Identification of Expression Quantitative Trait Loci (eQTL) for Adipose-Specific Regulatory Mechanisms in Hanwoo (Korean Cattle)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Tissue Collection
2.2. RNA Sequencing and Gene Expression Quantification
2.3. Genotype Data Preparation and Functional Annotation
2.4. Identification of eQTLs and Regulatory Hotspot Mapping
2.5. Functional Enrichment Analysis Using ClueGO
3. Results
3.1. RNA-Seq Data Processing and Variant Calling for eQTL Analysis
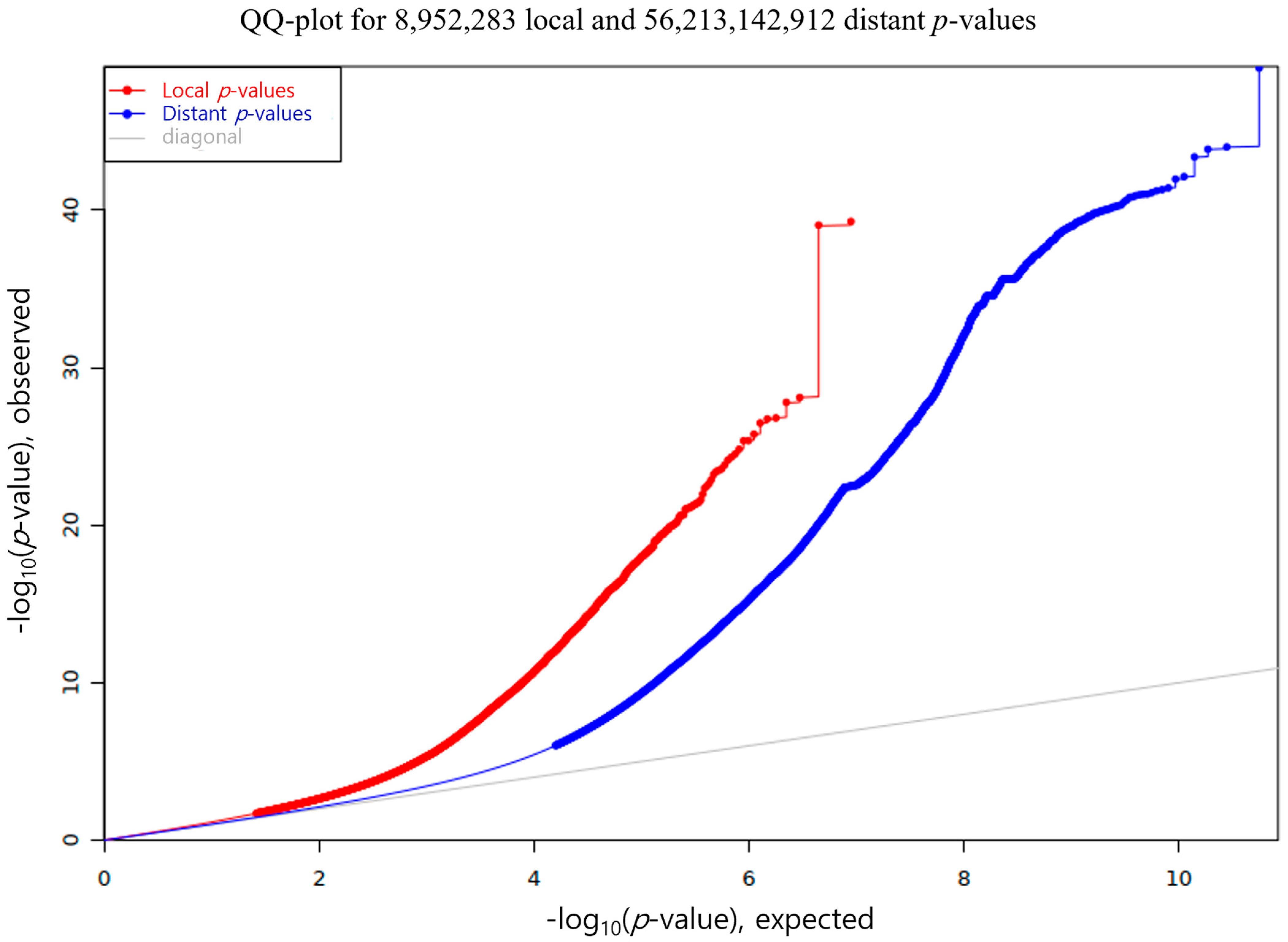
3.2. eQTL Mapping in Backfat Tissue
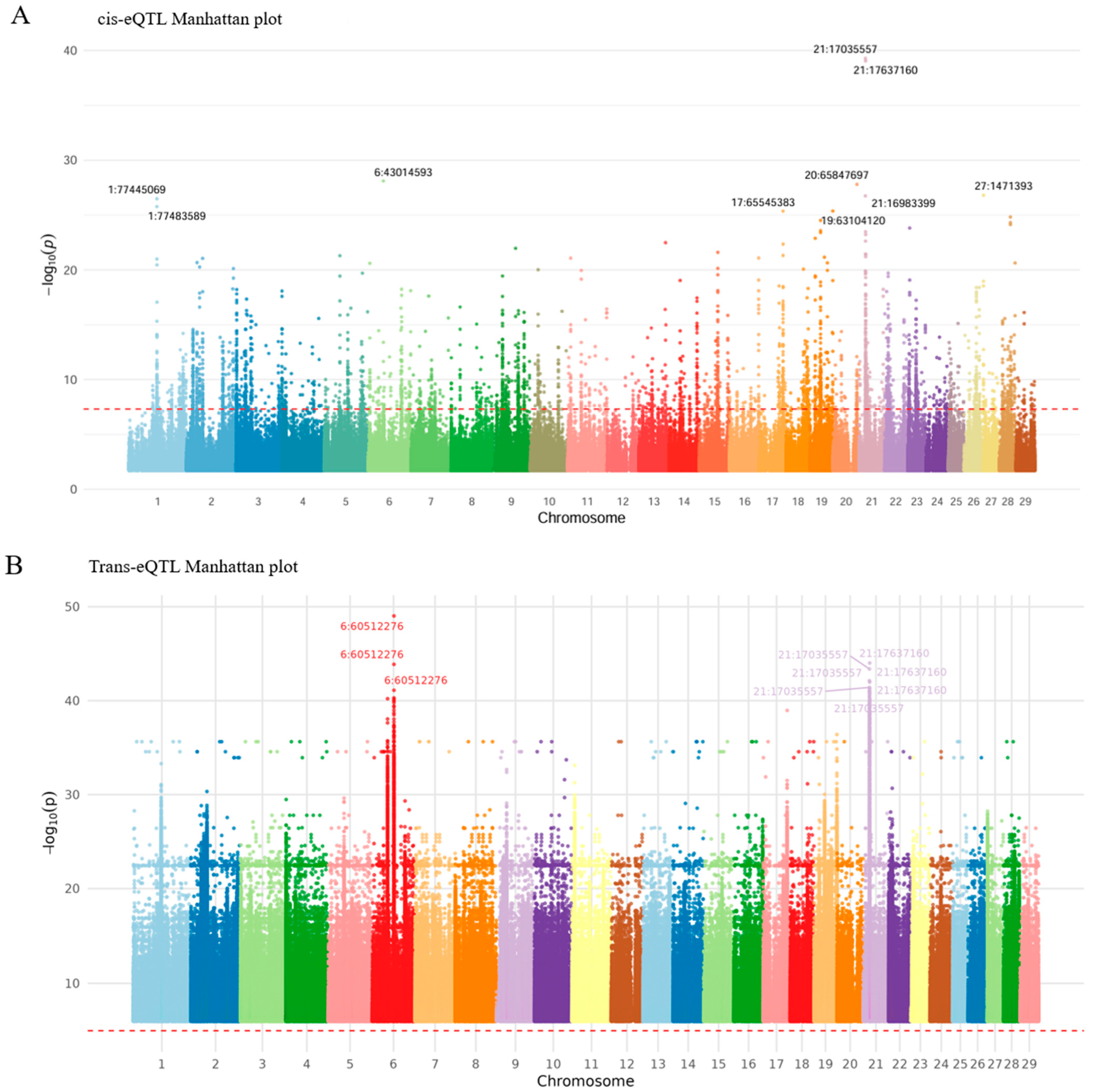
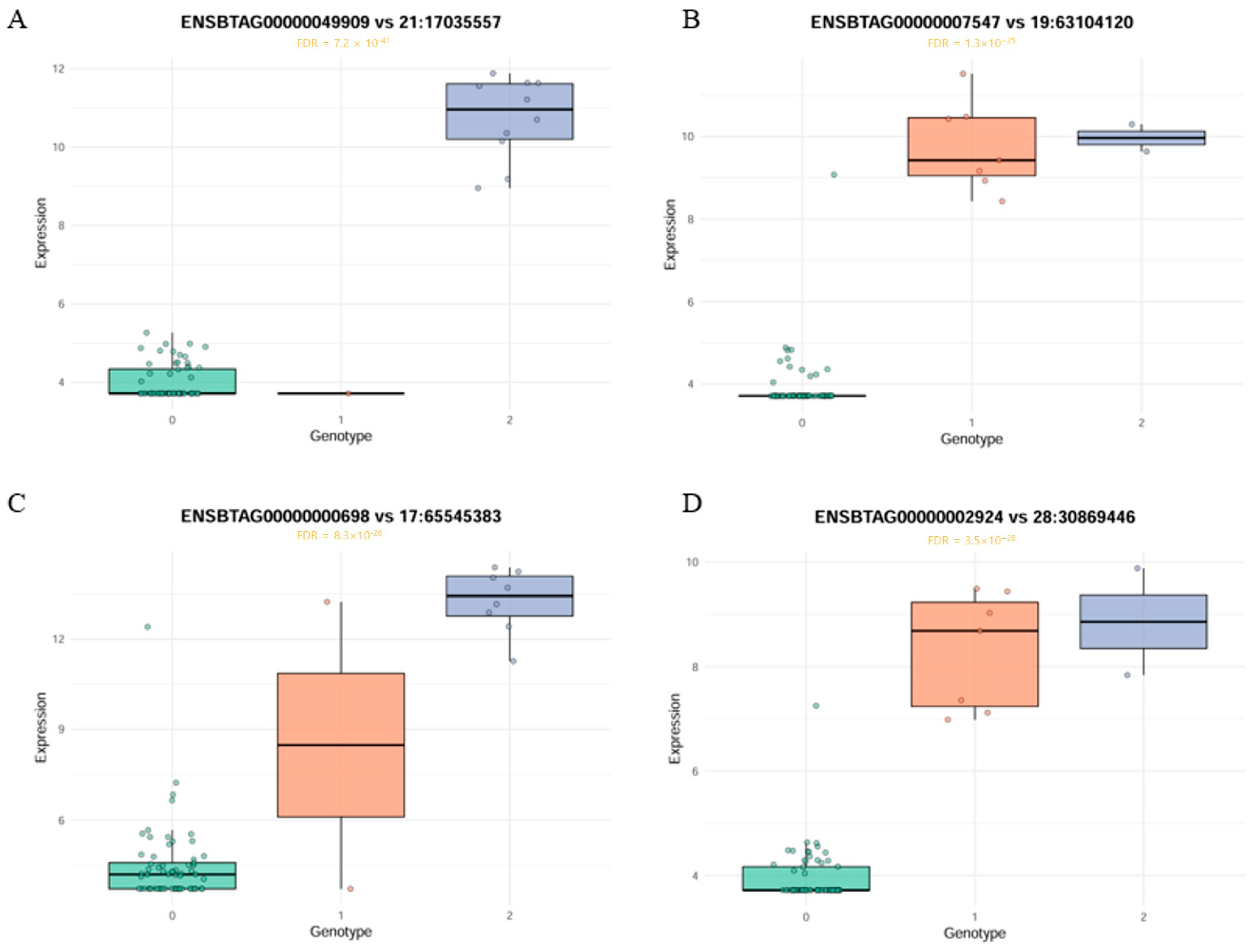
3.3. Identification of Significant cis-eQTL Associations and cis-eQTL Hotspot
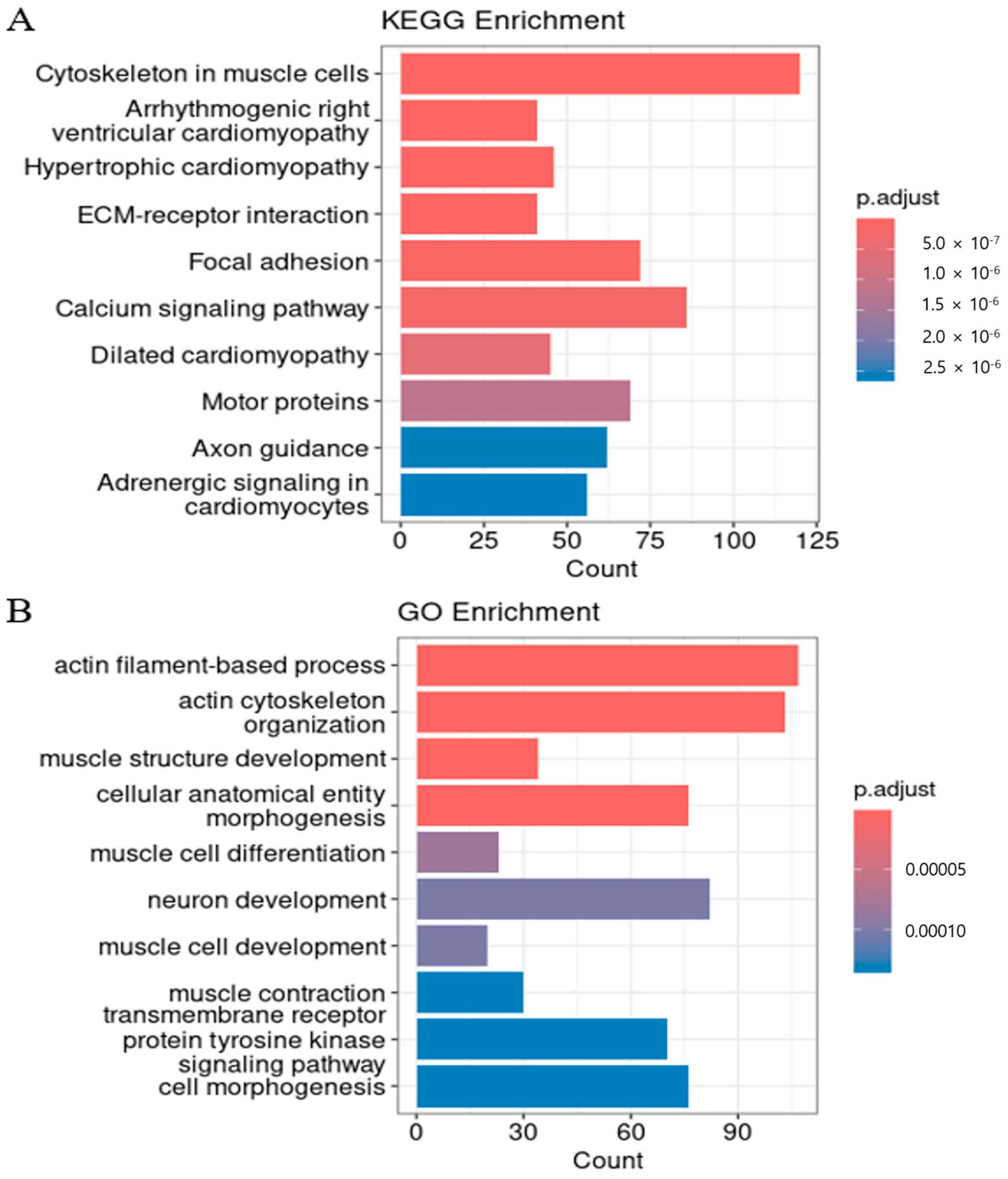
3.4. Functional Characterization of Genes Regulated by Cis-eQTL Genes
3.5. Identification and Functional Enrichment Analysis of a trans-eQTL Hotspot
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef]
- Abebe, B.K.; Wang, J.; Guo, J.; Wang, H.; Li, A.; Zan, L. A review of emerging technologies, nutritional practices, and management strategies to improve intramuscular fat composition in beef cattle. Anim. Biotechnol. 2024, 35, 2388704. [Google Scholar] [CrossRef] [PubMed]
- Tegeler, A.P.; Ford, H.R.; Fiallo-Diez, J.F.; Michelotti, T.C.; Johnson, B.J.; Benitez, O.J.; Woerner, D.R.; Strieder-Barboza, C. Transcriptome and Cellular Evidence of Depot-Specific Function in Beef Cattle Intramuscular, Subcutaneous, and Visceral Adipose Tissues. Biology 2025, 14, 848. [Google Scholar] [CrossRef] [PubMed]
- Sebo, Z.L.; Jeffery, E.; Holtrup, B.; Rodeheffer, M.S. A mesodermal fate map for adipose tissue. Development 2018, 145, dev166801. [Google Scholar] [CrossRef] [PubMed]
