A One Health Perspective on Heartworm Disease: Allergy Risk in Owners of Infected Dogs in Gran Canaria (Spain)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Gran Canaria
2.2. Samples
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCall, J.W.; Genchi, C.; Kramer, L.H.; Guerrero, J.; Venco, L. Heartworm disease in animals and humans. Adv. Parasitol. 2008, 66, 193–285. [Google Scholar]
- Simón, F.; Diosdado, A.; Siles-Lucas, M.; Kartashev, V.; González-Miguel, J. Human dirofilariosis in the 21st century: A scoping review of clinical cases reported in the literature. Transbound. Emerg. Dis. 2022, 69, 2424–2439. [Google Scholar] [CrossRef]
- Momčilović, S.; Jovanović, A.; Gasser, R.B. Human dirofilariasis—A potentially significant nematode zoonosis in an era of climate change. J. Infect. 2025, 90, 106460. [Google Scholar] [CrossRef] [PubMed]
- Savić, S.; Stosic, M.Z.; Marcic, D.; Hernández, I.; Potkonjak, A.; Otasevic, S.; Ruzic, M.; Morchón, R. Seroepidemiological Study of Canine and Human Dirofilariasis in the Endemic Region of Northern Serbia. Front. Vet. Sci. 2020, 7, 571. [Google Scholar] [CrossRef]
- Ciuca, L.; Simón, F.; Rinaldi, L.; Kramer, L.; Genchi, M.; Cringoli, G.; Acatrinei, D.; Miron, L.; Morchón, R. Seroepidemiological survey of human exposure to Dirofilaria spp. in Romania and Moldova. Acta Trop. 2018, 187, 169–174. [Google Scholar] [CrossRef]
- Zumaquero, L.; Simón, F.; Carretón, E.; Hernández, I.; Sandoval, C.; Morchón, R. Prevalence of canine and human dirofilariosis in Puebla, Mexico. Vet. Parasitol. 2020, 282, 109098. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Morchón, R.; García-Rodríguez, S.N.; Falcón-Cordón, Y.; Costa-Rodríguez, N.; Matos, J.I.; Rodríguez Escolar, I.; Carretón, E. Expansion of Canine Heartworm in Spain. Animals 2022, 12, 1268. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; García-Rodríguez, S.N.; Matos, J.I.; Costa-Rodríguez, N.; Falcón-Cordón, Y.; Carretón, E.; Morchón, R. Change in the Distribution Pattern of Dirofilaria immitis in Gran Canaria (Hyperendemic Island) between 1994 and 2020. Animals 2024, 14, 2037. [Google Scholar] [CrossRef]
- Rodríguez-Escolar, I.; Hernández-Lambraño, R.E.; Sánchez-Agudo, J.Á.; Collado, M.; Pérez-Pérez, P.; Morchón, R. Current Risk of Dirofilariosis Transmission in the Iberian Peninsula (Spain and Portugal) and the Balearic Islands (Spain) and Its Future Projection under Climate Change Scenarios. Animals 2023, 13, 1764. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lerma, B.; Pérez-Yarza, E.G.; Bercedo-Sanz, A.; Blanco-Quirós, A.; Vázquez-López, M.; Esquilas-Hernández, M. High prevalence of asthma and allergic diseases in children aged 6 to7 years from the Canary Islands. Allergol. Immunopathol. 2009, 37, 79–85. [Google Scholar]
- Juliá-Serdá, G.; Cabrera-Navarro, P.; Acosta-Fernández, O.; Martín-Pérez, P.; Losada-Cabrera, P.; García-Bello, M.A.; Carrillo-Díaz, T.; Antó-Boqué, J. High prevalence of asthma and atopy in the Canary Islands, Spain. Int. J. Tuberc. Lung Dis. 2011, 15, 536–541. [Google Scholar] [CrossRef]
