16, 351–358. [Google Scholar] [CrossRef]
- Noor, M.A.F.; Grams, K.L.; Bertucci, L.A.; Reiland, J. Chromosomal Inversions and the Reproductive Isolation of Species. Proc. Natl. Acad. Sci. USA 2001, 98, 12084–12088. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Barton, N.H. Chromosomal Speciation and Molecular Divergence--Accelerated Evolution in Rearranged Chromosomes. Science 2003, 300, 321–324. [Google Scholar] [CrossRef]
- Eroğlu, H.E. The Comparison of the Felidae Species with Karyotype Symmetry/Asymmetry Index (S/AI). Punjab Univ. J. Zool. 2017, 32, 229–235. [Google Scholar]
- Reig, O.A.; Bush, C.; Ortells, M.O.; Contreras, J.R. An Overview of Evolution, Systematic, Population Biology, Cytogenetics, Molecular Biology and Speciation in Ctenomys. Prog. Clin. Biol. Res. 1990, 335, 71–96. [Google Scholar]
- Tomasco, I.H.; Lessa, E.P. Phylogeography of the Tuco-Tuco Ctenomys pearsoni: mtDNa Variation and Its Implication for Chromosomal Differentiation. In The Quintessential Naturalist: Honoring the Life and Legacy of Oliver P. Pearson; Kelt, D., Kaspin, D., Eds.; California Scholarship Online: Oakland, CA, USA, 2007. [Google Scholar]
- De Freitas, T.R.O. Ctenomys lami: The Highest Chromosome Variability in Ctenomys (Rodentia, Ctenomyidae) Due to a Centric Fusion/Fission and Pericentric Inversion System. Acta Theriol. 2007, 52, 171–180. [Google Scholar] [CrossRef]
- Lopes, C.M.; Ximenes, S.S.F.; Gava, A.; De Freitas, T.R.O. The Role of Chromosomal Rearrangements and Geographical Barriers in the Divergence of Lineages in a South American Subterranean Rodent (Rodentia: Ctenomyidae: Ctenomys minutus). Heredity 2013, 111, 293–305. [Google Scholar] [CrossRef]
- Teta, P.; D’Elía, G. Uncovering the Species Diversity of Subterranean Rodents at the End of the World: Three New Species of Patagonian Tuco-Tucos (Rodentia, Hystricomorpha, Ctenomys). PeerJ 2020, 8, e9259. [Google Scholar] [CrossRef] [PubMed]
- Parada, A.; D’Elía, G.; Bidau, C.J.; Lessa, E.P. Species Groups and the Evolutionary Diversification of Tuco-Tucos, Genus Ctenomys (Rodentia: Ctenomyidae). J. Mammal. 2011, 92, 671–682. [Google Scholar] [CrossRef]
- Buschiazzo, L.M.; Caraballo, D.A.; Labaroni, C.A.; Teta, P.; Rossi, M.S.; Bidau, C.J.; Lanzone, C. Comprehensive Cytogenetic Analysis of the Most Chromosomally Variable Mammalian Genus from South America: Ctenomys (Rodentia: Caviomorpha: Ctenomyidae). Mamm. Biol. 2022, 102, 1963–1979. [Google Scholar] [CrossRef]
- Freitas, T.R.O. Tuco-Tucos (Rodentia, Octodontidae) in Southern Brazil: Ctenomys lami Spec. Nov. Separated from C. minutus Nehring 1887. Stud. Neotrop. Fauna Environ. 2001, 36, 1–8. [Google Scholar] [CrossRef]
- Buschiazzo, L.M.; Caraballo, D.A.; Cálcena, E.; Longarzo, M.L.; Labaroni, C.A.; Ferro, J.M.; Rossi, M.S.; Bolzán, A.D.; Lanzone, C. Integrative Analysis of Chromosome Banding, Telomere Localization and Molecular Genetics in the Highly Variable Ctenomys of the Corrientes Group (Rodentia; Ctenomyidae). Genetica 2018, 146, 403–414. [Google Scholar] [CrossRef]