- Nica, A.C.; Dermitzakis, E.T. Expression quantitative trait loci: Present and future. Philos. Trans. R. Soc. Lond B Biol. Sci. 2013, 368, 20120362. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.G.; Fitzsimons, C.; McClure, M.C.; McKenna, C.; Conroy, S.; Kenny, D.A.; McGee, M.; Waters, S.M.; Morris, D.W. GWAS and eQTL analysis identifies a SNP associated with both residual feed intake and GFRA2 expression in beef cattle. Sci. Rep. 2018, 8, 14301. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, Y.; Chang, T.; Wang, Z.; Zhu, B.; Chen, Y.; Gao, X.; Xu, L.; Zhang, L.; Gao, H.; et al. The eQTL colocalization and transcriptome-wide association study identify potentially causal genes responsible for economic traits in Simmental beef cattle. J. Anim. Sci. Biotechnol. 2023, 14, 78. [Google Scholar] [CrossRef]
- Jang, S.; Jang, S.; Kim, J.; Park, W. Multi-tissue transcriptome analysis to identify candidate genes associated with weight regulation in Hanwoo cattle. Front. Genet. 2024, 14, 1304638. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Pirrung, M.; McCue, L.A. FQC Dashboard: Integrates FastQC results into a web-based, interactive, and extensible FASTQ quality control tool. Bioinformatics 2017, 33, 3137–3139. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; Del Angel, G.; Rivas, M.A.; Hanna, M. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.; Daly, M.J. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Shabalin, A.A. Matrix eQTL: Ultra fast eQTL analysis via large matrix operations. Bioinformatics 2012, 28, 1353–1358. [Google Scholar] [CrossRef]
- Qu, W.; Gurdziel, K.; Pique-Regi, R.; Ruden, D.M. Lead modulates trans-and cis-expression quantitative trait loci (eQTLs) in Drosophila melanogaster heads. Front. Genet. 2018, 9, 395. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Wu, Y.; Lemmon, Z.H.; Xu, G.; Huang, C.; Liang, Y.; Xu, D.; Li, D.; Doebley, J.F. Genome-wide analysis of transcriptional variability in a large maize-teosinte population. Mol. Plant 2018, 11, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Albert, F.W.; Bloom, J.S.; Siegel, J.; Day, L.; Kruglyak, L. Genetics of trans-regulatory variation in gene expression. eLife 2018, 7, e35471. [Google Scholar] [CrossRef]
