Improved Eggshell Quality in Aged Hens Through Circadian Gut Microbiota and Metabolite Changes Induced by a 28-h Ahemeral Light Cycle
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
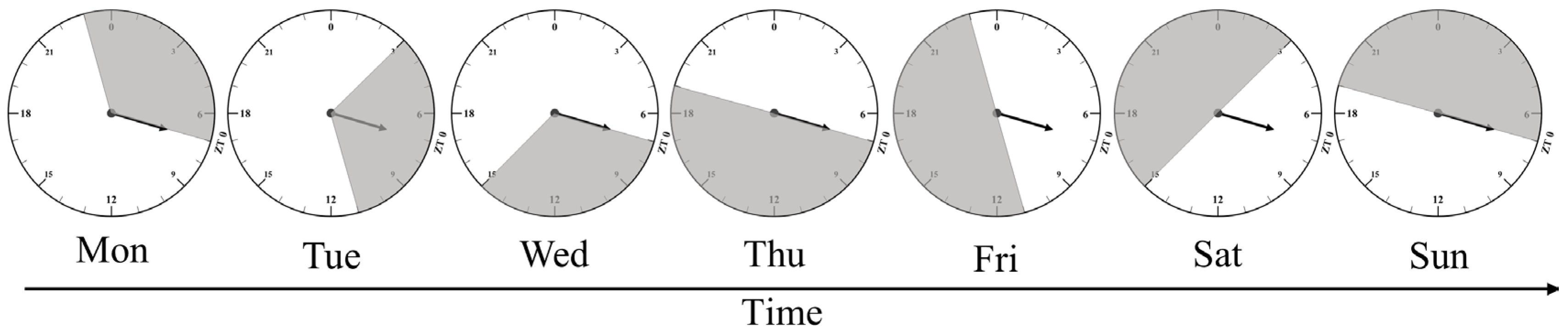
2.1. Experimental Design and Birds
2.2. Eggshell Quality and Serum Calcium
2.3. Histological Observations of Uterine Tissues
2.4. Cecal Contents Collection and 16S rRNA Sequences
2.5. Serum Collection and LC-MS/MS
2.6. Temporal Clustering Trend Analysis
2.7. Metabolite Enrichment Analysis
2.8. Data Analysis
3. Results
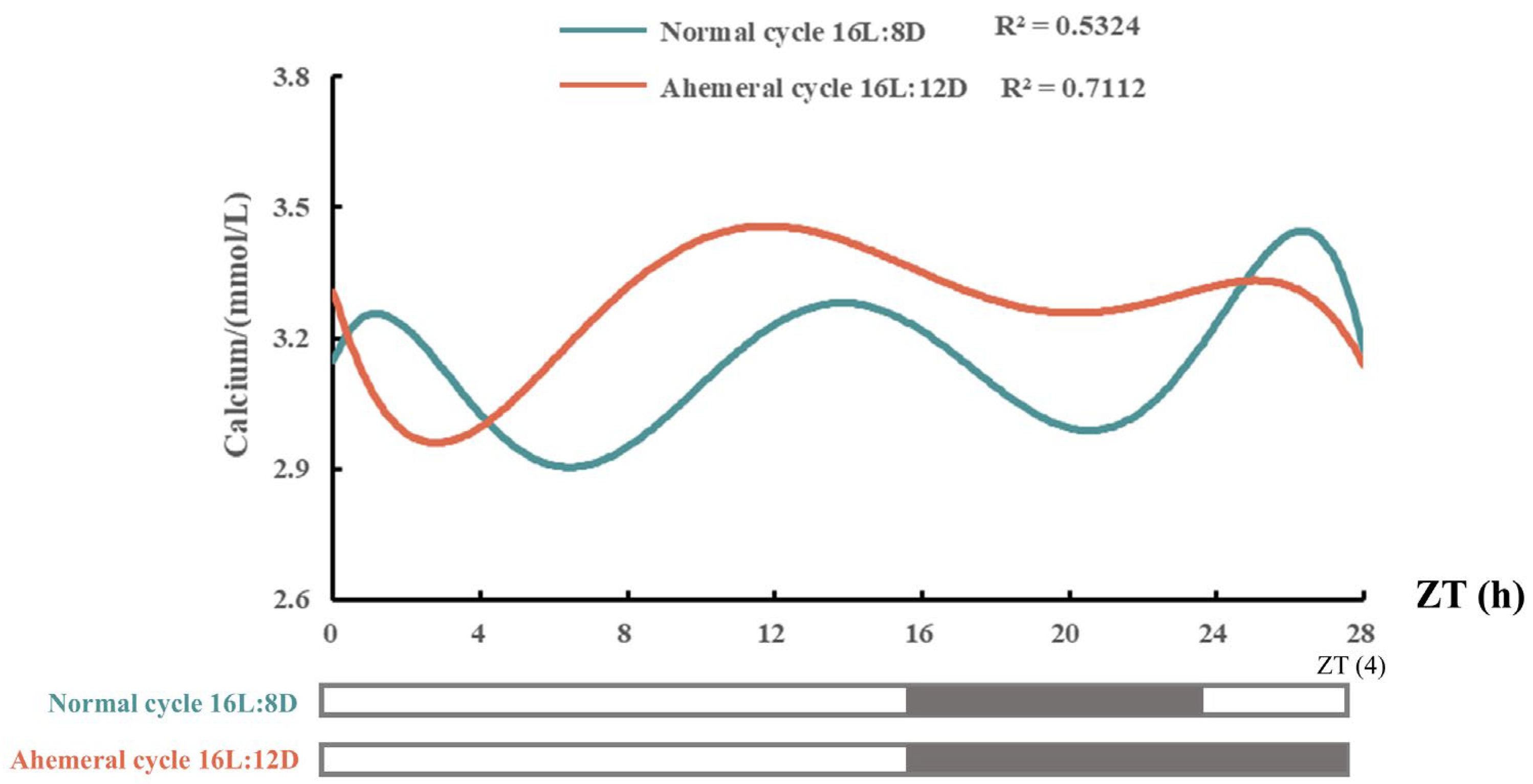
3.1. Eggshell Quality and Serum Calcium
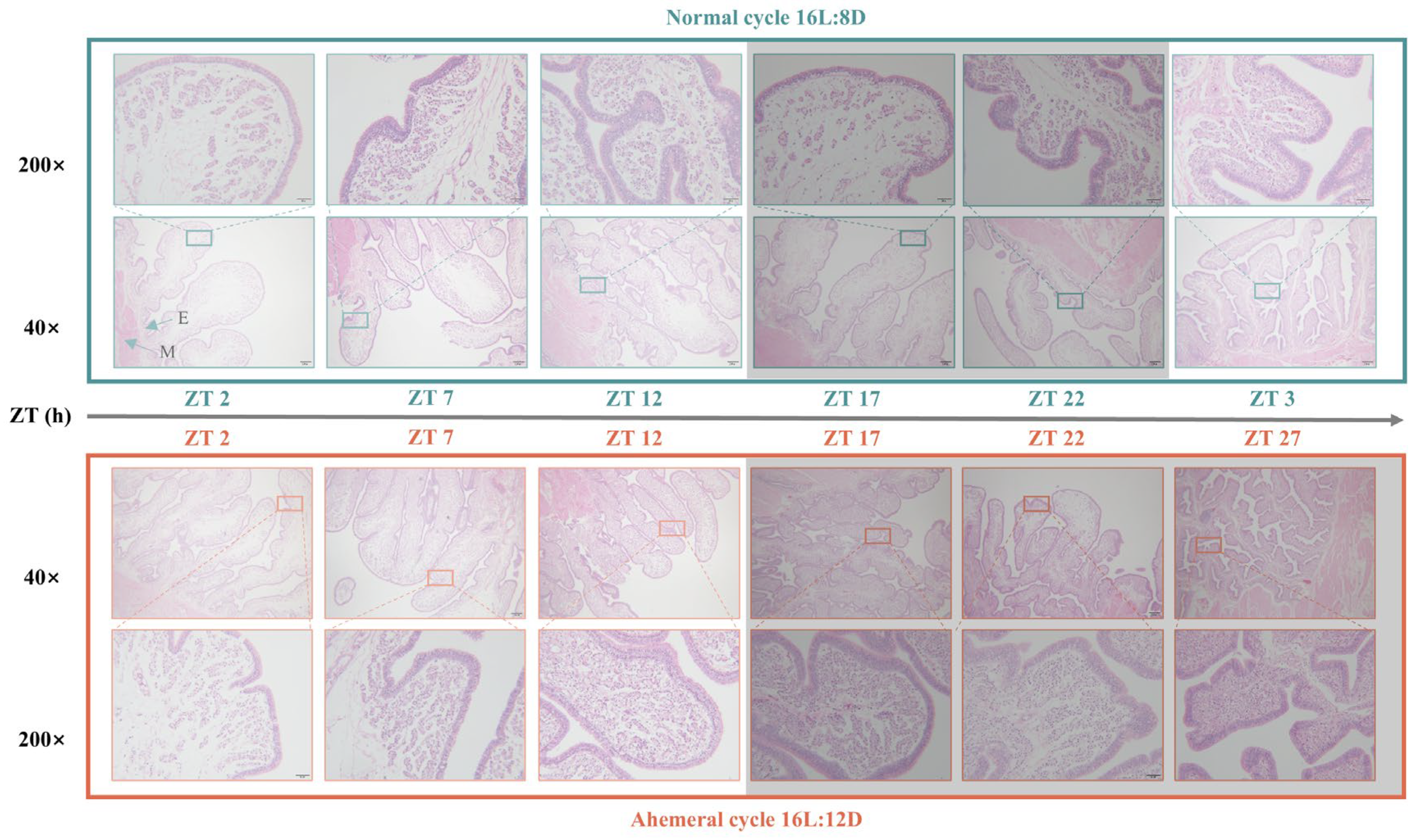
3.2. Morphological and Histological Observations
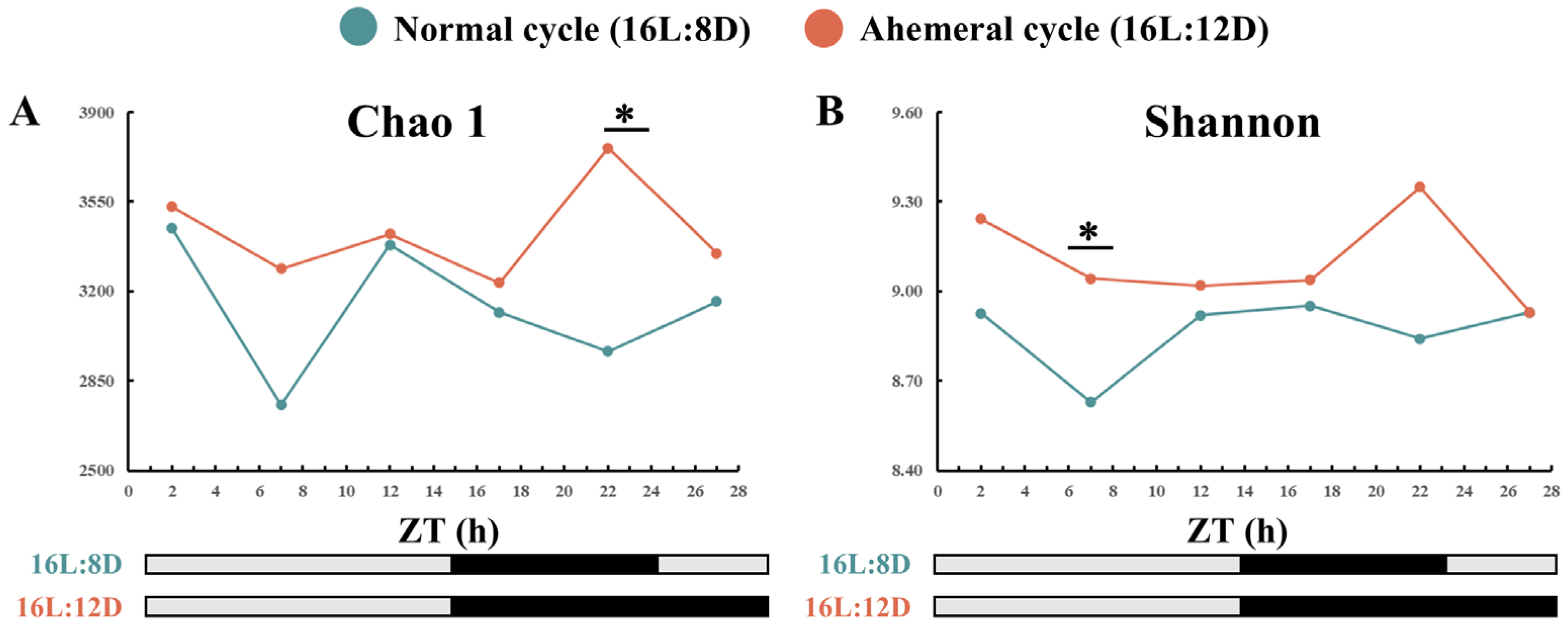
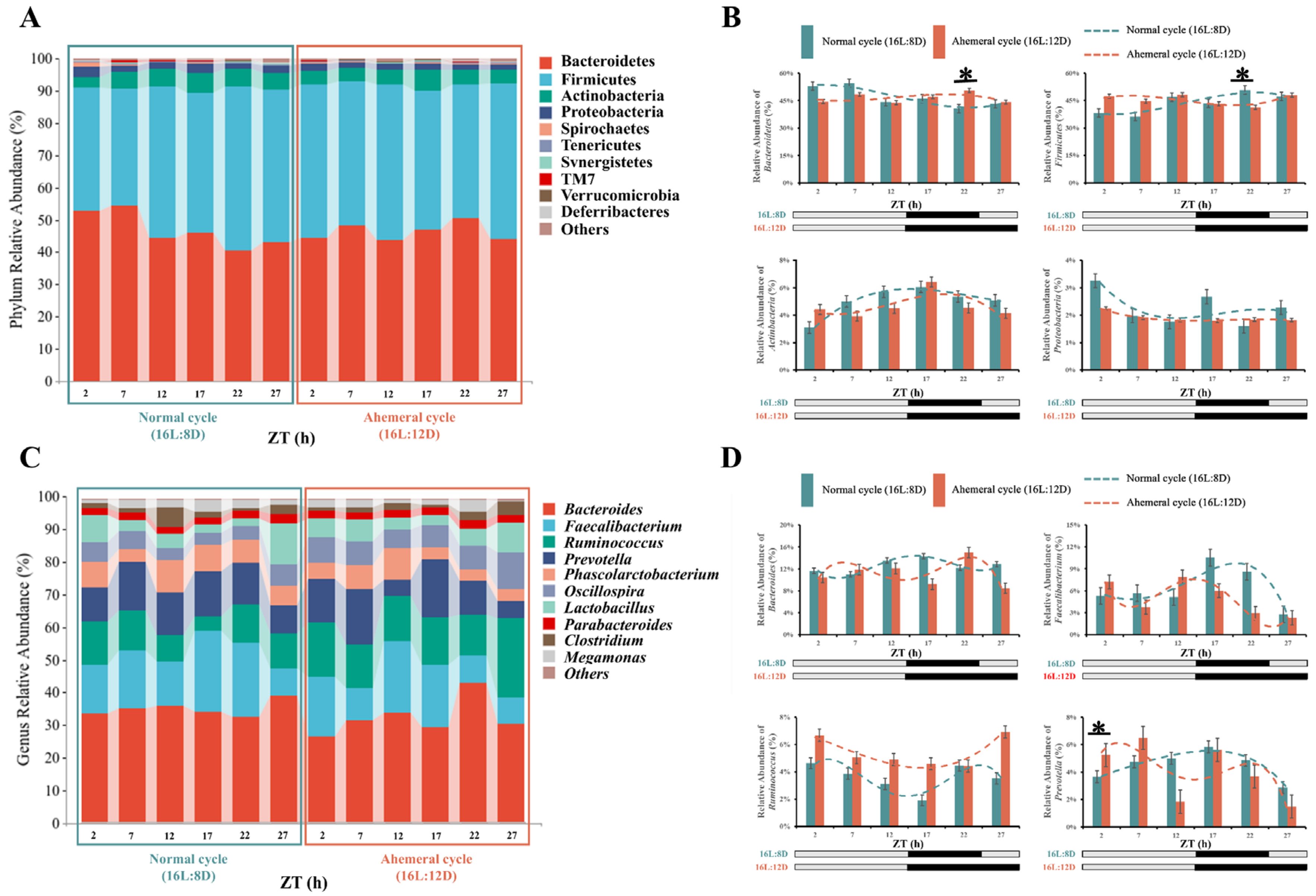
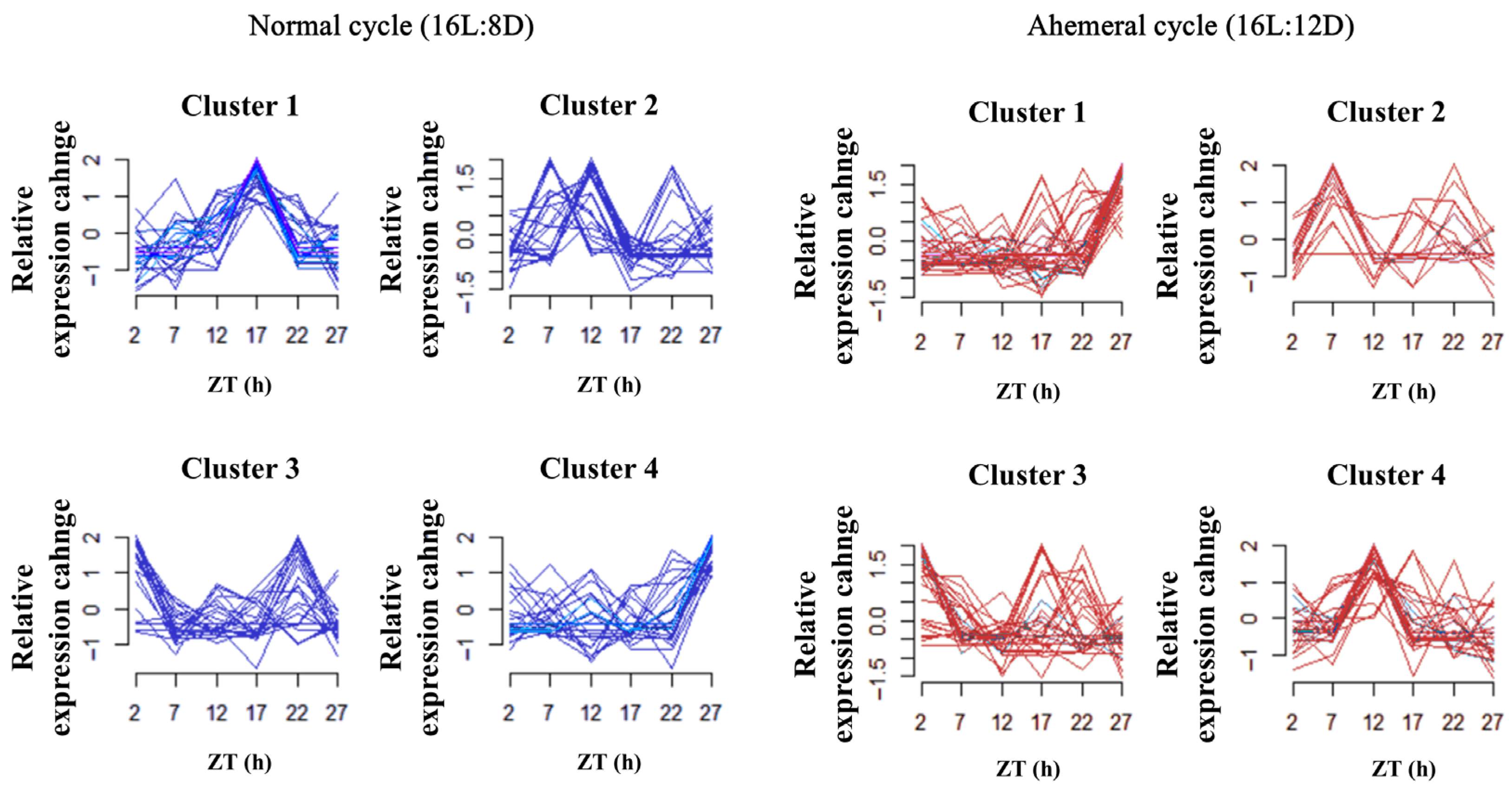
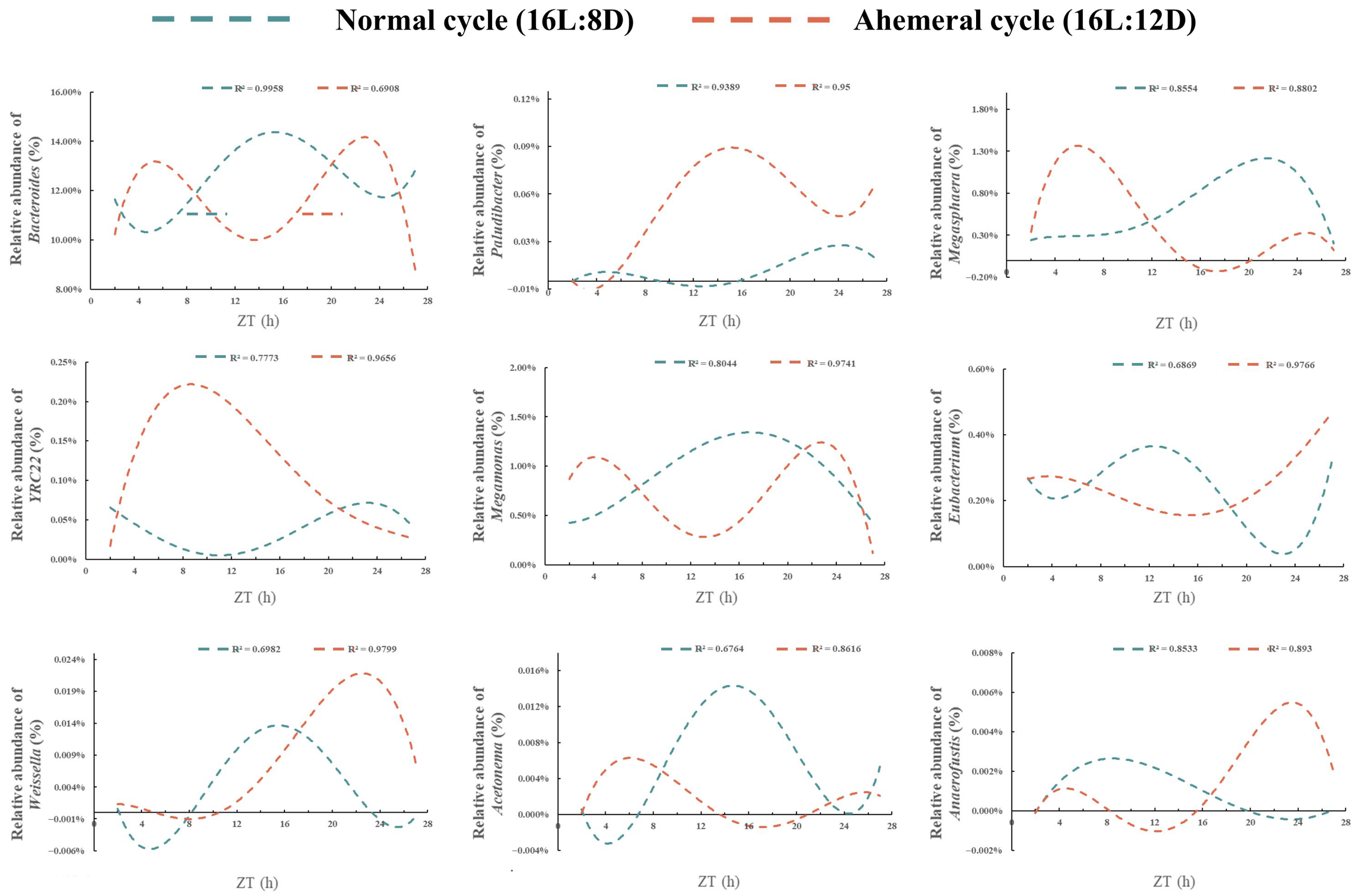
3.3. Gut Microbiology Analysis
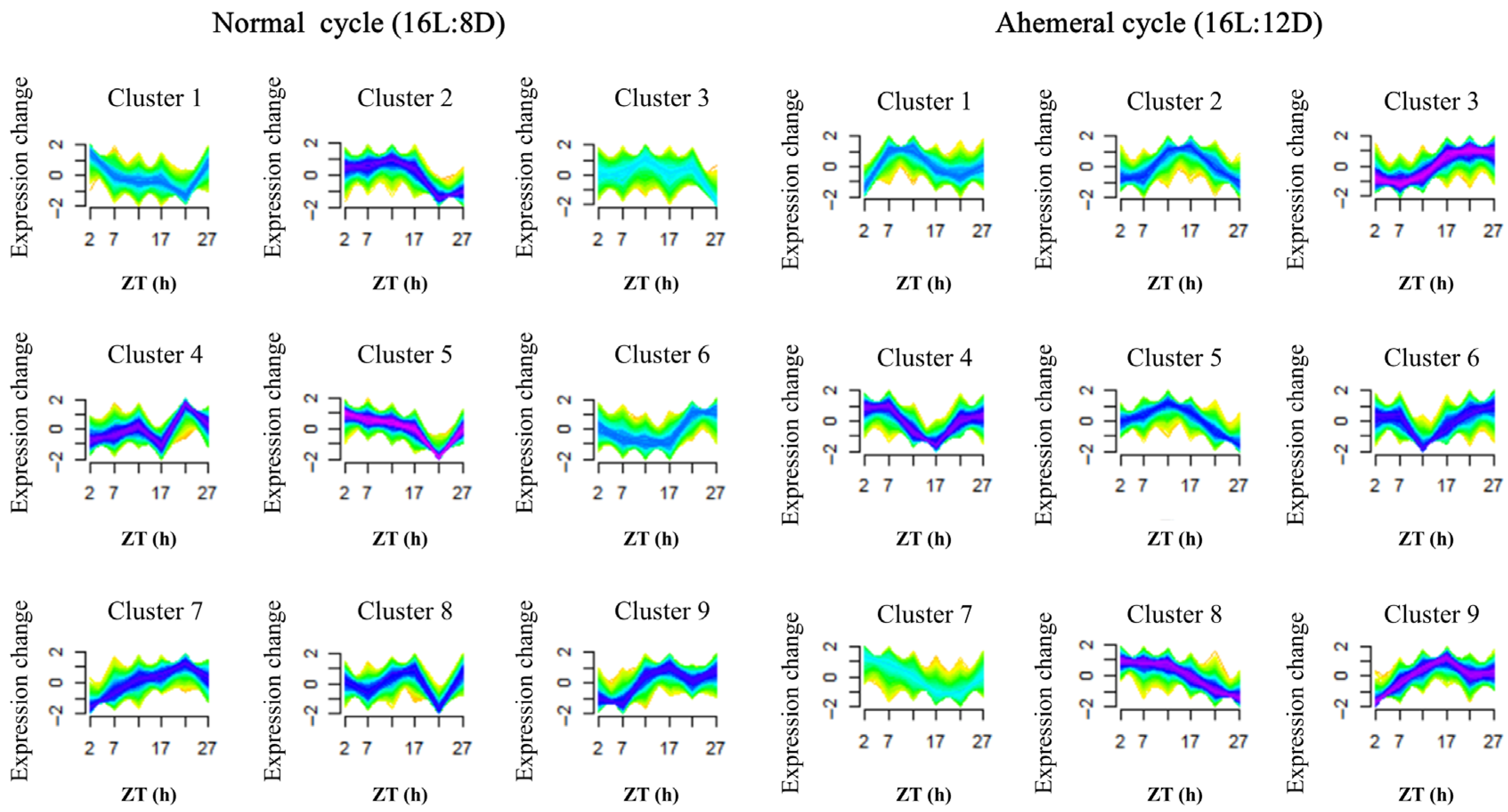
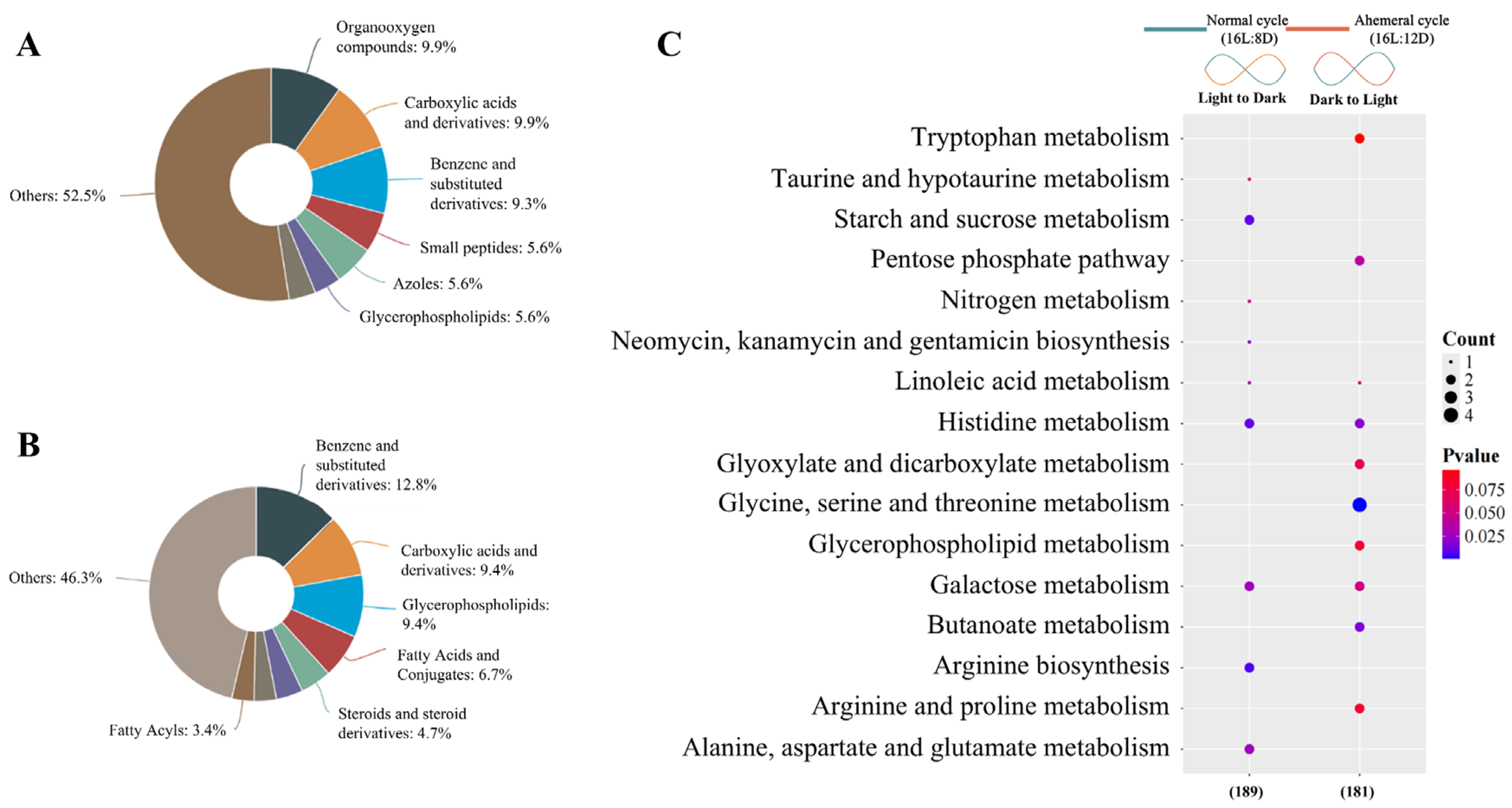
3.4. Metabolite Analysis
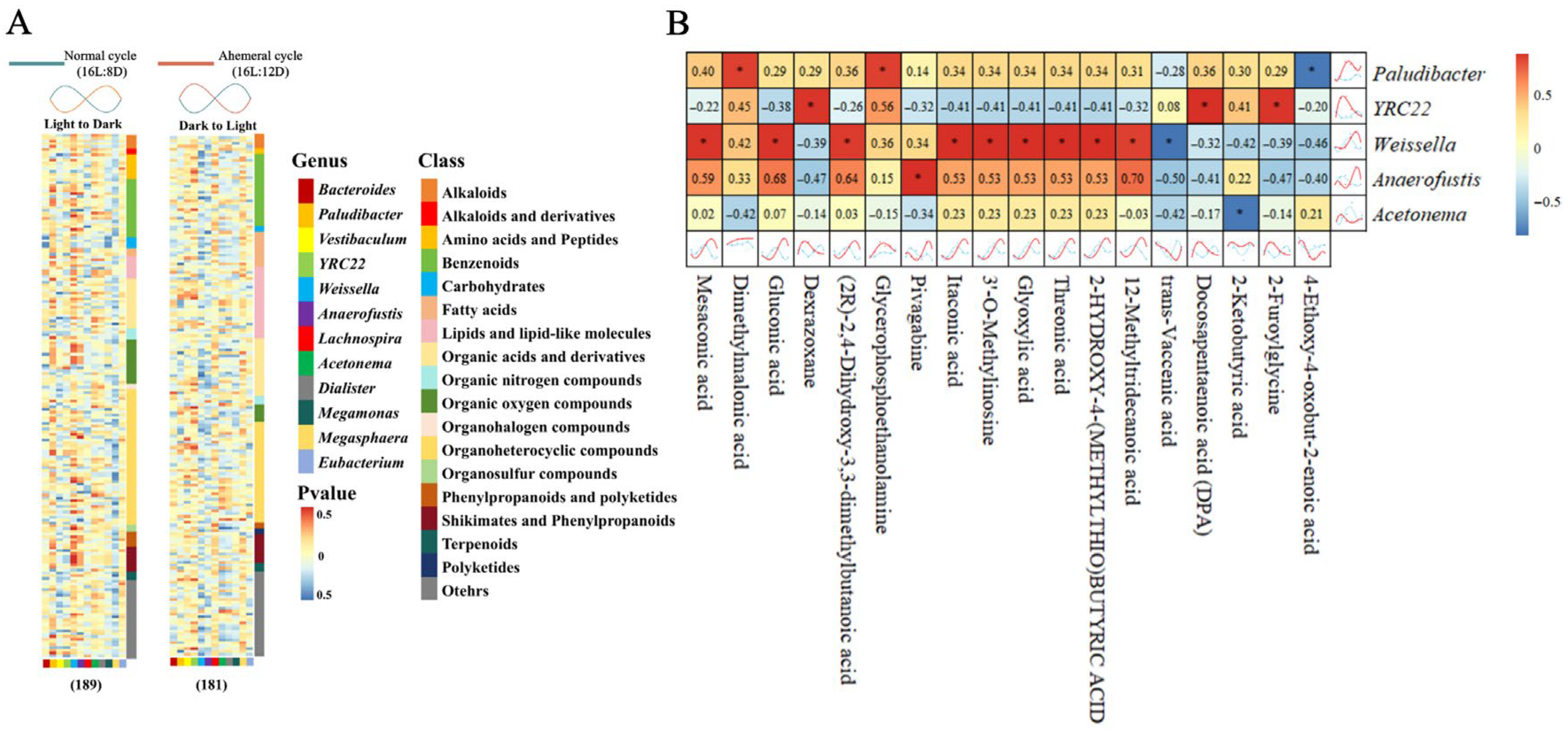
3.5. Correlation Analysis of Gut Microbiota and Metabolites
4. Discussion
4.1. Remodeling of Gut Microbial Rhythms and Diversity by the Light Cycle
4.2. Remodeling of Metabolite Rhythms by the Light Cycle
4.3. Remodeling of Uterine Epithelial Cells by the Light Cycle
4.4. Implications and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wistedt, A.; Ridderstrale, Y.; Wall, H.; Holm, L. Age-related changes in the shell gland and duodenum in relation to shell quality and bone strength in commercial laying hen hybrids. Acta Vet. Scand. 2019, 61, 14. [Google Scholar] [CrossRef]
- Benavides-Reyes, C.; Folegatti, E.; Dominguez-Gasca, N.; Litta, G.; Sanchez-Rodriguez, E.; Rodriguez-Navarro, A.B.; Faruk, M.U. Research note: Changes in eggshell quality and microstructure related to hen age during a production cycle. Poult. Sci. 2021, 100, 101287. [Google Scholar] [CrossRef]
- Samiullah, S.; Omar, A.S.; Roberts, J.; Chousalkar, K. Effect of production system and flock age on eggshell and egg internal quality measurements. Poult. Sci. 2017, 96, 246–258. [Google Scholar] [CrossRef]