- Freygang, C.C.; Marinho, J.R.; De Freitas, T.R.O. New Karyotypes and Some Considerations about the Chromosomal Diversification of Ctenomys minutus (Rodentia: Ctenomyidae) on the Coastal Plain of the Brazilian State of Rio Grande Do Sul. Genetica 2004, 121, 125–132. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, T.R.O. Cytogenetics Status of Four Ctenomys Species in the South of Brazil. Genetica 2006, 126, 227–235. [Google Scholar] [CrossRef]
- Freitas, T.R.O.D. Chromosome Polymorphism in Ctenomys minutus (Rodentia-Octodontidae). Braz. J. Genet. 1997, 20, 1–7. [Google Scholar] [CrossRef]
- Gava, A.; Freitas, T.R.O. Inter and Intra-Specific Hybridization in Tuco-Tucos (Ctenomys) from Brazilian Coastal Plains (Rodentia: Ctenomyidae). Genetica 2003, 119, 11–17. [Google Scholar] [CrossRef]
- Fornel, R.; Cordeiro-Estrela, P.; De Freitas, T.R.O. Skull Shape and Size Variation in Ctenomys minutus (Rodentia: Ctenomyidae) in Geographical, Chromosomal Polymorphism, and Environmental Contexts. Biol. J. Linn. Soc. 2010, 101, 705–720. [Google Scholar] [CrossRef]
- Weschenfelder, J.; Baitelli, R.; Corrêa, I.C.S.; Bortolin, E.C.; Dos Santos, C.B. Quaternary Incised Valleys in Southern Brazil Coastal Zone. J. S. Am. Earth Sci. 2014, 55, 83–93. [Google Scholar] [CrossRef]
- De Freitas, T.R.O. Speciation Within the Genus Ctenomys: An Attempt to Find Models. In Tuco-Tucos; Freitas, T.R.O.D., Gonçalves, G.L., Maestri, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 43–66. ISBN 978-3-030-61678-6. [Google Scholar]
- Lopes, C.M.; De Freitas, T.R.O. Human Impact in Naturally Patched Small Populations: Genetic Structure and Conservation of the Burrowing Rodent, Tuco-Tuco (Ctenomys lami). J. Hered. 2012, 103, 672–681. [Google Scholar] [CrossRef]
- Romanenko, S.A.; Perelman, P.L.; Trifonov, V.A.; Graphodatsky, A.S. Chromosomal Evolution in Rodentia. Heredity 2012, 108, 4–16. [Google Scholar] [CrossRef]
- Graphodatsky, A.S.; Perelman, P.L.; O’Brien, S.J. (Eds.) Atlas of Mammalian Chromosomes, 1st ed.; Wiley: Hoboken, NJ, USA, 2020; ISBN 978-1-119-41803-0. [Google Scholar]
- Kubiak, B.B.; Kretschmer, R.; Leipnitz, L.T.; Maestri, R.; De Almeida, T.S.; Borges, L.R.; Galiano, D.; Pereira, J.C.; De Oliveira, E.H.C.; Ferguson-Smith, M.A.; et al. Hybridization between Subterranean Tuco-Tucos (Rodentia, Ctenomyidae) with Contrasting Phylogenetic Positions. Sci. Rep. 2020, 10, 1502. [Google Scholar] [CrossRef]
- Sikes, R.S.; The Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the Use of Wild Mammals in Research and Education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef]
- Verma, R.; Babu, A. Human Chromosomes: Principles & Techniques; McGraw-Hill, Inc.: New York, NY, USA, 1996; Volume 43, p. 134. [Google Scholar]
- Reig, O.A.; Kiblisky, P. Chromosome Multiformity in the Genus Ctenomys (Rodentia, Octodontidae): A Progress Report. Chromosoma 1969, 28, 211–244. [Google Scholar] [CrossRef] [PubMed]
- Reig, O.A. Karyotypic Repatterning as One Triggering Factor in Cases of Explosive Speciation. In Evolutionary Biology of Transient Unstable Populations; Fontdevila, A., Ed.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 246–289. ISBN 978-3-642-74527-0. [Google Scholar]
- Ortells, M. Phylogenetic Analysis of G-Banded Karyotypes among the South American Subterranean Rodents of the Genus Ctenomys (Caviomorpha: Octodontidae), with Special Reference to Chromosomal Evolution and Speciation. Biol. J. Linn. Soc. 1995, 54, 43–70. [Google Scholar] [CrossRef]