- Suárez-Lorenzo, I.; Cruz-Niesvaara, D.; Rodríguez-Gallego, C.; Rodríguez de Castro, F.; Carrillo-Diaz, T. Epidemiological Study of the Allergic Population in the North of Gran Canaria. J. Investig. Allergol. Clin. Immunol. 2018, 28, 212–215. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Morchón, R.; Matos, J.I.; Falcón-Cordón, Y.; Costa-Rodríguez, N.; Carretón, E. Dirofilaria immitis Could Be a Risk Factor for the Development of Allergic Diseases in Humans. Animals 2020, 10, 1847. [Google Scholar] [CrossRef]
- Pou-Barreto, C.; Quispe-Ricalde, M.A.; Morchón, R.; Vázquez, C.; Genchi, M.; Postigo, I.; Valladares, B.; Simón, F. Galectin and aldolase-like molecules are responsible for the specific IgE response in humans exposed to Dirofilaria immitis. Parasite Immunol. 2008, 30, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.S.; Barbosa, T.C.; Barbosa, M.M.F.; Miyasato, P.A.; Nakano, E.; Leite, L.C.C.; Farias, L.P. Stage and tissue expression patterns of Schistosoma mansoni venom allergen-like proteins SmVAL 4, 13, 16 and 24. Parasites Vectors 2017, 10, 223. [Google Scholar] [CrossRef]
- Nkurunungi, G.; Kabagenyi, J.; Nampijja, M.; Sanya, R.E.; Walusimbi, B.; Nassuuna, J.; Webb, E.L.; Elliott, A.M.; LaVIISWA study team. Schistosoma mansoni-specific immune responses and allergy in Uganda. Parasite Immunol. 2018, 40, e12506. [Google Scholar] [PubMed]
- Brandt, O.; Wegenstein, B.; Müller, I.; Smith, D.; Nqweniso, S.; Adams, L.; Müller, S.; du Randt, R.; Pühse, U.; Gerber, M.; et al. Association between allergic sensitization and intestinal parasite infection in schoolchildren in Gqeberha, South Africa. Clin. Exp. Allergy 2022, 52, 670–683. [Google Scholar] [CrossRef]
- Pouryousef, A.; Abbasi, R.; Mehrabi, S.; Moshfe, A.; Mikaeili, F.; Rezaei, Z.; Rostamzadeh, D.; Saadat, A.; ArefKhah, N. Serosurvey of Toxocariasis and Its Association With Allergic Asthma in Children: A Case-Control Study in Southwest Iran. Parasite Immunol. 2025, 47, e70005. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Carmo, F.; Miranda, J.; Seixas, A.P.; Mota, M. Eosinophilia and IgE Elevation: An Uncommon Toxocara Infection. Cureus 2025, 17, e77965. [Google Scholar] [CrossRef]
- Santiago, L.F.; da Silva, E.S.; Dos Santos, P.S.; Salazar-Garcés, L.F.; Santos, S.P.O.; Fernandes, A.M.S.; Silva, R.C.; Alves, V.S.; Briza, P.; Ferreira, F.; et al. The proteome of human adult whipworm Trichuris trichiura: A source of potential immunomodulatory molecules. Acta Trop. 2025, 263, 107566. [Google Scholar] [CrossRef]
- Torres-Chable, O.M.; Brito-Argaez, L.G.; Islas-Flores, I.R.; Zaragoza-Vera, C.V.; Zaragoza-Vera, M.; Arjona-Jimenez, G.; Baak-Baak, C.M.; Cigarroa-Toledo, N.; Gonzalez-Garduño, R.; Machain-Williams, C.I.; et al. Dirofilaria immitis proteins recognized by antibodies from individuals living with microfilaremic dogs. J. Infect. Dev. Ctries. 2020, 14, 1442–1447. [Google Scholar] [CrossRef]
- Climate Shifts. Worldmaps of Köppen-Geiger Climate Classification. Available online: https://koeppen-geiger.vu-wien.ac.at/shifts.htm (accessed on 25 September 2025).
- The Canary Islands Statistics Institute (ISTAC). Available online: https://www.gobiernodecanarias.org/istac/ (accessed on 25 September 2025).