- Sirri, F.; Zampiga, M.; Berardinelli, A.; Meluzzi, A. Variability and interaction of some egg physical and eggshell quality attributes during the entire laying hen cycle. Poult. Sci. 2018, 97, 1818–1823. [Google Scholar] [CrossRef]
- Bell, D.D.; Weaver, W.D. Commercial chicken meat and egg production. In Egg Handling and Egg Breakage; Bell, D.D., Ed.; Springer: Boston, MA, USA, 2002; pp. 1091–1105. [Google Scholar]
- Ren, Z.Z.; Piepenburg, A.J.; Butz, D.E.; Claus, J.R.; Cook, M.E. Vaccine to fibroblast growth factor 23 peptides increases eggshell strength. Poult. Sci. 2018, 97, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Sun, Y.Y.; Yuan, J.W.; Li, X.C.; Shi, L.; Isa, A.M.; Wang, Y.M.; Ge, P.Z.; Zong, Y.H.; Wang, P.L.; et al. Fresh insights into the light-induced pineal gland circadian rhythm transmission mechanism derived from mRNA and miRNA profiling. Anim. Res. One Health 2024, 1–19. [Google Scholar] [CrossRef]
- Liu, X.L.; Shi, L.; Hao, E.Y.; Chen, X.Y.; Liu, Z.W.; Chen, Y.F.; Wang, D.H.; Huang, C.X.; Ai, J.W.; Wu, M.; et al. Effects of 28 h ahemeral light cycle on production performance, egg quality, blood parameters, and uterine characteristics of hens during the late laying period. Poult. Sci. 2024, 103, 103489. [Google Scholar] [CrossRef] [PubMed]
- Geng, A.L.; Zhang, Y.; Zhang, J.; Wang, H.H.; Chu, Q.; Liu, H.G. Effects of lighting pattern and photoperiod on egg production and egg quality of a native chicken under free-range condition. Poult. Sci. 2018, 97, 2378–2384. [Google Scholar] [CrossRef]
- Cui, Z.F.; Zhang, Z.C.; Amevor, F.K.; Du, X.X.; Li, L.; Tian, Y.F.; Kang, X.C.; Shu, G.; Zhu, Q.; Wang, Y.; et al. Circadian miR-449c-5p regulates uterine Ca(2+) transport during eggshell calcification in chickens. BMC Genom. 2021, 22, 764. [Google Scholar] [CrossRef]
- Qian, X.; Wang, M.H.; Jiao, H.C.; Zhao, J.P.; Li, H.F.; Wang, X.J.; Lin, H. Prolonged scotophase within a 24 hour light regime improves eggshell quality by enhancing calcium deposition in laying hens. Poult. Sci. 2021, 100, 101098. [Google Scholar] [CrossRef]
- Shanawany, M.M. Ahemeral light cycles and egg quality. World Poult. Sci. J. 1990, 46, 101–108. [Google Scholar] [CrossRef]
