Use of Contrast-Enhanced Ultrasound in Suspected Traumatic or Spontaneous Renal Injury in Cats: A Case Series
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
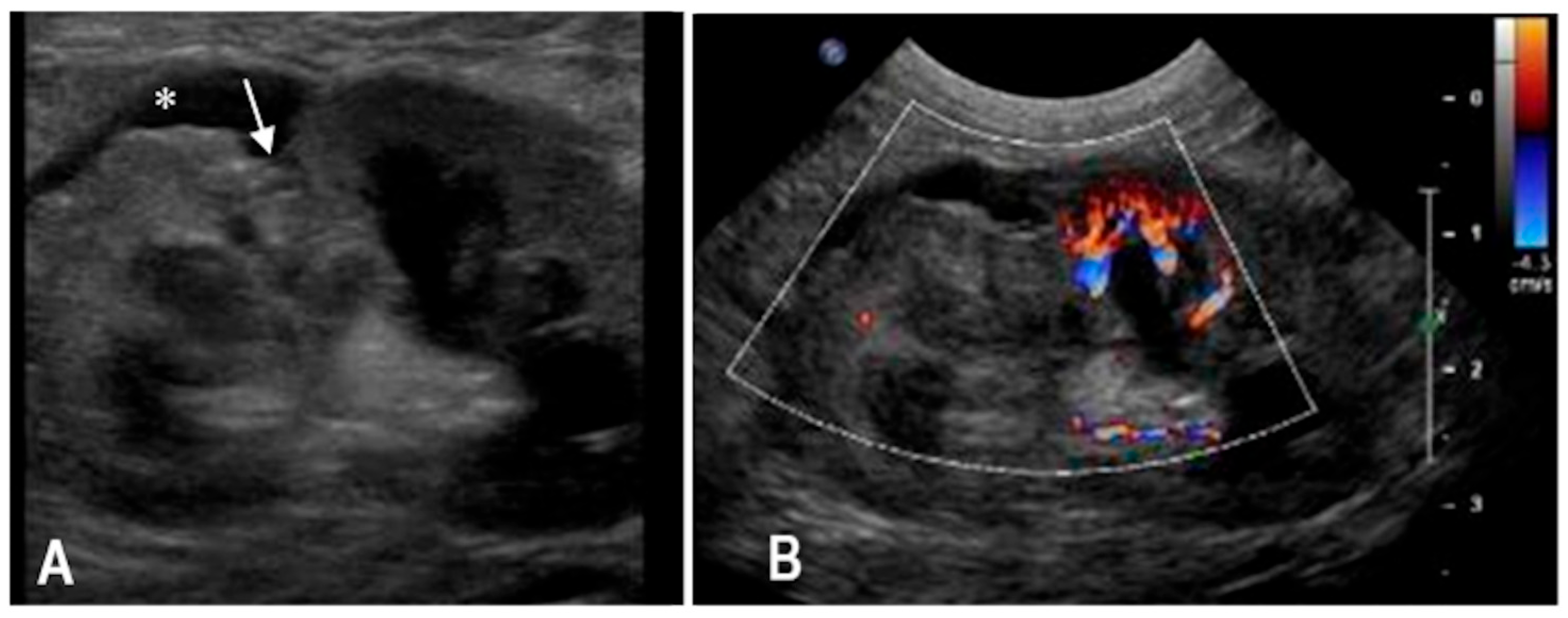
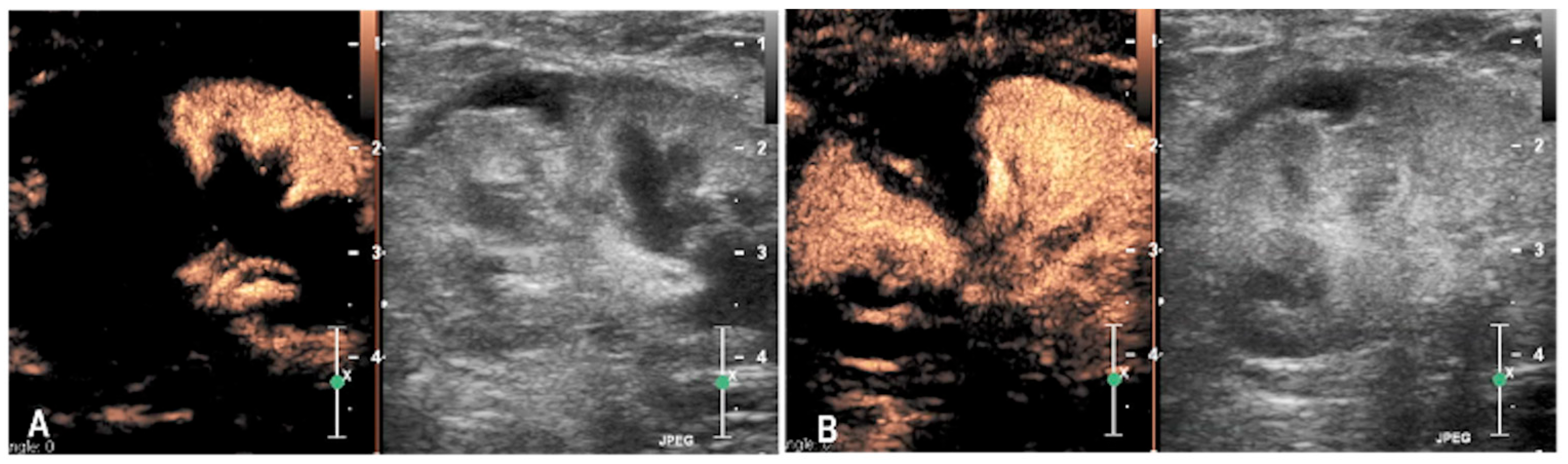
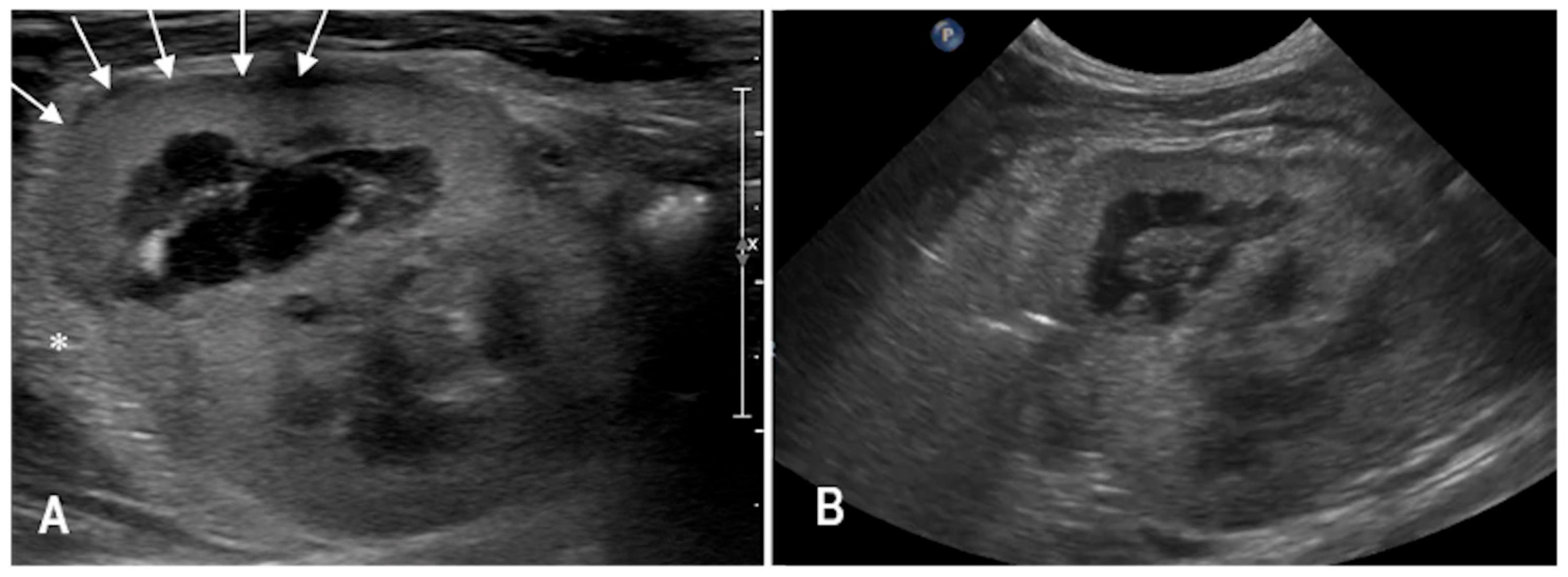
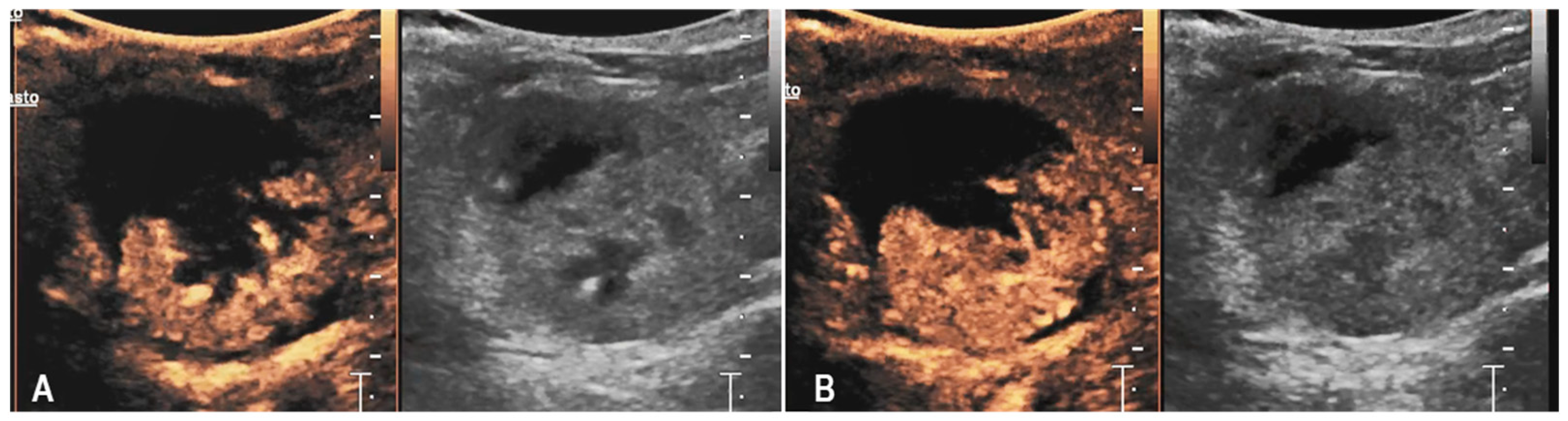
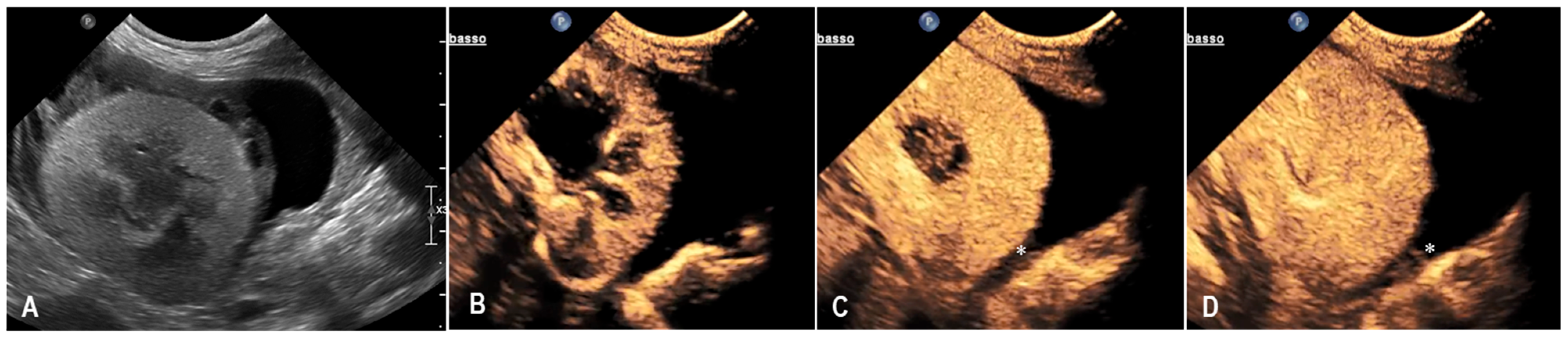
3.1. Case 1
3.2. Case 2
3.3. Case 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFAST | Abdominal Focused Assessment with Sonography for Trauma |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| aPTT | Activated partial thromboplastin time |
| B-mode | Brightness mode ultrasonography |
| CEUS | Contrast-enhanced ultrasonography |
| CT | Computed tomography |
| HCM | Hypertrophic cardiomyopathy |
| PCV | Packed cell volume |
| PT | Prothrombin time |
| RI | Reference interval |
| RBCs | Red blood cells |
References
- Vnuk, D.; Pirkić, B.; Matičić, D.; Radišić, B.; Stejskal, M.; Babić, T.; Kreszinger, M.; Lemo, N. Feline high-rise syndrome: 119 cases (1998–2001). J. Feline Med. Surg. 2004, 6, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Borse, N.; Gilchrist, J.; Dellinger, A.M.; Rudd, R.A.; Ballesteros, M.F.; Sleet, D.A. CDC Childhood Injury Report: Patterns of Unintentional Injuries Among 0–19 Year Olds in the United States, 2000–2006; Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA, USA, 2008. [Google Scholar]
