Simple Summary
Litopenaeus vannamei, as a high-quality protein source, is widely favored by aquaculturists. However, it is frequently affected by pathogens during cultivation, among which White Spot Syndrome Virus (WSSV) is one of the most lethal pathogens in shrimp aquaculture, severely restricting the development of the shrimp industry. In this study, a resistance gene (ALF-like) capable of inhibiting WSSV is described. To date, no antiviral drugs developed using this gene as a target have been identified. From this perspective, the molecular mechanism study on the ALF-like gene in L. vannamei conducted herein is of great significance. This work is aimed at evaluating the potential of the ALF-like gene for targeted antiviral drug development. As confirmed by the study, the ALF-like gene can play an important anti-WSSV role in disease resistance research of L. vannamei and can serve as a candidate gene for developing targeted antiviral drugs.
Abstract
Anti-lipopolysaccharide factors (ALFs) are an important molecular category within the antimicrobial peptide family. They play a crucial role in resisting pathogen infections and are of importance in the innate immune system of shrimp. A novel ALF-like gene was identified from L. vannamei in this study. Its expression profile was investigated after WSSV infection. Results demonstrated that the mRNA transcription level of the ALF-like gene was significantly upregulated in hemocytes, hepatopancreas, gills, and intestines of L. vannamei. When the mRNA transcription level of the ALF-like gene was inhibited, the expression levels of key WSSV genes (VP 28 and IE 1) were significantly upregulated, accompanied by a decrease in shrimp survival rate. Meanwhile, the expression of genes involved in the apoptotic pathway (Lv-Caspase 3, Lv-Caspase 8, and Lv-Bcl 2) and antioxidant enzyme pathway (Lv-GST, Lv-CAT, Lv-Prx, Lv-GPX, and Lv-SOD) was also significantly increased. Flow cytometry further revealed that the hemocyte apoptosis rate induced by WSSV infection was reduced when the transcription level of the target gene was inhibited. These results indicate that the Lv-ALF-like gene plays an important regulatory role in the resistance of L. vannamei to WSSV infection, and studying the function of this gene is of great significance for disease prevention and control of shrimp.
1. Introduction
Within the category of aquatic proteins, shrimp products occupy a very important position [1,2]. However, during the breeding process, bacterial (like Vibrio parahaemolyticus) and viral (WSSV) infections frequently occur, resulting in substantial mortality among farmed shrimp [3,4]. White spot syndrome disease, caused by the WSSV, frequently affects shrimp and crab species [5], leading to substantial economic losses in the crustacean aquaculture industry. To date, there is no effective method to prevent the widespread transmission of this pathogen. Therefore, employing molecular biology techniques to investigate the molecular mechanisms between pathogens and their hosts is of significant importance for pathogen prevention and control, as well as for the development of healthy breeding strategies. Invertebrates lack acquired immunity and are currently believed to rely solely on their innate immune system to combat pathogens [6]. However, research on the immune system of the model organism Drosophila has revealed that it is primarily focused on the Toll, IMD, and JAK-STAT signaling pathways [7,8,9]. Furthermore, various immune effector molecules have been identified, including antimicrobial peptides, antibiotics, lysozyme, anti-lipopolysaccharide factors (ALFs) and diverse lectin molecules. These molecules play crucial roles in both antibacterial and antiviral immune responses [10]. Among the molecules, antimicrobial peptides (AMPs) have been crucial components of the innate immune system [11]. Notably, ALF is a specific type of AMP originally isolated from the hemolymph of Horseshoe crabs that exhibits activity against Gram-negative bacteria and endotoxins [12].
Antimicrobial peptides known as ALFs in crustacean aquatic animals frequently exhibit significant antibacterial activity [13,14]. Various subtypes of ALF have been identified in species such as Penaeus vannamei, Penaeus monodon, and Fenneropenaeus chinensis among others [15,16,17,18,19,20]. In a study on Fenneropenaeus chinensis, infection with WSSV led to an upward trend in Fc-ALF expression levels throughout the period from incubation to rapid outbreak [17]. These findings suggest that Fc-ALF may play a significant role in the defense against WSSV infection. Additionally, it was observed that the LBD peptides of ALF1, ALF2, ALF5, and ALF7 molecules in Penaeus chinensis can effectively inhibit the proliferation of WSSV in in vivo application experiments [21]. The ALF molecule has also been successfully identified in studies involving L. vannamei, where the mRNA and protein levels of Lv-AV-K exhibited an increase following infection with WSSV and Vibrio anguillarum [22]. In the study of the molecular function of ALF in aquatic species, particularly shrimp, it was discovered that the biological role of this molecule is being progressively analyzed and revealed [23]. Following the infection of freshwater crayfish with the WSSV, the transcript level of ALF exhibited an upregulated expression trend. The application of RNA interference (RNAi) to knock down the ALF gene resulted in increased virus replication [24]. This study demonstrates that ALF plays a role in inhibiting the virus.
In this study, a novel Lv-ALF-like gene, identified in L. vannamei, regulates antiviral innate immunity by suppressing WSSV replication through the modulation of apoptosis and activation of antioxidant pathways. Knockdown of Lv-ALF-like enhances the expression of viral genes (VP 28, IE 1) and reduces shrimp survival rate; meanwhile, it inhibits the decrease in shrimp hemocyte apoptosis rate and is also involved in regulating the expression of genes related to apoptosis and antioxidant pathways. Its hemocyte-specific expression and functional divergence within the ALF family underscore its pivotal role in balancing immune responses; it plays a crucial role in pathogen prevention and control.
2. Materials and Methods
2.1. WSSV Challenge and Sample Collection
In this study, the experimental material consisted of L. vannamei (about 3.4 ± 0.6 g each), from Bangpu Seed Industry Technology Co., Ltd. (Weifang, China). Prior to the commencement of the experiment, a 5-day pre-incubation period was conducted, during which the salt concentration was maintained at 29‰ and the temperature at 24 ± 1 °C, and an air pump was utilized to supply oxygen, with normal feeding provided. The pathogenic WSSV, at a concentration of 4.5 × 106 copies, was stored in the laboratory of the Aquatic Genetics and Breeding Center of the Yellow Sea Fisheries Research Institute of the Chinese Academy of Fishery Sciences (Qingdao, China). The immunostimulation method employed was injection, performed slowly into the abdomen of each shrimp, with 20 μL of WSSV extracted administered per shrimp. A total of 50 shrimp were used in this experiment. Among them, 40 shrimp were infected with WSSV, and 10 shrimp were used as a control group before the immunization. At specified time points 0, 12, 24, 36, 48, and 72 h post injection (hpi), the hemocytes, hepatopancreas, gills, and intestinal tissues of the shrimp were collected. Anticoagulant (10% sodium citrate, pH 7.0) was extracted using a 5 mL sterile syringe and mixed with hemolymph in equal proportions. Then, at each time point, three shrimp were sampled in parallel, and hemolymph and hemocytes were separated by centrifugation at 800× g for 10 min at 4 °C. Some tissues were cryopreserved, while others were utilized for total RNA extraction experiments.