- Ibaraki, K.; Yoshida, S.; Kunimatsu, Y.; Kojima, Y. Effect of 28-h ahemeral light-dark cycles on egg production and egg shell qualities of laying hens ii. effect of 28-h ahemeral L-D cycle on egg qualities at mature and aged stages. Jpn. Poult. Sci. 1988, 25, 93–101. [Google Scholar] [CrossRef][Green Version]
- Liu, X.L.; Shi, L.; Su, B.F.; Liu, A.Y.; Wang, D.H.; Chen, Y.F.; Hao, E.Y.; Bai, H.; Sun, Y.Y.; Li, Y.L.; et al. Long-24-h ahemeral light cycle improved eggshell quality of hens in late laying period. Poult. Sci. 2025, 104, 104959. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.; Labas, V.; Brionne, A.; Harichaux, G.; Hennequet-Antier, C.; Nys, Y.; Gautron, J. Quantitative proteomics and bioinformatic analysis provide new insight into protein function during avian eggshell biomineralization. J. Proteom. 2015, 113, 178–193. [Google Scholar] [CrossRef]
- Liu, Y.; Uyanga, V.A.; Jiao, H.C.; Wang, X.J.; Zhao, J.P.; Zhou, Y.L.; Lin, H. Effects of feeding strategies on eggshell quality of laying hens during late laying period. Poult. Sci. 2023, 102, 102406. [Google Scholar] [CrossRef]
- Ma, O.; Dutta, A.; Bliss, D.W.; Nakatsu, C.H.; Weaver, C.M.; Whisner, C.M. Ldentifying gut microbiome features that predict responsiveness toward a prebiotic capable of increasing calcium absorption: A pilot study. Calcif. Tissue Int. 2024, 114, 513–523. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, Z.; Yang, P.K.; Zhang, M.R.; Xi, L.; Liu, Q.; Li, J.Q. Molecular mechanism underlying the effect of illumination time on the growth performance of broilers via changes in the intestinal bacterial community. Peer J. 2020, 8, e9638. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Yuan, Y.L.; Wang, J.; Zhang, S.Y.; Zhu, R.; Wang, Y.; Wu, Y.B.; Liao, X.D.; Mi, J.D. Reducing light exposure enhances the circadian rhythm of the biological clock through interactions with the gut microbiota. Sci. Total Environ. 2023, 858, 160041. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Karz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nesengani, L.T.; Gong, Y.S.; Yang, Y.J.; Lu, W.F. 16S rRNA gene sequencing reveals effects of photoperiod on cecal microbiota of broiler roosters. Peer J. 2018, 6, e4390. [Google Scholar] [CrossRef]