- Holmes, J.F.; Sokolove, P.E.; Brant, W.E.; Palchak, M.J.; Vance, C.W.; Owings, J.T.; Kuppermann, N. Identification of children with intra-abdominal injuries after blunt trauma. Ann. Emerg. Med. 2002, 39, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Culp, W.T.N.; Silverstein, D. Thoracic and abdominal trauma. In Small Animal Critical Care Medicine, 2nd ed.; Silverstein, D., Hopper, K., Eds.; Elsevier Health Sciences: St. Louis, MO, USA, 2014; pp. 728–733. [Google Scholar]
- Lisciandro, G.R.; Lagutchik, M.S.; Mann, K.A.; Fosgate, G.T.; Tiller, E.G.; Cabano, N.R.; Bauer, L.D.; Book, B.P.; Howard, P.K. Evaluation of an abdominal fluid scoring system determined using abdominal focused assessment with sonography for trauma in 101 dogs with motor vehicle trauma. J. Vet. Emerg. Crit. Care 2009, 19, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Walters, A.M.; O’Brien, M.A.; Selmic, L.E.; Hartman, S.; McMichael, M.; O’Brien, R.T. Evaluation of the agreement between focused assessment with sonography for trauma (AFAST/TFAST) and computed tomography in dogs and cats with recent trauma. J. Vet. Emerg. Crit. Care 2018, 28, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Paltiel, H.J.; Barth, R.A.; Bruno, C.; Chen, A.E.; Deganello, A.; Harkanyi, Z.; Henry, M.K.; Ključevšek, D.; Back, S.J. Contrast-enhanced ultrasound of blunt abdominal trauma in children. Pediatr. Radiol. 2021, 51, 2253–2269. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.; Miele, V.; Scaglione, M.; Pinto, A. The use of contrast-enhanced ultrasound in blunt abdominal trauma: Advantages and limitations. Acta Radiol. 