2.2. Total RNA Extraction and cDNA Preparation
In the experiment, we utilized the RNA extraction kit supplied by Vazyme Biotechnology Co., Ltd., (Nanjing, China) to extract total RNA from shrimp tissues (50 mg tissues) following a challenge with WSSV, adhering strictly to the manufacturer’s instructions. The concentration of the extracted total RNA was determined using the NanoPhotometer®N50 (Implen, Schast, Germany). 10 μL of the total RNA was allocated for reverse transcription experiments, while the remaining samples were stored in a −80 °C freezer.
2.3. Molecular Cloning and Bioinformatic Analysis
Using the hepatopancreas of L. vannamei 24 h post challenge as the cDNA template, primers were designed using Primers 3.0 software (https://primer3.ut.ee/, accessed on 15 August 2024). Primers (Lv-ALF-F1 and Lv-ALF-R1) were designed to amplify the ALF-like gene; primer information is shown in Table 1. The experiment mainly utilized Quick Taq HS DyeMix (TOYOBO, Shanghai, China) for PCR experiments. The total reaction system was 25 μL, including 12.5 μL of 2× Quick Taq HS DyeMix (TOYOBO, Shanghai, China), 0.5 μL of each F/R primer (0.2 μM), 1 μL of cDNA template (100 ng/μL), and 10.5 μL of RNase-free ddH2O. The reaction program consisted of an initial denaturation at 95 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 45 s, and extension at 72 °C for 50 s, concluded with a final extension at 72 °C for 5 min. The resulting products were analyzed using 1.0% agarose gel electrophoresis, purified with a purification kit, and subsequently sent to Sangon Biotech Co., Ltd. (Shanghai, China), for sequencing. The comparison of the amino acid sequence of the ALF molecule with those of other molecules is mainly analyzed using the basic local alignment search tool (BLAST 2.17.0) (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 27 August 2024) on the web. The ExPASy online tool (http://www.expasy.org, accessed on 30 August 2024) was then employed to translate the gene sequences and predict the corresponding proteins. Finally, a phylogenetic tree of the proteins was constructed using the Neighbor Joining (NJ) method in MEGA 11.0 software (https://www.megasoftware.net/, accessed on 20 October 2024), with 1000 bootstrap replicates.

Table 1.
The sequences of primers and probes used in this study.
2.4. Tissue Distribution and Expression Pattern Analysis
Hemocytes, hepatopancreas, gills, intestines, and muscle tissues of healthy shrimp were collected to examine the distribution of target genes across various tissues. Concurrently, we challenged L. vannamei with WSSV and collected samples at different time points post challenge. The relative expression of the target genes in each tissue segment was assessed using qRT-PCR technology, following the SYBR Green kit protocol (Shanghai, China). The experimental reaction system comprised a total volume of 20 μL, including 10 μL of SYBR Green, 0.5 μL of primers, and ddH2O to reach the final volume. Lv-ALF-like and 18 S rRNA primers are listed in Table 1, and 18 S rRNA primers served as the internal reference control. The data were calculated using the 2−ΔΔCT method. To enhance the stability of the experiment, three repeated trials were conducted (comprising three technical repetitions). Primer information is as shown in Table 1.
2.5. Double-Stranded RNA Preparation and RNA Interference
Design and synthesize specific primers, ALF and GFP, that contain the T7 promoter sequence, and follow the methodology outlined by Yang et al. [25]. Utilize the PCR-amplified product as a template for synthesizing double-stranded RNA (dsRNA), in accordance with the instructions provided in the in vitro transcription T7 kit (Takara, Japan). Prepare a total reaction volume of 50 μL, consisting of the following reagent components: 8 μg of DNA template, 20 μL of 5× transcription buffer, 2.4 μL of A/U/C/GTP mixture (10 μM each), 60 U of RNasin, and 40 U of T7 RNA polymerase. RNase-free water should be added to achieve a total volume of 50 μL. Mix the components thoroughly and incubate in a 42 °C water bath for 2 h. Subsequently, add 20 μL of 10× DNase I buffer, 6 μL of DNase I, and additional RNase-free water to reach a final volume of 100 μL. Mix well, adjust the temperature to 37 °C, and continue incubation in the water bath for 1 h. To ensure complete removal of the DNA template, purify the mixture using phenol/chloroform followed by ethanol precipitation. Prior to use, adjust the working concentration to 3 μg/μL and perform necessary interference efficiency testing before commencing formal experiments.
A total of 120 shrimp were randomly assigned to four groups, each containing 30 shrimp, and were pre-reared for 5 d during the initial phase of the experiment. During this period, the shrimp were fed normally twice a day. Subsequently, sterile 20 μL PBS and 20 μL WSSV (4.5 × 106 copies) were injected into the second and third abdominal segments of the shrimp, respectively. In the interference experiment, 10 μL of dsALF RNA and dsGFP RNA (as a control group) were first injected, followed by WSSV infection. To enhance the interference effect, a booster injection was administered 24 h after the dsRNA injection. The volume of dsRNA injected was 10 μL. Statistical analysis of the interference effect and shrimp survival rate was conducted at 0, 2, 6, 12, 24, 36, 48, and 72 hpi. The experiment was designed with three parallel experiments, each consisting of three biological replicates. The internal control reference used was 18 S rRNA.