- Zhang, G.; Roberto, N.M.; Lee, D.; Hahnel, S.R.; Andersen, E.C. The impact of species-wide gene expression variation on Caenorhabditis elegans complex traits. Nat. Commun. 2022, 13, 3462. [Google Scholar] [CrossRef]
- Van Dyke, K.; Lutz, S.; Mekonnen, G.; Myers, C.L.; Albert, F.W. Trans-acting genetic variation affects the expression of adjacent genes. Genetics 2021, 217, iyaa051. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Consortium, G. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- He, B.; Yin, J.; Gong, S.; Gu, J.; Xiao, J.; Shi, W.; Ding, W.; He, Y. Bioinformatics analysis of key genes and pathways for hepatocellular carcinoma transformed from cirrhosis. Medicine 2017, 96, e6938. [Google Scholar] [CrossRef] [PubMed]
- Albert, F.W.; Kruglyak, L. The role of regulatory variation in complex traits and disease. Nat. Rev. Genet. 2015, 16, 197–212. [Google Scholar] [CrossRef]
- Potolitsyna, E.; Pickering, S.H.; Bellanger, A.; Germier, T.; Collas, P.; Briand, N. Cytoskeletal rearrangement precedes nucleolar remodeling during adipogenesis. Commun. Biol. 2024, 7, 458. [Google Scholar] [CrossRef]
- Yang, W.; Guo, X.; Thein, S.; Xu, F.; Sugii, S.; Baas, P.W.; Radda, G.K.; Han, W. Regulation of adipogenesis by cytoskeleton remodelling is facilitated by acetyltransferase MEC-17-dependent acetylation of α-tubulin. Biochem. J. 2013, 449, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.C.; Qi, X.; Gjeluci, K.; Leussis, M.P.; Basu, H.; Reis, S.A.; Zhao, W.N.; Piguel, N.H.; Penzes, P.; Haggarty, S.J.; et al. Disruption of the psychiatric risk gene Ankyrin 3 enhances microtubule dynamics through GSK3/CRMP2 signaling. Transl. Psychiatry 2018, 8, 135. [Google Scholar] [CrossRef]