- Ma, D.D.; Yu, M.; Zhang, M.H.; Feng, J.H. Research Note: The effect of photoperiod on the NLRP3 inflammasome and gut microbiota in broiler chickens. Poult. Sci. 2024, 103, 103507. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Zhang, M.H.; Feng, J.H. Gut microbiota dysbiosis exaggerates ammonia-induced tracheal injury via TLR4 signaling pathway. Ecotoxicol. Environ. Saf. 2022, 246, 114206. [Google Scholar] [CrossRef]
- Malinen, E.; Krogius-Kurikka, L.; Lyra, A.; Nikkila, J.; Jaaskelainen, A.; Rinttila, T.; Vilpponen-Salmela, T.; von Wroght, A.J.; Palva, A. Association of symptoms with gastrointestinal microbiota in irritable bowel syndrome. World J. Gastroenterol. 2010, 16, 4532–4540. [Google Scholar] [CrossRef]
- De-Cesare, A.; Sirri, F.; Manfreda, G.; Moniaci, P.; Giardini, A.; Zampiga, M.; Meluzzi, A. Effect of dietary supplementation with Lactobacillus acidophilus D2/CSL (CECT 4529) on caecum microbioma and productive performance in broiler chickens. PLoS ONE 2017, 12, e0176309. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Jones, R.M.; Schett, G.; Pacifici, R. The gut-bone axis: How bacterial metabolites bridge the distance. J. Clin. Investig. 2019, 129, 3018–3028. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Qi, G.H.; Wang, J.; Zhang, H.J.; Qiu, K.; Wu, S.G. Intestinal microbiota of layer hens and its association with egg quality and safety. Poult. Sci. 2022, 101, 102008. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef]
- Bedrosian, T.A.; Fonken, L.K.; Nelson, R.J. Endocrine effects of circadian disruption. Annu. Rev. Physiol. 2016, 78, 109–131. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Zwighaft, Z.; Aviram, R.; Shalev, M.; Rousso-Noori, L.; Kraut-Cohen, J.; Golik, M.; Brandis, A.; Reinke, H.; Aharoni, A.; Kahana, C.; et al. Circadian clock control by polyamine levels through a mechanism that declines with age. Cell Metab. 2015, 22, 874–885. [Google Scholar] [CrossRef]
- Krishnaiah, S.Y.; Wu, G.; Altman, B.J.; Growe, J.; Rhoades, S.D.; Coldren, F.; Venkataraman, A.; Olarerin-George, A.O.; Francey, L.J.; Mukherjee, S.; et al. Clock regulation of metabolites reveals coupling between transcription and metabolism. Cell Metab. 2017, 25, 961–974.e4. [Google Scholar] [CrossRef]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef]