2.6. Detection of Immune Pathway Genes
In this experiment, 80 shrimp were randomly divided into two groups, with 40 shrimp in each group. They were fed normally twice a day. The control was dsGFP + WSSV, and the experimental group was dsALF-like + WSSV. Thirty μg of dsRNA was injected into the second and third abdominal segments of the shrimp, and then an booster injection was given 24 h later. Subsequently, 20 μL of WSSV (4.5 × 106 copies) was injected for immune stimulation. Hepatopancreas tissues were collected from both groups at 0, 12, 24, 36, and 48 hpi, and total RNA was extracted for cDNA synthesis. Reverse transcribed cDNA would be used to measure relative gene expression levels.
2.7. Detection of Hemocytes Apoptosis Rate
To extract shrimp hemocytes from the dsGFP + WSSV and dsALF-like + WSSV groups, we mixed the hemocytes with an equal volume of anticoagulant (10% sodium citrate, pH 7.0). Subsequently, we separated the shrimp hemocytes by centrifuging at 800× g for 5 min at 4 °C in sterile PBS. Then, the cells were resuspended using PBS and the concentration was adjusted to 3 × 105 cells/mL. For apoptosis detection, we utilized the Annexin-FITC/PI (Nanjing, China G003-1-2) apoptosis detection kit, following the manufacturer’s instructions. We combined the resuspended cells with 500 μL of binding solution, added 5 μL of Annexin-FITC, followed by 5 μL of PI (Propidium iodide), and mixed them. We incubated the mixture at room temperature in the dark for 10 min before analyzing the samples using a flow cytometer (CytoFLEX, Shanghai, China). The obtained data were analyzed using the FlowJo v10 software (https://www.flowjo.com/learn/flowjo-university/flowjo, accessed on 11 October 2024).
2.8. Statistical Analysis
All data in the experiment are expressed as mean ± SD (standard deviation). The data from the experiment were statistically analyzed, and then a unpaired sample t-test was conducted. For survival rates, we using GraphPad Prism 5.0 (https://www.graphpad.com/, accessed on 2 December 2024) software to generate the Kaplan ± Meier plot (log-rank χ2 test). p values were considered to be statistically significant as follows: * p < 0.05; ** p < 0.01; *** p < 0.001. The same letters indicate no significant difference, and different letters indicate significant differences between groups.
3. Results
3.1. Sequence Feature of Lv-ALF
The sequence of 824 bp was obtained through PCR amplification, resulting in an ORF of 375 bp that encodes a total of 124 amino acids. Based on an analysis using BLAST 2.17.0 software (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 27 August 2024), this sequence was designated as the Lv-ALF-like gene. Its sequence features include a signal peptide (the gray shaded part in Figure 1), an ORF encoding 124 amino acids and a lipopolysaccharide binding domain (LBD). The LBD is indicated by an underline in Figure 1.
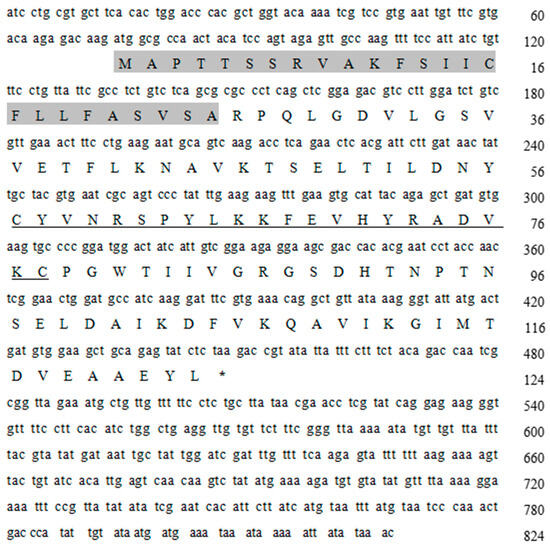
Figure 1.
The Lv-ALF-like gene cDNA sequence and amino acid sequence. (The gray shaded region represents the signal peptide sequence, and the underlined sequence represents the LBD sequence. Uppercase letters indicate amino acids, *: Represents the termination codon).
3.2. Multiple Sequence Alignment and Phylogenetic Tree
To study the similarity between the Lv-ALF-like molecule and other molecules, we compared them with homologous sequences using DNAMAN 6.0 software (https://www.lynnon.com/, accessed on 15 August 2024), and these molecules include Penaeus monodon ALF-like (XP_037785278.1), Penaeus indicus ALF-like (XP_063590858.1), Penaeus chinensis ALF-like (XP_047475653.1), Penaeus japonicus ALF-like (XP_042866095.1), Portunus trituberculatus ALF-like (XP_045120962.1), Scylla paramamosain ALF-like (XP_063877528.1), Chionoecetes opilio ALF (KAG0717214.1), Homarus americanus ALF-like (XP_042230805.1), Macrobrachium rosenbergii ALF-like (XP_066959710.1), Macrobrachium nipponense ALF-like (XP_064081001.1), Eriocheir sinensis ALF-like (XP_050711929.1), and Cherax quadricarinatus ALF-like (XP_053652897.1). The similarity of these sequences to ALF-like molecules was found to be 53.16% (Figure 2).
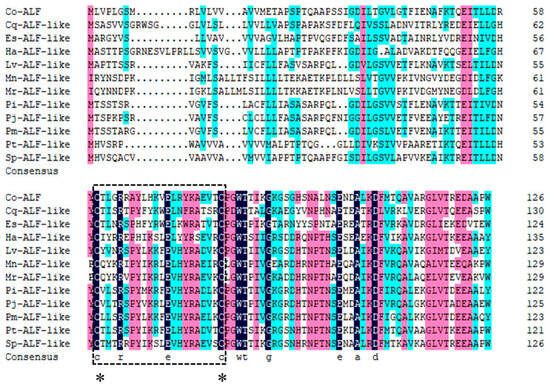
Figure 2.
Multiple sequence alignment of ALF-like molecules. Different colors represent different conservations of amino acids (black represents fully conserved amino acid sites, red represents highly conserved amino acid sites, and blue represents moderately conserved amino acid sites, *: represents the conservative cysteine residue), and the lipopolysaccharide binding domain is marked with a square box.
We further constructed a phylogenetic tree. The analysis revealed that the Lv-ALF-like molecule, P. monodon ALF-like (XP_037785278.1), P. indicus ALF-like (XP_063590858.1), and P. japonicus ALF-like (XP_042866095.1) were located on the same branch. It shared the highest similarity with P. indicus ALF-like (XP_063590858.1) and P. monodon ALF-like (XP_037785278.1). In contrast, it has been found to have lower similarity to C. opilio ALF (KAG0717214.1) (Figure 3).