2014, 55, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Deftereos, S.P.; Foutzitzi, S.; Skarentzos, K.; Aggelidou, M.; Oikonomou, P.; Kambouri, K. Role of contrast-enhanced ultrasound (CEUS) in the pediatric population with blunt abdominal trauma: A prospective study from a single-center experience. Maedica 2022, 17, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.J.; Hesta, M.; Stock, E.; Bogaerts, E.; Broeckx, B.J.; Saunders, J.H.; Vanderperren, K. Renal perfusion parameters measured by contrast-enhanced ultrasound in healthy dogs demonstrate a wide range of variability in the long term. Vet. Radiol. Ultrasound 2019, 60, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, T.; Lippi, I.; Rota, A.; Citi, S. Contrast-enhanced ultrasound of renal perfusion in dogs with acute kidney injury. J. Small Anim. Pract. 2019, 60, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Stock, E.; Paepe, D.; Daminet, S.; Vandermeulen, E.; Duchateau, L.; Saunders, J.H.; Vanderperren, K. Contrast-enhanced ultrasound examination for the assessment of renal perfusion in cats with chronic kidney disease. J. Vet. Intern. Med. 2018, 32, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Hall, G.B.; Schwarz, T.; Liuti, T.; Salgado, J.P.; Ferreira, M.F.; Willems, A.L. Arterial malformations leading to bilateral spontaneous renal hemorrhage in a dog. J. Vet. Emerg. Crit. Care 2022, 32, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Lv, F.; Luo, Y.; Song, Q.; Xu, Q.; Su, Y.; Tang, Y.; Tang, J. Contrast-enhanced ultrasound for evaluation of renal trauma during acute hemorrhagic shock: A canine model. J. Med. Ultrason. 