- Foley, M.H.; Cockburn, D.W.; Koropatkin, N.M. The Sus operon: A model system for starch uptake by the human gut Bacteroidetes. Cell Mol. Life Sci. 2016, 73, 2603–2617. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Thompson, S.V.; Holscher, H.D. Complex interactions of circadian rhythms, eating behaviors, and the gastrointestinal microbiota and their potential impact on health. Nutr. Rev. 2017, 75, 673–682. [Google Scholar] [CrossRef]
- Vacilotto, M.M.; Pellegrini, V.O.A.; Sepulchro, A.G.V.; Capetti, C.C.D.M.; Curvelo, A.A.S.; Marcondes, W.F.; Arantes, A.; Polikarpov, I. Paludibacter propionicigenes GH10 xylanase as a tool for enzymatic xylooligosaccharides production from heteroxylans. Carbohydr. Polym. 2022, 275, 118684. [Google Scholar] [CrossRef]
- Liu, X.F.; Zheng, Y.Q.; Li, H.; Ma, Y.Y.; Cao, R.M.; Zheng, Z.K.; Tian, Y.C.; Du, L.; Zhang, J.S.; Zhang, C.Q.; et al. The role of metabolites in the progression of osteoarthritis: Mechanisms and advances in therapy. J. Orthop. Translat. 2025, 50, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Ma, X.Y.; Li, X.M.; Zhang, X.G.; Sun, Y.F.; Ning, C.; Zhang, Q.; Wang, D.; Tang, H. Integrating proteomics and metabolomics to elucidate the regulatory mechanisms of pimpled egg production in chickens: Multi-omics analysis of the mechanism of pimpled egg formation. Poult. Sci. 2025, 104, 104818. [Google Scholar] [CrossRef]
- Jonchere, V.; Brionne, A.; Gautron, J.; Nys, Y. Identification of uterine ion transporters for mineralisation precursors of the avian eggshell. BMC Physiol. 2012, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Wang, S.; Ruan, D.; Zheng, C.T. Calcium metabolism mechanism of eggshell formation and its influencing factors. J. Anim. Nutr. 2021, 3, 1201–1207. [Google Scholar]
- Gautron, J.; Dombre, C.; Nau, F.; Feidt, C.; Guillier, L. Review: Production factors affecting the quality of chicken table eggs and egg products in Europe. Animal 2022, 16, 1100425. [Google Scholar] [CrossRef]