- Shi, H.; Halvorsen, Y.-D.; Ellis, P.N.; Wilkison, W.O.; Zemel, M.B. Role of intracellular calcium in human adipocyte differentiation. Physiol. Genom. 2000, 3, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, K.; Lee, S.-H.; Chung, K.-Y.; Park, J.-E.; Jang, G.-W.; Park, M.-R.; Kim, N.Y.; Kim, T.-H.; Chai, H.-H.; Park, W.C.; et al. A Gene-Set Enrichment and Protein-Protein Interaction Network-Based GWAS with Regulatory SNPs Identifies Candidate Genes and Pathways Associated with Carcass Traits in Hanwoo Cattle. Genes 2020, 11, 316. [Google Scholar] [CrossRef]
- Mariman, E.C.; Wang, P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell. Mol. Life Sci. 2010, 67, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Mor-Yossef Moldovan, L.; Lustig, M.; Naftaly, A.; Mardamshina, M.; Geiger, T.; Gefen, A.; Benayahu, D. Cell shape alteration during adipogenesis is associated with coordinated matrix cues. J. Cell. Physiol. 2019, 234, 3850–3863. [Google Scholar] [CrossRef]
- Ramírez-Zamudio, G.D.; Ganga, M.J.G.; Pereira, G.L.; Nociti, R.P.; Chiaratti, M.R.; Cooke, R.F.; Chardulo, L.A.L.; Baldassini, W.A.; Machado-Neto, O.R.; Curi, R.A. Effect of Cow-Calf Supplementation on Gene Expression, Processes, and Pathways Related to Adipogenesis and Lipogenesis in Longissimus thoracis Muscle of F1 Angus × Nellore Cattle at Weaning. Metabolites 2023, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.C.F.; Silva, E.N.; Frezarim, G.B.; Salatta, B.M.; Baldi, F.; Fonseca, L.F.S.; De Albuquerque, L.G.; Muniz, M.M.M.; Silva, D.B.D.S. Cis-eQTL analysis reveals genes involved in biological processes of the immune system in Nelore cattle. Gene 2025, 937, 149138. [Google Scholar]
- Brümmer, A.; Bergmann, S. Disentangling genetic effects on transcriptional and post-transcriptional gene regulation through integrating exon and intron expression QTLs. Nat. Commun. 2024, 15, 3786. [Google Scholar] [CrossRef]
- Rogowski, K.; van Dijk, J.; Magiera, M.M.; Bosc, C.; Deloulme, J.-C.; Bosson, A.; Peris, L.; Gold, N.D.; Lacroix, B.; Grau, M.B.; et al. A Family of Protein-Deglutamylating Enzymes Associated with Neurodegeneration. Cell 2010, 143, 564–578. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, W.; Shao, W.; Li, F.; Gao, Y.; Yao, K.; Yang, M.; Zhang, X.; Wang, Y.; Yang, L. Exploration of non-coding RNAs related to intramuscular fat deposition Xinjiang Brown cattle and Angus × Wagyu cattle. BMC Genom. 2025, 26, 249. [Google Scholar] [CrossRef]