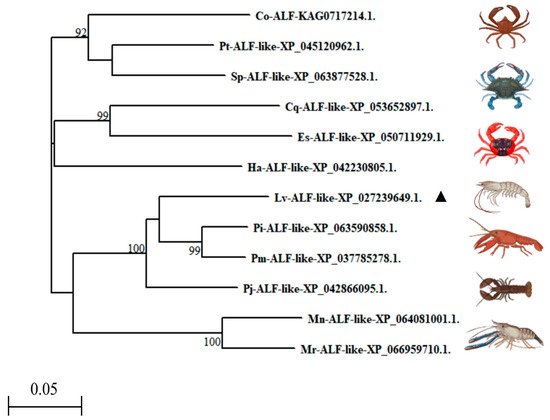
Figure 3.
Phylogenetic tree analysis of Lv-ALF-like with other ALF molecules from invertebrates (▲: Representative of the Litopenaeus vannamei).
3.3. Tissue Distribution and Lv-ALF like Gene mRNA Expression Pattern After WSSV Challenge
The results indicated that the Lv-ALF like gene exhibited the highest expression levels in hemolymph, gill, and muscle tissues, while the lowest levels were observed in the hepatopancreas and intestine, with negligible expression in intestinal tissue (Figure 4).
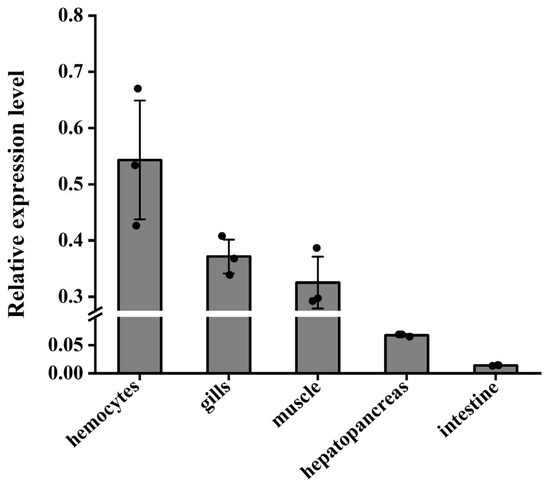
Figure 4.
Distribution and relative expression levels of Lv-ALF like genes in the hemocytes, hepatopancreas, gills, intestines, and muscle tissues of normal L. vannamei. Tissue distribution of ALF-like mRNA. With 18S rRNA as the internal reference, qRT-PCR was used to detect the expression levels of ALF-like. Three independent experiments were performed, and the standard deviation was the average of three samples.
Following the challenge with WSSV injection, the mRNA expression of the Lv-ALF like gene in hemocytes tissue demonstrated a trend of initial increase followed by a decrease, peaking at 24 h with a relative expression level which was increased by nearly 18.5-fold (Figure 5A); gene expression in hepatopancreas tissue increased 18.2-fold at 36 h (Figure 5B). In gill tissue, gene expression peaked at 48 h, exhibiting a 48.3-fold increase (Figure 5C). Conversely, relative gene expression in intestinal tissue demonstrated an initial increase followed by a decrease, with the highest expression level occurring at 12 h, where it increased by 376-fold (Figure 5D). The expression levels of ALF-like in hemocytes, hepatopancreas, gills, and intestinal tissues showed an overall trend of first increasing and then decreasing at different time points after WSSV infection.
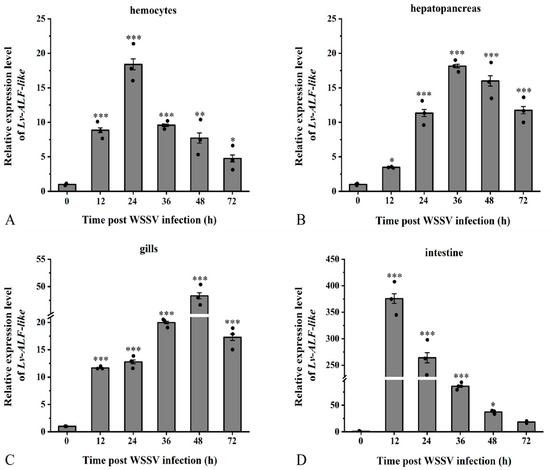
Figure 5.
The relative expression level of the Lv-ALF-like gene. Study on the expression patterns in (A) hemocytes, (B) hepatopancreas, (C) gills, and (D) intestinal tissues of L. vannamei challenged with WSSV at different time points. Significant differences are indicated by asterisks (*: p <0.05, **: p < 0.01, ***: p < 0.001). Errors represent ± SD of amplification and quantitation data for three samples.
3.4. RNAi Interference Effect and Survival Rate Analysis
QRT-PCR results indicated that compared to the control injection, the expression levels of Lv-ALF-like were significantly reduced. It is worth noting that at 24 h and 36 h, the interference rate of Lv-ALF-like remained at approximately 79% and 85%, respectively (Figure 6A). In contrast, the effect of dsGFP injection on the expression levels of Lv-ALF-like gene was negligible.
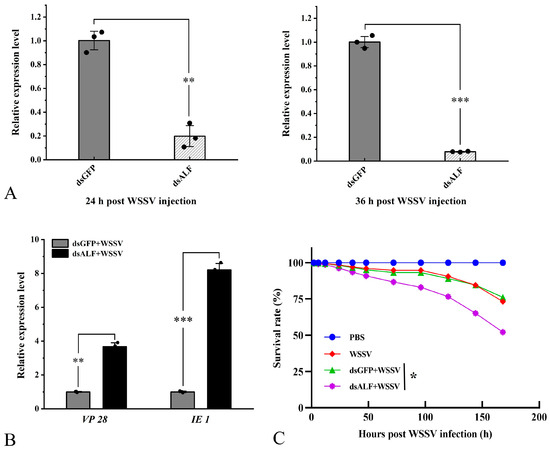
Figure 6.