- ZOOCAN. Animal Canary Identification Register. Available online: https://zoocan.net (accessed on 25 September 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 2 May 2025).
- Galletta, F.; Klain, A.; Manti, S.; Mori, F.; Grella, C.; Tomei, L.; Senatore, A.A.; Licari, A.; Miraglia Del Giudice, M.; Indolfi, C. Parental Knowledge and Preventive Strategies in Pediatric IgE-Mediated Food Allergy-Results from a Cross-Sectional Survey. Nutrients 2025, 17, 2387. [Google Scholar] [CrossRef]
- Zakzuk, J.; Donado, K.; Mondol, E.; Marrugo, V.; Regino, R.; López, J.F.; Hernandez, K.; Mercado, D.; Dennis, R.; Puerta, L.; et al. IgE-Mediated Sensitization to Blo t 21 and Blo t 5 Is Associated With Asthma in the Tropics: A Case-Control Study. J. Investig. Allercol. Clin. Immunol. 2024, 34, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Mihai, C.M.; Lupu, A.; Chisnoiu, T.; Balasa, A.L.; Baciu, G.; Lupu, V.V.; Popovici, V.; Suciu, F.; Enache, F.D.; Cambrea, S.C.; et al. A Comprehensive Analysis of Echinococcus granulosus Infections in Children and Adolescents: Results of a 7-Year Retrospective Study and Literature Review. Pathogens 2025, 14, 53. [Google Scholar] [CrossRef]
- Sitcharungsi, R.; Sirivichayakul, C. Allergic diseases and helminth infections. Pathog. Glob. Health 2013, 107, 110–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cho, S.W.; Lee, H.N. Immune reactions and allergy in experimental anisakiasis. Korean J. Parasitol. 2006, 44, 271–283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Acevedo, N.; Sánchez, J.; Erler, A.; Mercado, D.; Briza, P.; Kennedy, M.; Fernandez, A.; Gutierrez, M.; Chua, K.Y.; Cheong, N.; et al. IgE cross-reactivity between Ascaris and domestic mite allergens: The role of tropomyosin and the nematode polyprotein ABA-1. Allergy 2009, 64, 1635–1643. [Google Scholar] [CrossRef]