- Price, M.G.; Davis, C.F.; Deng, F.; Burgess, D.L. The α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate receptor trafficking regulator “stargazin” is related to the claudin family of proteins by its ability to mediate cell-cell adhesion. J. Biol. Chem. 2005, 280, 19711–19720. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guo, D.; Zhu, R.; Wang, H.; Yang, C.; Shi, D.; Shang, J. Identification of highly expressed genes and efficient core promoters specific to buffalo skeletal muscles. Arch. Anim. Breed. 2025, 68, 67–76. [Google Scholar] [CrossRef]
- Berger, J.; Berger, S.; Li, M.; Currie, P.D. Myo18b is essential for sarcomere assembly in fast skeletal muscle. Hum. Mol. Genet. 2017, 26, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.M.; West, R.C.; Hayes, C.S.; Waddell, D.S. Dual-specificity phosphatase 29 is induced during neurogenic skeletal muscle atrophy and attenuates glucocorticoid receptor activity in muscle cell culture. Am. J. Physiol. Cell Physiol. 2020, 319, C441–C454. [Google Scholar] [CrossRef] [PubMed]
- Ogunbawo, A.R.; Mulim, H.A.; Campos, G.S.; Oliveira, H.R. Genetic foundations of Nellore traits: A gene prioritization and Functional Analyses of Genome-Wide Association Study results. Genes 2024, 15, 1131. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G. The developmental basis of mesenchymal stem/stromal cells (MSCs). BMC Dev. Biol. 2015, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.; Simmons, R.A. The role of adipocyte precursors in development and obesity. Front. Endocrinol. 2021, 11, 613606. [Google Scholar] [CrossRef]
- Smolková, K.; Ježek, P. The Role of Mitochondrial NADPH-Dependent Isocitrate Dehydrogenase in Cancer Cells. Int. J. Cell Biol. 2012, 2012, 273947. [Google Scholar] [CrossRef]