- Slamovits, C.H.; Cook, J.A.; Lessa, E.P.; Susana Rossi, M. Recurrent Amplifications and Deletions of Satellite DNA Accompanied Chromosomal Diversification in South American Tuco-Tucos (Genus Ctenomys, Rodentia: Octodontidae): A Phylogenetic Approach. Mol. Biol. Evol. 2001, 18, 1708–1719. [Google Scholar] [CrossRef] [PubMed]
- Bidau, C.J.; Martí, D.A.; Giménez, M.D. Two Exceptional South American Models for the Study of Chromosomal Evolution: The Tucura Dichroplus Pratensis and the Tucotucos of the Genus Ctenomys. Hist. Nat. 2003, II, 53–72. [Google Scholar]
- Romanenko, S.A.; Sitnikova, N.A.; Serdukova, N.A.; Perelman, P.L.; Rubtsova, N.V.; Bakloushinskaya, I.Y.; Lyapunova, E.A.; Just, W.; Ferguson-Smith, M.A.; Yang, F.; et al. Chromosomal Evolution of Arvicolinae (Cricetidae, Rodentia). II. The Genome Homology of Two Mole Voles (Genus ellobius), the Field Vole and Golden Hamster Revealed by Comparative Chromosome Painting. Chromosome Res. 2007, 15, 891–897. [Google Scholar] [CrossRef]
- Romanenko, S.A.; Lyapunova, E.A.; Saidov, A.S.; O’Brien, P.C.M.; Serdyukova, N.A.; Ferguson-Smith, M.A.; Graphodatsky, A.S.; Bakloushinskaya, I. Chromosome Translocations as a Driver of Diversification in Mole Voles Ellobius (Rodentia, Mammalia). Int. J. Mol. Sci. 2019, 20, 4466. [Google Scholar] [CrossRef]
- Oliveira Da Silva, W.; Malcher, S.M.; Pereira, A.L.; Pieczarka, J.C.; Ferguson-Smith, M.A.; O’Brien, P.C.M.; Mendes-Oliveira, A.C.; Geise, L.; Nagamachi, C.Y. Chromosomal Signatures Corroborate the Phylogenetic Relationships within Akodontini (Rodentia, Sigmodontinae). Int. J. Mol. Sci. 2020, 21, 2415. [Google Scholar] [CrossRef]
- Trifonov, V.A.; Kosyakova, N.; Romanenko, S.A.; Stanyon, R.; Graphodatsky, A.S.; Liehr, T. New Insights into the Karyotypic Evolution in Muroid Rodents Revealed by Multicolor Banding Applying Murine Probes. Chromosome Res. 2010, 18, 265–275. [Google Scholar] [CrossRef]
- Rocchi, M.; Archidiacono, N.; Schempp, W.; Capozzi, O.; Stanyon, R. Centromere Repositioning in Mammals. Heredity 2012, 108, 59–67. [Google Scholar] [CrossRef]
- Romanenko, S.; Serdyukova, N.; Perelman, P.; Pavlova, S.; Bulatova, N.; Golenishchev, F.; Stanyon, R.; Graphodatsky, A. Intrachromosomal Rearrangements in Rodents from the Perspective of Comparative Region-Specific Painting. Genes 2017, 8, 215. [Google Scholar] [CrossRef]
- Torgasheva, A.A.; Basheva, E.A.; Gomez Fernandez, M.J.; Mirol, P.; Borodin, P.M. Chromosomes and Speciation in Tuco-Tuco (Ctenomys, Hystricognathi, Rodentia). Vestn. VOGiS 2016, 20, 408–415. [Google Scholar] [CrossRef]
- Baker, R.J.; Bickham, J.W. Karyotypic Evolution in Bats: Evidence of Extensive and Conservative Chromosomal Evolution in Closely Related Taxa. Syst. Biol. 1980, 29, 239–253. [Google Scholar] [CrossRef]
- Le Scouarnec, S.; Gribble, S.M. Characterising Chromosome Rearrangements: Recent Technical Advances in Molecular Cytogenetics. Heredity 2012, 108, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Warren, W.C.; Jasinska, A.J.; García-Pérez, R.; Svardal, H.; Tomlinson, C.; Rocchi, M.; Archidiacono, N.; Capozzi, O.; Minx, P.; Montague, M.J.; et al. The Genome of the Vervet (Chlorocebus aethiops sabaeus). Genome Res. 2015, 25, 1921–1933. [Google Scholar] [CrossRef] [PubMed]