- Minciullo, P.L.; Cascio, A.; Gangemi, S. Association between urticaria and nematode infections. Allergy Asthma Proc. 2018, 39, 86–95. [Google Scholar] [CrossRef]
- Pontone, M.; Giovannini, M.; Barni, S.; Mori, F.; Venturini, E.; Galli, L.; Valleriani, C.; Vecillas, L.L.; Sackesen, C.; Lopata, A.L.; et al. IgE-mediated Anisakis allergy in children. Allergol. Immunopathol. 2023, 51, 98–109. [Google Scholar] [CrossRef]
- Wilson, M.S.; Maizels, R.M. Regulation of allergy and autoimmunity in helminth infection. Clin. Rev. Allergy Immunol. 2004, 26, 35–50. [Google Scholar] [CrossRef]
- Fitzsimmons, C.M.; Falcone, F.H.; Dunne, D.W. Helminth Allergens, Parasite-Specific IgE, and Its Protective Role in Human Immunity. Front. Immunol. 2014, 5, 61. [Google Scholar] [CrossRef]
- Santiago, H.C.; Nutman, T.B. Human Helminths and Allergic Disease: The Hygiene Hypothesis and Beyond. Am. J. Trop. Med. Hyg. 2016, 95, 746–753. [Google Scholar] [CrossRef]
- Cruz, A.A.; Cooper, P.J.; Figueiredo, C.A.; Alcantara-Neves, N.M.; Rodrigues, L.C.; Barreto, M.L. Global issues in allergy and immunology: Parasitic infections and allergy. J. Allergy Clin. Immunol. 2017, 140, 1217–1228. [Google Scholar] [CrossRef]
- Yazdanbakhsh, M.; Kremsner, P.G.; van Ree, R. Allergy, parasites, and the hygiene hypothesis. Science 2002, 296, 490–494. [Google Scholar] [CrossRef]