- Urbani, G.; Distrutti, E.; Biagioli, M.; Marchianò, S.; Fiorucci, S. How Smell Regulates Metabolism: The Role of Ectopically Expressed Olfactory Receptors in Lipid and Glucose Homeostasis. J. Transl. Sci. 2022, 8, 470. [Google Scholar] [CrossRef]
- Hasin-Brumshtein, Y.; Khan, A.H.; Hormozdiari, F.; Pan, C.; Parks, B.W.; Petyuk, V.A.; Piehowski, P.D.; Brümmer, A.; Pellegrini, M.; Xiao, X. Hypothalamic transcriptomes of 99 mouse strains reveal trans eQTL hotspots, splicing QTLs and novel non-coding genes. eLife 2016, 5, e15614. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Petsch, K.; Shimizu, R.; Liu, S.; Xu, W.W.; Ying, K.; Yu, J.; Scanlon, M.J.; Schnable, P.S.; Timmermans, M.C. Mendelian and non-Mendelian regulation of gene expression in maize. PLoS Genet. 2013, 9, e1003202. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.M.; White, M.A.; Tautz, D.; Payseur, B.A. Genomic networks of hybrid sterility. PLoS Genet. 2014, 10, e1004162. [Google Scholar] [CrossRef] [PubMed]

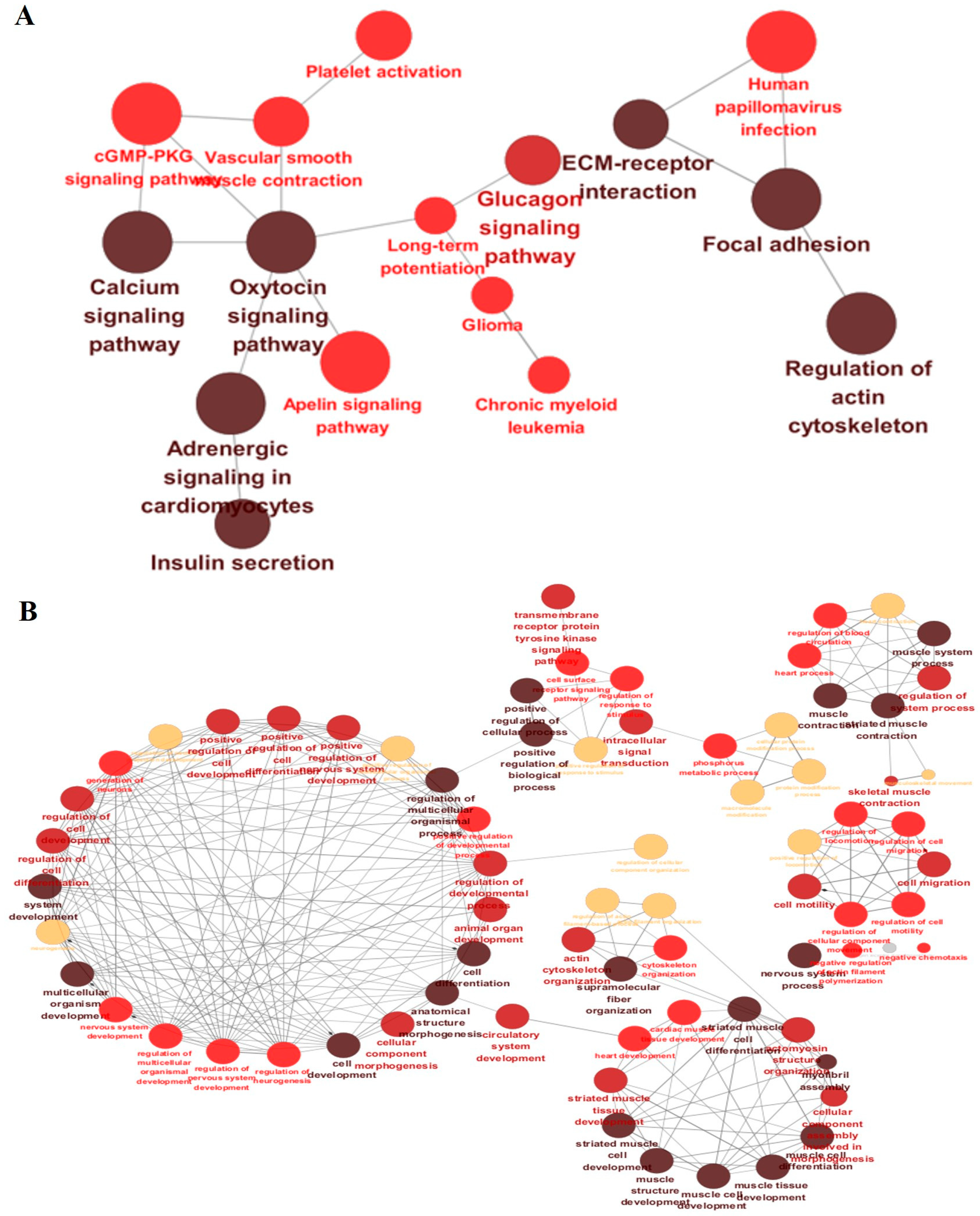
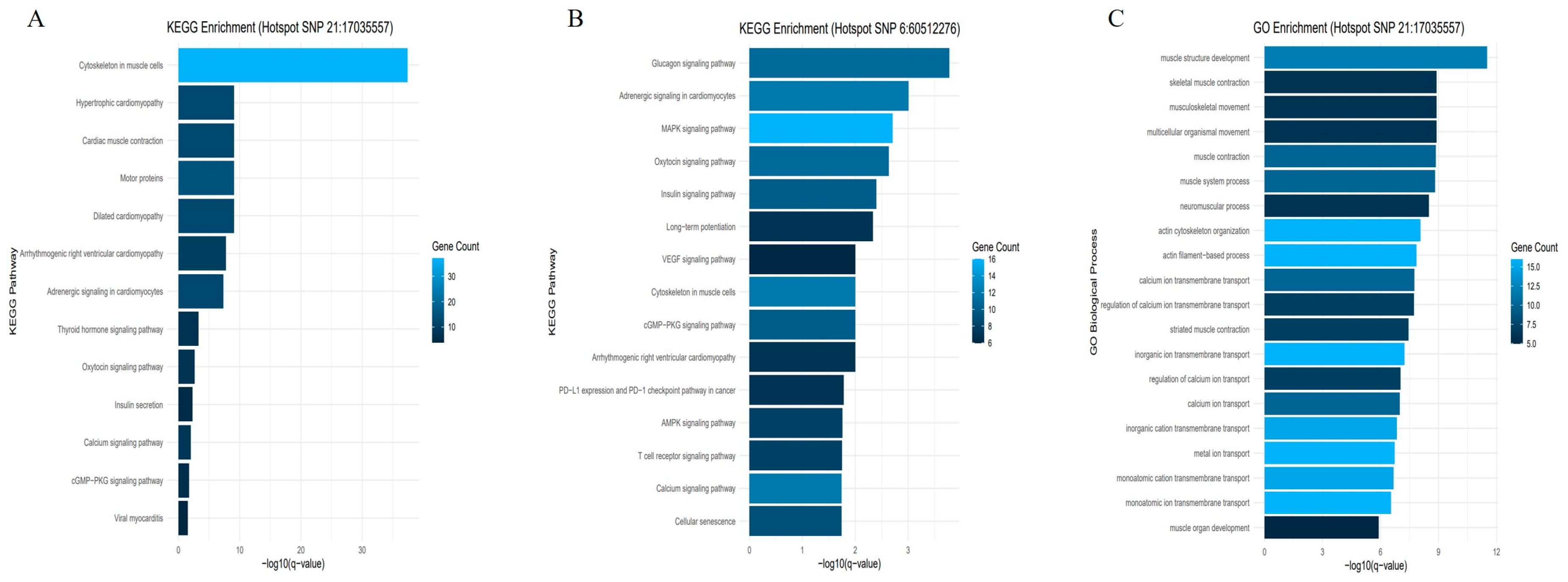
| Rank | CHR | POS | SNP | Gene ID | p-Value | FDR | Beta | MAF | Symbol | Variant Type |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | 17035557 | 21:17035557 | ENSBTAG00000049909 | 5.25 x 10−40 | 4 × 10−33 | 3.264562 | 0.14 | AGBL1 | Intron variant |
| 2 | 6 | 43014593 | 6:43014593 | ENSBTAG00000067097 | 7.92 × 10−29 | 2.36 × 10−22 | 2.255543 | 0.113333 | NA | Intron variant |
| 3 | 20 | 65847697 | 20:65847697 | ENSBTAG00000051967 | 1.61 × 10−28 | 3.6 × 10−22 | 2.54634 | 0.02 | NA | Upstream gene variant |
| 4 | 27 | 1471393 | 27:1471393 | ENSBTAG00000009387 | 1.58 × 10−27 | 2.71 × 10−21 | 4.559134 | 0.173333 | MYOM2 | Intron variant |
| 5 | 1 | 77445069 | 1:77445069 | ENSBTAG00000015460 | 3.3 × 10−27 | 4.23 × 10−21 | 2.994177 | 0.113333 | TP63 | Intron variant |
| 6 | 19 | 63104120 | 19:63104120 | ENSBTAG00000007547 | 4.37 × 10−26 | 3.98 × 10−20 | 4.236663 | 0.073333 | CACNG1 | Intron variant |