- Shi, L.; Sun, Y.Y.; Li, Y.L.; Bai, H.; Yuan, J.W.; Ma, H.; Wang, Y.M.; Wang, P.L.; Ni, A.X.; Jiang, L.L.; et al. Asymmetric expression of CA2 and CA13 linked to calcification in the bilateral mandibular condyles cause crossed beaks in chickens. Integr. Agric. 2024, 23, 2379–2390. [Google Scholar] [CrossRef]
- Melaku, M.; Zhong, R.Q.; Han, H.; Wan, F.; Yi, B.; Zhang, H.F. Butyric and citric acids and their salts in poultry nutrition: Effects on gut health and intestinal microbiota. Int. J. Mol. Sci. 2021, 22, 10392. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Yaqoob, M.U.; Hassan, F.; Alagawany, M.; Arif, M.; Taha, A.E.; Elnesr, S.S.; El-Tarabily, K.A.; Abd El-Hack, M.E. Applications of butyric acid in poultry production: The dynamics of gut health, performance, nutrient utilization, egg quality, and osteoporosis. Anim. Health Res. Rev. 2022, 23, 136–146. [Google Scholar] [CrossRef]
- Holmsen, H.; Hindenes, J.O.; Fukami, M. Glycerophospholipid metabolism: Back to the future. Thromb. Res. 1992, 67, 313–323. [Google Scholar] [CrossRef]
- Ma, Y.; Li, B.; Zhang, X.Y.; Wang, C.; Chen, W. Production of gluconic acid and its derivatives by microbial fermentation: Process improvement based on integrated routes. Front. Bioeng. Biotechnol. 2022, 10, 864787. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Nomura, S.; Higashibata, Y.; Hirose, M.; Gao, B.; Yoshimura, M.; Itoh, Y.; Yasui, T.; Tozawa, K.; Kohri, K. Successful formation of calcium oxalate crystal deposition in mouse kidney by intraabdominal glyoxylate injection. Urol. Res. 2007, 35, 89–99. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, J.; Liu, H.Y.; Yuan, J.H.; Yin, Y.P.; Wang, T.T.; Cheng, B.F.; Sun, S.H.; Guo, Z.Y. Curcumin ameliorates glyoxylate-induced calcium oxalate deposition and renal injuries in mice. Phytomedicine 2019, 61, 152861. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hu, P.; Jiang, J. Pharmacokinetics and safety of calcium L-threonate in healthy volunteers after single and multiple oral administrations. Acta Pharmacol. Sin. 2011, 32, 1555–1560. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hu, P.; Jiang, J. Calcium bioavailability of calcium L-threonate in healthy Chinese subjects measured with stable isotopes (44Ca and 42Ca). Eur. J. Clin. Pharmacol. 2013, 69, 1121–1126. [Google Scholar] [CrossRef]