- Genchi, C.; Kramer, L.H. The prevalence of Dirofilaria immitis and D. repens in the Old World. Vet. Parasitol. 2020, 280, 108995. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Carretón, E.; Morchón, R.; Silveira-Viera, L.; Falcón, Y.; Simón, F. The impact of the climate on the epidemiology of Dirofilaria immitis in the pet population of the Canary Islands. Vet. Parasitol. 2016, 216, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Morchón, R.; Rodríguez-Escolar, I.; Lambraño, R.E.H.; Agudo, J.Á.S.; Montoya-Alonso, J.A.; Serafín-Pérez, I.; Fernández-Serafín, C.; Carretón, E. Assessment Heartworm Disease in the Canary Islands (Spain): Risk of Transmission in a Hyperendemic Area by Ecological Niche Modeling and Its Future Projection. Animals 2023, 13, 3251. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, N.; Lopata, A.L.; Jeebhay, M.F.; Herbert, D.R.; Robins, T.G.; Brombacher, F. Exposure to the fish parasite Anisakis causes allergic airway disease and hypersensitivity reactions in fish-processing workers. J. Allergy Clin. Immunol. 2006, 117, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Armentia, A.; Martín-Armentia, S.; Pineda, F.; Martín-Armentia, B.; Castro, M.; Fernández, S.; Moro, A.; Castillo, M. Allergic hypersensitivity to garlic and onion in children and adults. Allergol. Immunopathol. 2020, 48, 232–236. [Google Scholar] [CrossRef]
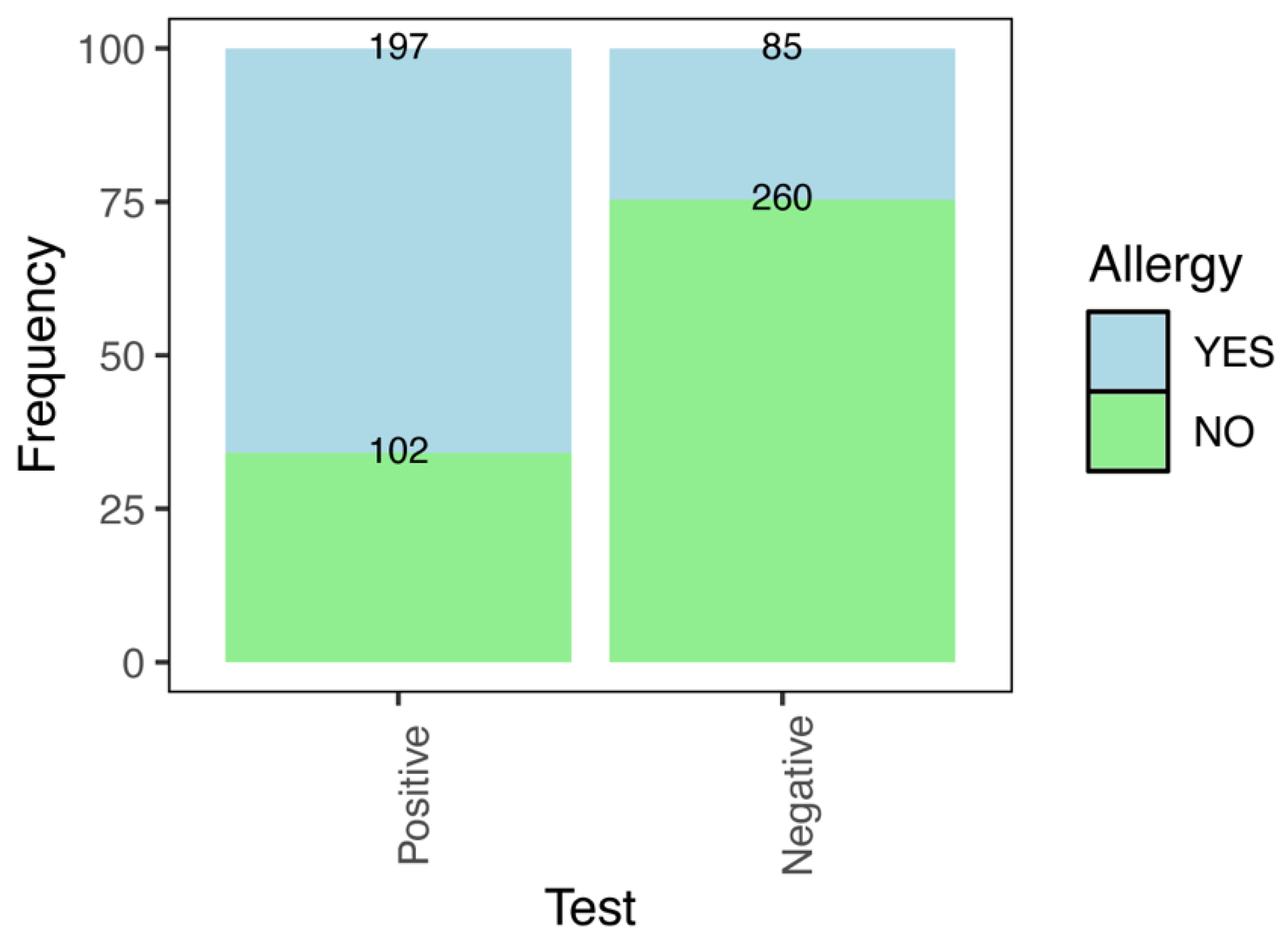
| Heartworm (Test) | Total Cases | Allergy in Owners (Frequency) | Odds Ratio | |
|---|---|---|---|---|
| Absolute | Relative (%) | (CI 2.5–97.5%) | ||
| Negative | 345 | 85 | 24.64 | Ref. |
| Positive | 299 | 197 | 65.89 | 5.91 (4.21–8.35) |
| Total | 644 | 282 | 43.79 | |