| 7 | 17 | 65545383 | 17:65545383 | ENSBTAG00000000698 | 4.44 × 10−26 | 3.98 × 10−20 | 4.30032 | 0.12 | MYO18B | Intron variant |
| 8 | 28 | 30869446 | 28:30869446 | ENSBTAG00000002924 | 1.54 × 10−25 | 1.25 × 10−19 | 3.264954 | 0.073333 | DUSP29 | Synonymous variant |
| 9 | 19 | 29483289 | 19:29483289 | ENSBTAG00000009702 | 3.15 × 10−25 | 2.35 × 10−19 | 2.823308 | 0.16 | MYH8 | Splice region & Intron variant |
| 10 | 28 | 30869446 | 28:30869446 | ENSBTAG00000059987 | 4.95 × 10−25 | 3.41 × 10−19 | 3.610908 | 0.073333 | DUSP13A | Synonymous variant |
| Functional Enrichment | Gene Ontology (GO) Term | Genes (Truncated) | False Discovery Rate (FDR) | p-Value |
|---|---|---|---|---|
| Biological Process | GO:0030029 ~ actin filament-based process | PLS1, ECT2, MARCKSL1, MYO1B, VIL1, ARHGEF10L, NEB, XIRP2, KANK4, ESPNL (+97 more) | 2.48939 × 10−7 | 1.07394 × 10−10 |
| Biological Process | GO:0061061 ~ muscle structure development | DES, SPEG, NEB, TMOD4, MYF6, MYF5, LMOD2, PDLIM5, PGM5, TMOD1 (+24 more) | 2.75832 × 10−7 | 3.56988 × 10−10 |
| Biological Process | GO:0031032 ~ actomyosin structure organization | ECT2, ARHGEF10L, NEB, TMOD4, LMOD2, LIMCH1, PGM5, TMOD1, ROCK2, RTKN (+14 more) | 0.000136244 | 7.57241 × 10−7 |
| Biological Process | GO:0007169 ~ transmembrane receptor protein tyrosine kinase signaling pathway | EPHA3, EPHB1, EPHB3, CBLB, FGR, NRP2, ERBB4, PID1, EPHA4, IGFBP5 (+60 more) | 0.000136244 | 5.73298 × 10−7 |
| Cellular Component | GO:0030017 ~ sarcomere | SYNC, DES, NEB, FHL3, TMOD4, PPP1R12A, LMOD2, MYOZ2, PDLIM5, TMOD1 (+28 more) | 5.4 × 10−13 | 3 × 10−15 |
| Cellular Component | GO:0016459 ~ myosin complex | MYH15, MYL1, MYO1B, MYO1A, MYO9B, MYO1F, MYO6, MYO1E, MYO5C, BMF (+19 more) | 3.10439 × 10−8 | 4.77598 × 10−10 |
| Cellular Component | GO:0005925 ~ focal adhesion | LPP, ITGB2, LIMS2, ASAP3, ITGB6, XIRP2, PARVB, PARVG, ARHGAP24, PTK2B (+9 more) | 0.001318107 | 4.05571 × 10−5 |
| Molecular Function | GO:0003774 ~ cytoskeletal motor activity | MYH15, DNAH7, MYO1B, KIF5C, KIF1A, MYO1A, DNAH11, CENPE, KIF24, KIF13B (+38 more) | 1.29268 × 10−8 | 4.5601 × 10−11 |