- Garg, M.K.; Mahalle, N. Calcium Supplementation: Why, Which, and How? Indian. J. Endocrinol. Metab. 2019, 23, 387–390. [Google Scholar] [PubMed]
- Kaur, G.; Cameron-Smith, D.; Garg, M.; Sinclair, A.J. Docosapentaenoic acid (22:5n-3): A review of its biological effects. Prog. Lipid Res. 2011, 50, 28–34. [Google Scholar] [CrossRef]
- Van, M.G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Mcmahon, H.T.; Gallop, J.L. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 2005, 438, 590–596. [Google Scholar] [CrossRef]
- Wu, H.Q.; Xie, S.; Miao, J.F.; Li, Y.C.; Wang, Z.H.; Wang, M.J.; Yu, Q.H. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes 2020, 11, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kurihara, S.; Takahashi, D.; Ohashi, W.; Nakamura, Y.; Kimura, S.; Onuki, M.; Kume, A.; Furusawa, Y.; Obata, Y.; et al. Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon. Nat. Commun. 2021, 12, 2105. [Google Scholar] [CrossRef] [PubMed]
- Di, L.B.; Acampora, V.; Saggese, A.; Calabro, V.; Vivo, M.; Angrisano, T.; Baccigalupi, L.; Ricca, E.; Pollice, A. Modulation of intestinal epithelial cell proliferation and apoptosis by Lactobacillus gasseri SF1183. Sci. Rep. 2022, 12, 20248. [Google Scholar] [CrossRef] [PubMed]
- Escorcia-Mora, P.; Valbuena, D.; Diez-Juan, A. The role of the gut microbiota in female reproductive and gynecological health: Insights into endometrial signaling pathways. Life 2025, 15, 762. [Google Scholar] [CrossRef]
- Rao, F.; Xue, T. Circadian-independent light regulation of mammalian metabolism. Nat. Metab. 2024, 6, 1000–1007. [Google Scholar] [CrossRef]
| Items | Content % |
|---|---|
| Ingredients | |
| Corn | 62.60 |
| Soybean meal | 23.90 |
| Wheat barn | 1.80 |
| Soybean | 0.90 |
| Limestone | 8.00 |
| CaHPO4 | 1.50 |
| NaCl | 0.30 |
| Premix (1) | 1.00 |
| Total | 100.00 |
| Nutrient levels (2) | |
| ME/(MJ/kg) | 11.24 |
| CP | 16.70 |
| Ca | 3.50 |
| AP | 0.44 |
| Met | 0.34 |
| Lys | 0.81 |
| Items | Normal Cycle 16L:8D | Ahemeral Cycle 16L:12D | SEM | p-Value |
|---|---|---|---|---|
| Laying rate/% | 64.13 | 64.60 | 0.81 | 0.814 |