The ALF-like gene has the ability to inhibit viral proliferation and also affects the survival rate of shrimp. (A) Injection of dsALF-like RNA. qRT-PCR results show that it can significantly knock down the mRNA expression level of the target gene. (B) After knockdown of the ALF-like gene, a significant increase in the expression levels of VP 28 and IE 1 was observed 36 h later. (C) Knocking down the target gene significantly reduces the survival rate of L. vannamei after WSSV infection. The RNAi experiment control group was designed with dsGFP. The survival rate statistical experiment was repeated three times independently. Significant differences are indicated by asterisks (*: p <0.05, **: p < 0.01, ***: p < 0.001). Errors represent ± SD of amplification and quantitation data for three samples.
Additionally, the proliferation of WSSV was assessed during the RNAi experiment. The results demonstrated that following the knockdown of Lv-ALF-like, the expression levels of VP 28 and IE 1 were significantly upregulated at 36 hpi (Figure 6B). Subsequently, the survival rate of shrimp in the 1 × PBS injection group, WSSV injection group, dsALF-like + WSSV injection group, and dsGFP + WSSV injection group was statistically analyzed. The experimental results indicated that the survival rate of shrimp following WSSV infection was significantly reduced after the injection of dsALF-like, compared to the other three groups (Figure 6C).
3.5. Lv-ALF-like Was Involved in Regulating the Antiviral Innate Immune Response
In this study, compared with the control group (dsGFP + WSSV), after Lv-ALF-like gene knockdown, the expression levels of apoptotic-related genes Lv-Caspase 3 and Lv-Caspase 8 in the experimental group (dsALF-like + WSSV) generally showed an upward trend at different time points. Compared with 12 h, their expression levels transiently decreased at 24 h, then increased again at 36 h, and gradually decreased at 48 h. This trend reflects the dynamic immune response process of “stress–compensation–reactivation–homeostasis” in L. vannamei after ALF-like knockdown. The overall upregulation represents the core activation of apoptotic clearance mechanisms, while the fluctuations at different time points may reflect the hosts’ precise temporal regulation of apoptotic pathways through negative feedback regulation, alternative pathway compensation, and damage repair mechanisms.
For the Lv-Bax gene, its expression in the experimental group showed an overall downward trend at 12, 24, 36, and 48 h compared with the control group. In the experimental group, the expression level decreased at 24 h compared with 12 h, recovered at 36 h, and significantly decreased at 48 h. In contrast, the Lv-Bcl 2 gene was generally upregulated at all time points in the experimental group compared with the control group, with the highest upregulation at 12 h. These results suggest that ALF-like is not only an immune effector molecule but also participates in “immune–apoptosis crosstalk” by regulating Bcl 2 family members. Its functional deficiency disrupts the homeostatic balance of apoptotic pathways, triggering dynamic stress responses in the host.
Following knockdown of the Lv-ALF-like gene, the antioxidant enzyme genes Lv-GST, Lv-CAT, Lv-Prx, Lv-GPX, and Lv-SOD in the experimental group were generally upregulated at 12, 24, 36, and 48 h time points compared with the control group. In the experimental group, the Lv-GST expression level was downregulated at 24 h compared with 12 h, then gradually recovered at 36 h and 48 h. The Lv-CAT expression level was highest at 12 h compared with other time points, decreased at 24 h, slightly recovered at 36 h, and dropped to the lowest level at 48 h. The Lv-Prx expression level continuously increased over time, reaching the highest at 48 h. The Lv-GPX expression level was highest at 12 h, decreased at 24 h, and then recovered at 36 h and 48 h. The Lv-SOD expression level was highest at 12 h, decreased at 24 h, showed a recovery trend at 36 h, and downregulated at 48 h compared with the control group (Figure 7). These results suggest that ALF-like is not only an effector molecule but also participates in host stress balance through immune–apoptosis cross-regulation. The fluctuating trend after knockdown may represent an “adaptive survival strategy” of shrimp under immune deficiency.
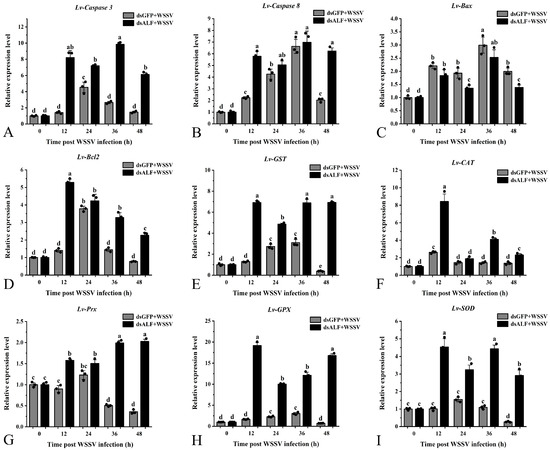
Figure 7.
Detection of genes related to antioxidant enzyme apoptosis pathway after RNAi knockdown of Lv-ALF like gene. After knocking down the target gene, WSSV was injected for immune stimulation, and the expression of related genes in the shrimp hepatopancreas was detected within 0, 12, 24, 36, and 48 h later. The dsGFP + WSSV group was used as the control. The same letters indicate no differences among the groups. Different letters represent significant differences between groups. Error bars represent ± SD of three independent PCR amplifications in quantitation ((A) Lv-Caspase 3; (B) Lv-Caspase 8; (C) Lv-Bax; (D) Lv-Bcl2; (E) Lv-GST; (F) Lv-CAT; (G) Lv-Prx; (H) Lv- GPX; (I) Lv-SOD).
3.6. Apoptosis in Hemocytes
The results revealed that the apoptosis rate in the PBS group was 38.41%, while in the WSSV-infected group, it was 58.32%. The dsGFP + WSSV group exhibited an apoptosis rate of 56.08%, and the dsALF-like + WSSV group showed a rate of 37.76%. Compared to the PBS group, the WSSV-infected group displayed a significant increase in apoptosis rate. In contrast, the experimental group (dsALF-like + WSSV) demonstrated a significant reduction in apoptosis rate compared to the RNAi control group (dsGFP + WSSV) (Figure 8A,B).
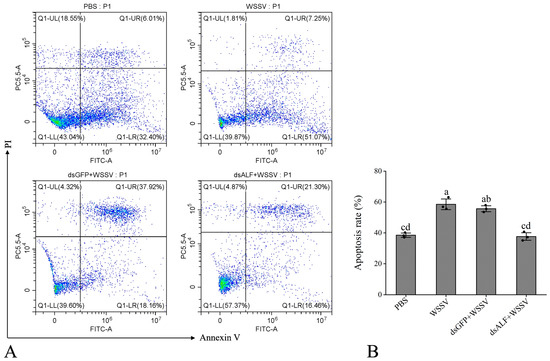
Figure 8.