| Molecular Function | GO:0019199 ~ transmembrane receptor protein kinase activity | EPHA3, EPHB1, EPHB3, NRP2, ERBB4, EPHA4, ACVR1, ACVR1C, DDR2, STYK1 (+18 more) | 0.000891088 | 7.02566 × 10−6 |
| Molecular Function | GO:0005261 ~ monoatomic cation channel activity | KCNAB1, KCNH8, CATSPER4, CHRND, CHRNG, SCN3A, SCN9A, CHRNA1, SCN7A, KCNJ13 (+65 more) | 0.003135404 | 3.7081 × 10−5 |
| Molecular Function | GO:0005201 ~ extracellular matrix structural constituent | COL6A5, COL6A6, COL4A4, COL4A3, COL11A1, COL1A2, MMRN1, MEGF9, COL5A1, LAMA2 (+6 more) | 0.00667519 | 1.20924 × 10−4 |
| Molecular Function | GO:0004683 ~ calmodulin-dependent protein kinase activity | MKNK1, CAMK2B, CAMK2D, CAMK4, CAMK2A, CAMK1D, MAPKAPK2, MAPKAPK3, CAMK2G, EEF2K (+1 more) | 0.00527478 | 6.93138 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Jeong, T.; Park, W.; Jang, S.; Lee, P.-Y.; Lim, D. Identification of Expression Quantitative Trait Loci (eQTL) for Adipose-Specific Regulatory Mechanisms in Hanwoo (Korean Cattle). Animals 2025, 15, 3082. https://doi.org/10.3390/ani15213082
Lee J, Jeong T, Park W, Jang S, Lee P-Y, Lim D. Identification of Expression Quantitative Trait Loci (eQTL) for Adipose-Specific Regulatory Mechanisms in Hanwoo (Korean Cattle). Animals. 2025; 15(21):3082. https://doi.org/10.3390/ani15213082
Chicago/Turabian StyleLee, Junyoung, Taejoon Jeong, Woncheoul Park, Sunsik Jang, Poong-Yeon Lee, and Dajeong Lim. 2025. "Identification of Expression Quantitative Trait Loci (eQTL) for Adipose-Specific Regulatory Mechanisms in Hanwoo (Korean Cattle)" Animals 15, no. 21: 3082. https://doi.org/10.3390/ani15213082
APA StyleLee, J., Jeong, T., Park, W., Jang, S., Lee, P.-Y., & Lim, D. (2025). Identification of Expression Quantitative Trait Loci (eQTL) for Adipose-Specific Regulatory Mechanisms in Hanwoo (Korean Cattle). Animals, 15(21), 3082. https://doi.org/10.3390/ani15213082