| Qualified egg rate/% | 92.90 | 93.58 | 0.23 | 0.145 |
| FER | 3.26 | 3.12 | 0.08 | 0.502 |
| Week | Items | Normal Cycle 16L:8D | Ahemeral Cycle 16L:12D | SEM | p-Value |
|---|---|---|---|---|---|
| 79 wk | Eggshell weight/g | 59.41 a | 62.85 b | 0.50 | 0.001 |
| Shape index/% | 1.30 | 1.33 | 0.01 | 0.176 | |
| Eggshell percentage/% | 8.59 a | 9.26 b | 0.09 | 0.001 | |
| Yolk percentage/% | 27.81 | 28.71 | 0.23 | 0.053 | |
| Eggshell thickness/mm | 0.31 a | 0.35 b | 0.01 | 0.001 | |
| Eggshell strength/N | 29.75 a | 33.94 b | 0.84 | 0.012 | |
| 84 wk | Eggshell weight/g | 58.54 a | 61.47 b | 0.39 | 0.001 |
| Shape index/% | 1.33 | 1.33 | 0.01 | 0.877 | |
| Eggshell percentage/% | 8.87 a | 9.28 b | 0.09 | 0.004 | |
| Yolk percentage/% | 27.40 | 27.90 | 0.27 | 0.145 | |
| Eggshell thickness/mm | 0.32 a | 0.34 b | 0.01 | 0.001 | |
| Eggshell strength/N | 30.42 a | 35.18 b | 0.66 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Li, X.; Liu, X.; Wu, X.; Fan, Y.; Yao, Y.; Zhang, R.; Wang, D.; Chen, Y.; Hao, E.; et al. Improved Eggshell Quality in Aged Hens Through Circadian Gut Microbiota and Metabolite Changes Induced by a 28-h Ahemeral Light Cycle. Animals 2025, 15, 3086. https://doi.org/10.3390/ani15213086
Xu J, Li X, Liu X, Wu X, Fan Y, Yao Y, Zhang R, Wang D, Chen Y, Hao E, et al. Improved Eggshell Quality in Aged Hens Through Circadian Gut Microbiota and Metabolite Changes Induced by a 28-h Ahemeral Light Cycle. Animals. 2025; 15(21):3086. https://doi.org/10.3390/ani15213086
Chicago/Turabian StyleXu, Junjie, Xinxin Li, Xuelu Liu, Xinling Wu, Yihao Fan, Yichun Yao, Rongcai Zhang, Dehe Wang, Yifan Chen, Erying Hao, and et al. 2025. "Improved Eggshell Quality in Aged Hens Through Circadian Gut Microbiota and Metabolite Changes Induced by a 28-h Ahemeral Light Cycle" Animals 15, no. 21: 3086. https://doi.org/10.3390/ani15213086
APA StyleXu, J., Li, X., Liu, X., Wu, X., Fan, Y., Yao, Y., Zhang, R., Wang, D., Chen, Y., Hao, E., Sun, Y., Chen, J., Chen, H., & Shi, L. (2025). Improved Eggshell Quality in Aged Hens Through Circadian Gut Microbiota and Metabolite Changes Induced by a 28-h Ahemeral Light Cycle. Animals, 15(21), 3086. https://doi.org/10.3390/ani15213086