The knockdown of the Lv-ALF-like gene mediates the detection of the apoptosis rate in shrimp hemocytes. (A) Scatter plots of the experimental samples. Different quadrants in the flow cytometry analysis represent cell conditions, where Q1-UL represents cell debris; Q1-UR represents late apoptosis; Q1-LR represents early apoptosis; and Q1-LL represents normal cells. (B) Apoptosis rate, the total fluorescence intensity of Q1-UR and Q1-LR was used to calculate the apoptotic rate of the sample. The same letters indicate no significant difference between the groups, while different letters indicate the existence of significant differences between the groups.
4. Discussion
Crustaceans primarily rely on innate immune responses to defend against pathogenic microorganisms [26]. Pattern recognition receptors (PRRs) in host cells identify pathogen associated molecular patterns (PAMPs), which subsequently stimulate specific signaling pathways and lead to the production of immune factors, including the AMP family [27]. Notably, research on shrimp has revealed that the ALF is a member of this antimicrobial peptide family [11]. ALF is one of the most extensively studied antimicrobial peptides in crustaceans [28] and is currently recognized as the most prevalent antibacterial factor in shrimp. While it has been demonstrated to possess both antibacterial and antiviral properties, the disease resistance mechanisms regulated by this molecule require further investigation. In our research, we identified a conserved amino acid sequence through sequence comparison, confirming that this molecule belongs to the ALF family, which we designated as the Lv-ALF-like gene.
Cloning and expression analysis of ALF molecules have been identified in various species, including Pacifastacus leniusculus [24], Marsupenaeus japonicus [29], and Penaeus monodon [13]. In this study, we analyzed the mRNA expression of ALF-like and found that, among the tissues examined, relative expression levels were higher in hemocyte, gills, and muscle tissues, while expression levels were either lower or not detected in the hepatopancreas and intestinal tissues. In studies involving Penaeus monodon, ALF was found to be constitutively expressed in hemocytes, heart, gills, intestines, and lymphoid organs, with no expression detected in the hepatopancreas [15]. This finding aligns with our results, although we observed trace expression in the hepatopancreas and intestinal tissues. Research on shrimp indicates that, despite variations in ALF transcript levels across different tissues, it still plays a protective role during the body’s pathogen defense processes [30].
When L. vannamei was stimulated by WSSV, the expression of Lv-ALF-like demonstrated a significant upregulation in response to pathogen stimulation. The expression profile of nLvALF 1 in shrimp also exhibited an upregulation trend following WSSV infection [31], although variations were noted across different tissues. Previous research on AMPs has indicated that their distinct functions primarily depend on amino acid sequences [11]. Notably, when comparing the amino acid sequence of the Lv-ALF-like molecule with those in previous studies, it was found that they are not the same protein.
To investigate the potential role of ALF-like in the immune response following WSSV infection in shrimp, sequence-specific dsRNA was synthesized, leading to the successful downregulation of target gene expression via microinjection. As the Lv-ALF-like gene was knocked down, this resulted in the upregulation of VP 28 and IE 1 expression and a significant increase in cumulative mortality, which adversely affected shrimp survival rates. Through the above experiments, it was found that the Lv-ALF-like gene has the effect of inhibiting the proliferation of pathogens. Knockdown of the ALF gene led to a decrease in the survival rate of shrimp, indicating that the Lv-ALF-like gene plays an important inhibitory role in the shrimp’s resistance to WSSV infection. This experiment has preliminarily demonstrated that the Lv-ALF-like gene may play a regulatory role in the innate immune system of shrimp against viruses. In a related study by de la Vega et al., knockdown of the Lv-ALF1 gene also led to a significant increase in shrimp mortality upon WSSV stimulation [16]. Crustins molecules belonging to the antimicrobial peptide family were observed after knocking down target gene expression by RNAi technology before injecting vibrio infection, and a significant increase in mortality rate in shrimp was found [32]. This is consistent with our findings. But the difference is that the specificity of the sequence may lead to them having different functions. There are similar studies on ALF antiviral in freshwater crayfish. By injecting dsRNA to interfere with the Lv-ALF-like gene, it was found that the WSSV content in the shrimp was significantly increased, which is consistent with the upregulated expression of VP 28 and IE 1 genes after knocking down the target genes [24].
In addition, after knocking down the target gene, the expression of antioxidant enzymes and apoptosis pathway genes was significantly upregulated compared with the control group dsGFP. These findings suggest that ALF are typically involved in modulating the immune response. When the expression of the Lv-ALF-like gene decreases, it may regulate the innate immune system by upregulating the expression of apoptotic-related signaling pathways and antioxidant enzymes, in order to enhance the organism’s defense against pathogens and maintain survival under viral infection conditions. After shrimp hemocytes were infected with WSSV, the apoptosis rate was significantly increased, indicating that WSSV infection can induce shrimp hemocyte apoptosis. After knockdown of the target gene, a significant decrease in apoptosis was also observed. It is possible that the Lv-ALF-like gene affects cell apoptosis by regulating the expression of the Lv-Bcl 2 family proteins. The knockdown of Lv-ALF-like resulted in decreased expression of Lv-Bax and increased expression of Lv-Bcl 2, ultimately leading to a decreased hemocyte apoptosis rate. This reveals an important role of Lv-ALF-like in the regulation of apoptosis and provides new insights into understanding the multiple functions of Lv-ALF-like in the immune response. Indeed, in addition to being resistant to multiple pathogenic microorganisms, antimicrobial peptides are involved in apoptosis and promote the production of chemokines and the expression of other immune genes [33]. However, antioxidant enzyme genes (Lv-GST, Lv-CAT, Lv-Prx, Lv-GPX, Lv-SOD) are upregulated in response to high levels of Reactive Oxygen Species (ROS). ROS is a reactive molecule that directly kills bacteria, but excessive ROS causes tissue damage and inflammation. This could be attributed to shrimp immune deficiency caused by Lv-ALF-like knockdown, which leads to persistent pathogen infection, triggering ROS burst and oxidative stress, thereby driving the overall upregulation of antioxidant enzyme genes. The dynamic fluctuations of each gene reflect functional division of labor (CAT/SOD for acute response, Prx for long-term clearance, GST/GPX for metabolic detoxification), compensatory balance, and metabolic feedback, so as to maintain redox homeostasis. Upregulated expression of antioxidant enzymes (SpSOD, SpCAT, SpGPX) can be used to remove ROS in vivo to protect the body [34]. This is consistent with our research findings. The results of the above studies show that this may be because when the expression of ALF-like is knocked down, the immune system may be upregulated. This upregulation is related to the expression of apoptosis-related signaling pathways and antioxidant enzymes, which serves as compensation to enhance the body’s defense against pathogens and ensure cell survival under stress conditions.