2015, 42, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Gerboni, G.M.; Capra, G.; Ferro, S.; Bellino, C.; Perego, M.; Zanet, S.; D’Angelo, A.; Gianella, P. The use of contrast-enhanced ultrasonography for the detection of active renal hemorrhage in a dog with spontaneous kidney rupture resulting in hemoperitoneum. J. Vet. Emerg. Crit. Care 2015, 25, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Scarabelli, S.; Cripps, P.; Rioja, E.; Alderson, B. Adverse reactions following administration of contrast media for diagnostic imaging in anaesthetized dogs and cats: A retrospective study. Vet. Anaesth. Analg. 2016, 43, 502–510. [Google Scholar] [CrossRef] [PubMed]
| Case | Age/Sex/Breed | History/Trauma | Main Clinical Findings | Clinical Pathology Findings (RI) |
|---|---|---|---|---|
| 1 | 5 y/F/Domestic shorthair | Fall from 2nd floor | Tachypnea pale mucosae pain left kidney palpation | HCT 17% (32–48) WBC 4.47 × 103/μL (4.8–14.93) Creatine 2.62 mg/dL (0.8–1.8) Urea 127 mg/dL (30–65) ALT 490 U/L (20–72) AST 607 U/L (9–40) Alb 1.81 g/dL (2.6–4.0) RBCs in urine sediment |
| 2 | 3 y/F spayed/Domestic shorthair | Suspected blunt trauma Diaphragmatic rupture | Lethargy paradoxical breathing pink urine | HCT 21% (32–48) Urea 90 mg/dL (30–65) ALT 591 U/L (20–72) AST 418 U/L (9–40) RBCs in urine sediment |