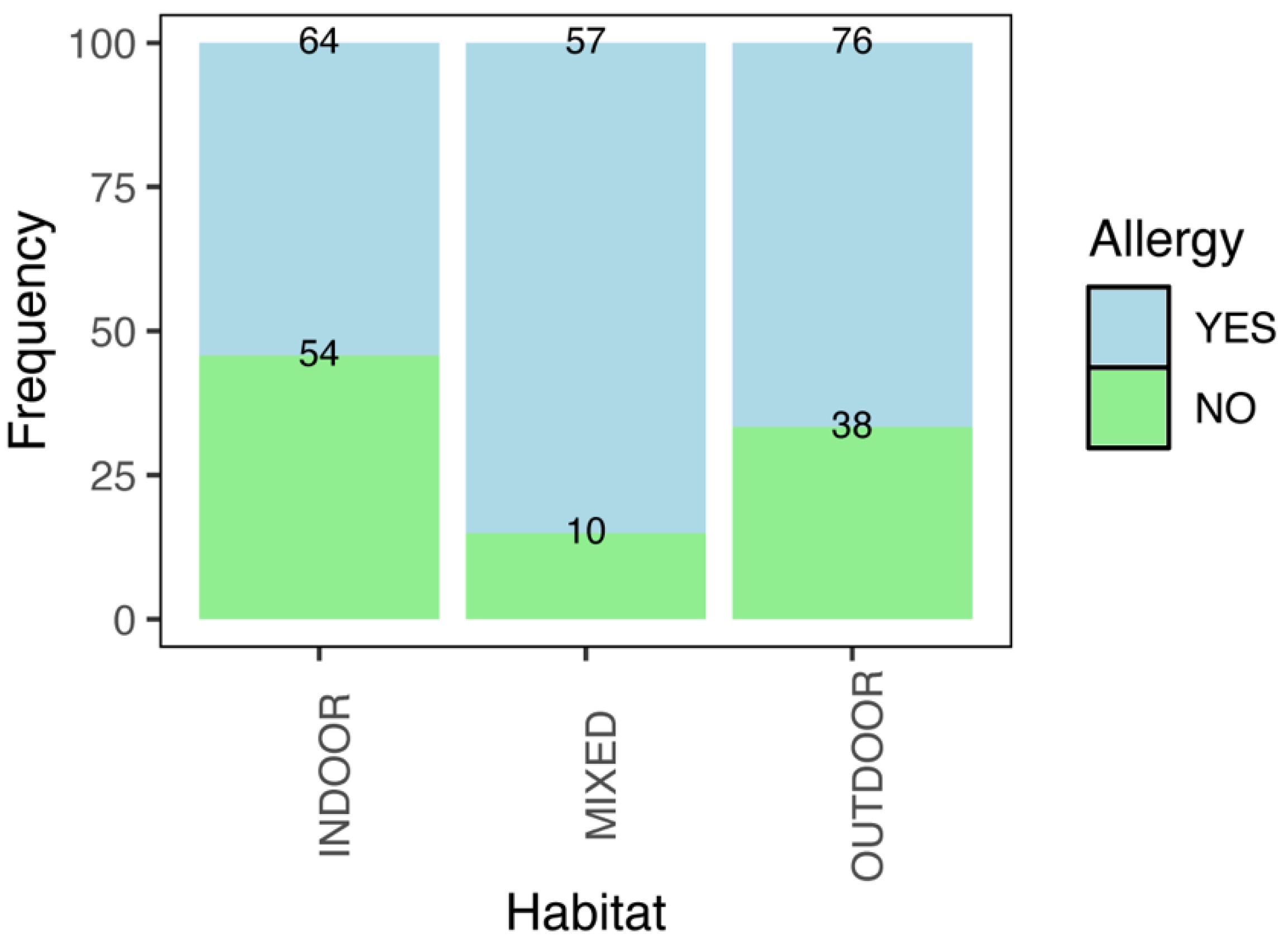
| Habitat of Heartworm | Total Cases | Allergy in Owners (Frequency) | Odds Ratio | |
|---|---|---|---|---|
| Positive Test | Absolute | Relative (%) | (CI 2.5–97.5%) | |
| Indoor | 118 | 64 | 54.24 | Ref. |
| Outdoor | 114 | 76 | 66.67 | 1.69 (0.99–2.89) |
| Mixed | 67 | 57 | 85.07 | 4.81 (2.32–10.82) |
| Total | 299 | 197 | 65.89 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoya-Alonso, J.A.; Balmori-de la Puente, A.; Costa-Rodríguez, N.; Matos, J.I.; Carretón, E.; Morchón, R. A One Health Perspective on Heartworm Disease: Allergy Risk in Owners of Infected Dogs in Gran Canaria (Spain). Animals 2025, 15, 3084. https://doi.org/10.3390/ani15213084
Montoya-Alonso JA, Balmori-de la Puente A, Costa-Rodríguez N, Matos JI, Carretón E, Morchón R. A One Health Perspective on Heartworm Disease: Allergy Risk in Owners of Infected Dogs in Gran Canaria (Spain). Animals. 2025; 15(21):3084. https://doi.org/10.3390/ani15213084
Chicago/Turabian StyleMontoya-Alonso, José Alberto, Alfonso Balmori-de la Puente, Noelia Costa-Rodríguez, Jorge Isidoro Matos, Elena Carretón, and Rodrigo Morchón. 2025. "A One Health Perspective on Heartworm Disease: Allergy Risk in Owners of Infected Dogs in Gran Canaria (Spain)" Animals 15, no. 21: 3084. https://doi.org/10.3390/ani15213084
APA StyleMontoya-Alonso, J. A., Balmori-de la Puente, A., Costa-Rodríguez, N., Matos, J. I., Carretón, E., & Morchón, R. (2025). A One Health Perspective on Heartworm Disease: Allergy Risk in Owners of Infected Dogs in Gran Canaria (Spain). Animals, 15(21), 3084. https://doi.org/10.3390/ani15213084