In summary, this study reveals that the Lv-ALF-like modulates anti-WSSV immunity in shrimp through a dual mechanism: regulating antioxidant enzyme expression to neutralize oxidative stress and controlling apoptosis-related genes to maintain cellular homeostasis. These compensatory effects enable Lv-ALF-like to exert potent anti-disease functions. The discovery of the antiviral action of Lv-ALF-like provides novel insights for developing targeted therapeutics and advancing preventive strategies against viral outbreaks in aquaculture.
5. Conclusions
This study characterized the Lv-ALF-like gene in L. vannamei; the tissue distribution analysis revealed that the expression level was highest in hemocytes and lowest in intestinal tissues. WSSV infection induced significant upregulation of Lv-ALF-like in immune relevant tissues (hemocytes, hepatopancreas, gills, intestines). Gene knockdown exacerbated WSSV replication, as evidenced by elevated viral gene (VP 28, IE 1) expression and reduced host survival rates, concurrently triggering upregulation of antioxidant- (Lv-GST, Lv-CAT, Lv-Prx, Lv-GPX, Lv-SOD) and apoptosis-related genes (Lv-Caspase 3, Lv-Caspase 8, Lv-Bcl 2), alongside suppressed hemocyte apoptosis. In general, the Lv-ALF-like protein acts as a key antiviral effector. It may play a role in inhibiting virus proliferation and enhancing host viability through the dual regulation of oxidative and apoptotic signals. However, its regulatory network remains unclear, and further mechanistic studies are needed to elucidate it.
Author Contributions
B.Y.: Original draft, Methodology, Formal analysis, Data curation, Conceptualization; L.Z.: Visualization, Validation, Software, Investigation; F.F.: Software, Conceptualization; K.L.: Investigation; S.L.: Software, Conceptualization; J.K.: Supervision, Methodology; Q.F.: Conceptualization; J.C.: Software; B.C.: Methodology; P.D.: Investigation; Q.X.: Visualization, Investigation; X.L.: Supervision, Methodology, Conceptualization; X.M.: Writing—review and editing, Resources, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Key R&D Program of Shandong Province, China (2024LZGC038), the National Key R&D Program of China (2022YFF1000304), the National Natural Science Foundation of China (32172960), the China Agriculture Research System (CARS-48), the Central Public-interest Scientific Institution Basal Research Fund, CAFS (2020TD26), and the Open Competition Program of Top Ten Critical Priorities of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province (2022SDZG01).
Institutional Review Board Statement
Under the approval number YSFRI-2024005 2024.1.31, this study was conducted with the approval of the committee at the Yellow Sea Fisheries Research Institute.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Author Qun Xing was employed by the company BLUP Aquabreed Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Li, E.; Xu, C.; Wang, X.D.; Wang, S.F.; Zhao, Q.; Zhang, M.L.; Qin, J.G.; Chen, L.Q. Gut Microbiota and its Modulation for Healthy Farming of Pacific White Shrimp Litopenaeus vannamei. Rev. Fish. Sci. Aquac. 2018, 26, 381–399. [Google Scholar] [CrossRef]
- Yin, T.; Shi, L. Processing and Preservation of Aquatic Products. Foods 2023, 12, 2061. [Google Scholar] [CrossRef]
- Angthong, P.; Uengwetwanit, T.; Arayamethakorn, S.; Rungrassamee, W. Transcriptomic analysis of the black tiger shrimp (Penaeus monodon) reveals insights into immune development in their early life stages. Sci. Rep. 2021, 11, 13881. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, G.; Liu, B.; Zhou, C.; Wang, H.; Qin, W.; Jiang, Z.; Wan, X.; Ren, Q. Characterization and functional analysis of tandem threonine containing C-type lectin (Thr-Lec) from the ridgetail white prawn Exopalaemon carinicauda. Fish Shellfish Immunol. Rep. 2021, 2, 100018. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Y.; Zhao, Z.; Weng, S.; Yang, J.; Liu, S.; Liu, C.; Yuan, F.; Ai, B.; Zhang, H.; et al. A convenient polyculture system that controls a shrimp viral disease with a high transmission rate. Commun. Biol. 2021, 4, 1276. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Yang, B.; Luo, K.; Luan, S.; Kong, J.; Li, X.; Meng, X. Molecular Characterization and Expression Analysis of the C-Type Lectin Domain Family 4 Member F in Litopenaeus vannamei against White Spot Syndrome Virus. Animals 2024, 14, 1137. [Google Scholar] [CrossRef] [PubMed]
- Waring, A.L.; Hill, J.; Allen, B.M.; Bretz, N.M.; Le, N.; Kr, P.; Fuss, D.; Mortimer, N.T. Meta-Analysis of Immune Induced Gene Expression Changes in Diverse Drosophila melanogaster Innate Immune Responses. Insects 2022, 13, 490. [Google Scholar] [CrossRef]
- Shah, E.J.; Hüttemann, M.; Sanderson, T.H.; Gurdziel, K.; Ruden, D.M. Inhibiting Mitochondrial Cytochrome c Oxidase Downregulates Gene Transcription After Traumatic Brain Injury in Drosophila. Front. Physiol. 2021, 12, 628777. [Google Scholar] [CrossRef]