| 3 | 10 y/M neutered/Chartreux | No trauma Addison’s disease HCM | Lethargy | PCV 11% (32–48) Creat 3.42 mg/dL (0.8–1.8) Urea 124 mg/dL (30–65) ALT 87 U/L (20–72) AST 114 U/L (9–40) PT 15.8 s (9–15) aPTT 25.3 s (9–20) |
| Summary (median/range) | Age: 5 y (3–10) Sex: 2F/1M | Lesion types: 2 traumatic 1 spontaneous; | HCT/PCV: 17% (11–21) Creatinine: 3.02 mg/dL (2.62–3.42) Urea: 124 mg/dL (90–127) ALT: 490 U/L (87–591) AST: 418 U/L (114–607) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perfetti, S.; Gai, C.; Linta, N.; Tamburini, G.; Monari, E.; Ciuffoli, E.; Diana, A. Use of Contrast-Enhanced Ultrasound in Suspected Traumatic or Spontaneous Renal Injury in Cats: A Case Series. Animals 2025, 15, 3089. https://doi.org/10.3390/ani15213089
Perfetti S, Gai C, Linta N, Tamburini G, Monari E, Ciuffoli E, Diana A. Use of Contrast-Enhanced Ultrasound in Suspected Traumatic or Spontaneous Renal Injury in Cats: A Case Series. Animals. 2025; 15(21):3089. https://doi.org/10.3390/ani15213089
Chicago/Turabian StylePerfetti, Simone, Carolina Gai, Nikolina Linta, Giacomo Tamburini, Erika Monari, Elena Ciuffoli, and Alessia Diana. 2025. "Use of Contrast-Enhanced Ultrasound in Suspected Traumatic or Spontaneous Renal Injury in Cats: A Case Series" Animals 15, no. 21: 3089. https://doi.org/10.3390/ani15213089
APA StylePerfetti, S., Gai, C., Linta, N., Tamburini, G., Monari, E., Ciuffoli, E., & Diana, A. (2025). Use of Contrast-Enhanced Ultrasound in Suspected Traumatic or Spontaneous Renal Injury in Cats: A Case Series. Animals, 15(21), 3089. https://doi.org/10.3390/ani15213089