| Species | Blocks/Cytotypes | Individuals/Sex | Locality | Geographic Coordinate |
|---|---|---|---|---|
| C. lami | A (54) | 2 ♀ | Parque do Itapuã (RS) | 30°20′47.4″ S 51°01′35.6″ W |
| B (58) | 2 ♀ | Passo do Vigário (RS) | 30°13′13.1″ S 50°59′43.6″ W | |
| C (54) | 2 ♀ | Lombas (RS) | 30°01′32.4″ S 50°39′16.6″ W | |
| D (56b) | 2 ♀ | Chico Lomã (RS) | 29°56′49.8″ S 50°35′48.7″ W | |
| C. minutus | I (50a) | 1 ♀ | Jaguaruna (SC) | 28°41′53.02″ S 49°01′33.86″ W |
| II (48a) | 2 ♀ | Praia do barro (RS) | 29°42′14.86″ S 49°58′51.86″ W | |
| III (46a) | 2 ♂♀ | Bacupari (RS) | 30°28′41.01″ S 50°27′13.92″ W | |
| IV (42) | 2 ♀ | Mostardas (RS) | 31°06′ 17″ S 50°55′20″ W | |
| V (46b) | 2 ♂♀ | Tavares (RS) | 31°17′ 58,9″ S 51°05′47,6″ W | |
| VI (48b) | 2 ♀ | Bojuru (RS) | 31°39′10.7″ S 51°26′14.8″ W | |
| VII (50b) | 2 ♀ | São José do Norte (RS) | 32°04′34.47″ S 52°02′31.47″ W |
| C. flamarioni (2n = 48) | C. minutus I (2n = 50a) | C. lami A (2n = 54) |
|---|---|---|
| 1 | 6q, 10, 22 | 4q, 8, 21 |
| 2 | 11, 15, 24 | 9, 14, 26 |
| 3 | 14p, 18 | 12p, 15 |
| 4 | 5p, 19 | 2p, 3q |
| 5 | 1q | 13 |
| 6 | 7 | 6 |
| 7 | 1p | 23 |
| 8 | 3q | 18 |
| 9 | 9 | 7 |
| 10 | 17 | 1q |
| 11 | 4q | 19 |
| 12 | 12 | 10 |
| 13 | 13q, 14q | 11q, 12q |
| 14 | 2q, 8p, 13p | 5p, 11p, 16 |
| 15 | 20 | 1p |
| 16 | 4p, 16 | 20, 22 |
| 17 | 21 | 17 |
| 18 | 5q | 2q |
| 19 | 2p | 24 |
| 20 | 3p | 25 |
| 21 | 8q | 5q |
| 22 | 23 | 3p |
| 23 | 6p | 4p |
| X | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, T.D.; Bertocchi, N.Á.; Pozzobon, L.C.; Furo, I.d.O.; de Oliveira, E.H.C.; Pereira, J.C.; Ferguson-Smith, M.A.; Kretschmer, R.; de Freitas, T.R.O. Comparative Chromosome Painting Clarifies the Intraspecific Chromosomal Variation in Two Ctenomys Species (Rodentia: Ctenomyidae). Animals 2025, 15, 3091. https://doi.org/10.3390/ani15213091
de Oliveira TD, Bertocchi NÁ, Pozzobon LC, Furo IdO, de Oliveira EHC, Pereira JC, Ferguson-Smith MA, Kretschmer R, de Freitas TRO. Comparative Chromosome Painting Clarifies the Intraspecific Chromosomal Variation in Two Ctenomys Species (Rodentia: Ctenomyidae). Animals. 2025; 15(21):3091. https://doi.org/10.3390/ani15213091
Chicago/Turabian Stylede Oliveira, Thays Duarte, Natasha Ávila Bertocchi, Luciano Cesar Pozzobon, Ivanete de Oliveira Furo, Edivaldo Herculano Corrêa de Oliveira, Jorge C. Pereira, Malcolm A. Ferguson-Smith, Rafael Kretschmer, and Thales R. O. de Freitas. 2025. "Comparative Chromosome Painting Clarifies the Intraspecific Chromosomal Variation in Two Ctenomys Species (Rodentia: Ctenomyidae)" Animals 15, no. 21: 3091. https://doi.org/10.3390/ani15213091
APA Stylede Oliveira, T. D., Bertocchi, N. Á., Pozzobon, L. C., Furo, I. d. O., de Oliveira, E. H. C., Pereira, J. C., Ferguson-Smith, M. A., Kretschmer, R., & de Freitas, T. R. O. (2025). Comparative Chromosome Painting Clarifies the Intraspecific Chromosomal Variation in Two Ctenomys Species (Rodentia: Ctenomyidae). Animals, 15(21), 3091. https://doi.org/10.3390/ani15213091