- van Alphen, B.; Stewart, S.; Iwanaszko, M.; Xu, F.; Li, K.; Rozenfeld, S.; Ramakrishnan, A.; Itoh, T.Q.; Sisobhan, S.; Qin, Z.; et al. Glial immune-related pathways mediate effects of closed head traumatic brain injury on behavior and lethality in Drosophila. PLoS Biol. 2022, 20, e3001456. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, J.; Cheng, W.; Mao, Y.; Yu, X. Antibacterial activity of an anti-lipopolysaccharide factor (MjALF-D) identified from kuruma prawn (Marsupenaeus japonicus). Fish Shellfish Immunol. 2022, 127, 295–305. [Google Scholar] [CrossRef]
- Marín-Parra, C.; Serna-Duque, J.A.; Espinosa-Ruiz, C.; Esteban, M.Á. Analysis of In Silico Properties and In Vitro Immunomodulatory Effects of Seven Synthetic Host Defence Peptides in Gilthead Seabream (Sparus aurata) Leucocytes. Mar. Biotechnol. 2025, 27, 109. [Google Scholar] [CrossRef]
- Tanaka, S.; Nakamura, T.; Morita, T.; Iwanaga, S. Limulus anti-LPS factor: An anticoagulant which inhibits the endotoxin mediated activation of Limulus coagulation system. Biochem. Biophys. Res. Commun. 1982, 105, 717–723. [Google Scholar] [CrossRef]
- Somboonwiwat, K.; Marcos, M.; Tassanakajon, A.; Klinbunga, S.; Aumelas, A.; Romestand, B.; Gueguen, Y.; Boze, H.; Moulin, G.; Bachère, E. Recombinant expression and anti-microbial activity of anti-lipopolysaccharide factor (ALF) from the black tiger shrimp Penaeus monodon. Dev. Comp. Immunol. 2005, 29, 841–851. [Google Scholar] [CrossRef]
- Ma, L.; Wang, H.; Liu, Y.; Sun, J.; Yan, X.; Lu, Z.; Hao, C.; Qie, X. Single von Willebrand factor C-domain protein-2 confers immune defense against bacterial infections in the silkworm, Bombyx mori. Int. J. Biol. Macromol. 2024, 279 Pt 2, 135241. [Google Scholar] [CrossRef] [PubMed]
- Supungul, P.; Klinbunga, S.; Pichyangkura, R.; Hirono, I.; Aoki, T.; Tassanakajon, A. Antimicrobial peptides discovered in the black tiger shrimp Penaeus monodon using the EST approach. Dis. Aquat. Organ. 2004, 61, 123–135. [Google Scholar] [CrossRef] [PubMed]
- de la Vega, E.; O’Leary, N.A.; Shockey, J.E.; Robalino, J.; Payne, C.; Browdy, C.L.; Warr, G.W.; Gross, P.S. Anti-lipopolysaccharide factor in Litopenaeus vannamei (LvALF): A broad spectrum antimicrobial peptide essential for shrimp immunity against bacterial and fungal infection. Mol. Immunol. 2008, 45, 1916–1925. [Google Scholar] [CrossRef]
- Li, S.H.; Zhang, X.J.; Sun, Z.; Li, F.H.; Xiang, J.H. Transcriptome analysis on Chinese shrimp Fenneropenaeus chinensis during WSSV acute infection. PLoS ONE 2013, 8, e58627. [Google Scholar] [CrossRef]
- Jimenez-Vega, F.; Vargas-Albores, F. Isoforms of Litopenaeus vannamei antilipopolysaccharide and its expression by bacterial challenge. J. Shellfish Res. 2007, 26, 1169–1175. [Google Scholar] [CrossRef]
- Gross, P.S.; Bartlett, T.C.; Browdy, C.L.; Chapman, R.W.; Warr, G.W. Immune gene discovery by expressed sequence tag analysis of hemocytes and hepatopancreas in the Pacific White Shrimp, Litopenaeus vannamei, and the Atlantic White Shrimp, L. setiferus. Dev. Comp. Immunol. 2001, 25, 565–577. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, Z.; Li, X.C.; Jie, D.; Hui, K.M.; Zhang, C.Y.; Wang, W. Three different anti-lipopolysaccharide factors identified from giant freshwater prawn, Macrobrachium rosenbergii. Fish Shellfish Immunol. 2012, 33, 766–774. [Google Scholar] [CrossRef]
- Li, S.H.; Guo, S.Y.; Li, F.H.; Xiang, J.H. Functional diversity of anti-lipopolysaccharide factor isoforms in shrimp and their characters related to antiviral activity. Mar. Drugs 2015, 13, 2602–2616. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.B.; He, L.Y.; Wei, X.M.; Wang, X.L.; Tang, X.Q. An Anti-Lipopolysaccharide Factor in Litopenaeus vannamei Par-ticipates in the Immune Defense Against WSSV and Vibrio Anguillarum. J. Crustac. Biology 2015, 35, 670–675. [Google Scholar] [CrossRef]
- Sun, M.; Li, S.; Lv, X.; Xiang, J.; Lu, Y.; Li, F. A Lymphoid Organ Specific Anti-Lipopolysaccharide Factor from Litopenaeus vannamei Exhibits Strong Antimicrobial Activities. Mar. Drugs 2021, 19, 250. [Google Scholar] [CrossRef]
- Liu, H.P.; Jiravanichpaisal, P.; Söderhäll, I.; Cerenius, L.; Söderhäll, K. Antilipopolysaccharide factor interferes with white spot syndrome virus replication in vitro and in vivo in the crayfish Pacifastacus leniusculus. J. Virol. 2006, 80, 10365–10371. [Google Scholar] [CrossRef]
- Yang, B.B.; Li, Q.Q.; Zhang, M.D.; Lin, S.H.; Shen, X.L.; Du, Z.Q. Molecular cloning and functional characterization of peroxiredoxin 4 (prx 4) in freshwater crayfish, Procambarus clarkii. Fish Shellfish Immunol. 2023, 137, 108781. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yang, Y.; Zhang, C.; Chen, H.Y.; Chen, F.; Wang, K.J. A Novel Antimicrobial Peptide Sparamosin26-54 From the Mud Crab Scylla paramamosain Showing Potent Antifungal Activity Against Cryptococcus neoformans. Front. Microbiol. 2021, 12, 746006. [Google Scholar] [CrossRef]
- Lu, Y.; Su, F.; Li, Q.; Zhang, J.; Li, Y.; Tang, T.; Hu, Q.; Yu, X.Q. Pattern recognition receptors in Drosophila immune responses. Dev. Comp. Immunol. 2020, 102, 103468. [Google Scholar] [CrossRef]
- Wang, F.; Li, S.; Li, F. Different Immune Responses of the Lymphoid Organ in Shrimp at Early Challenge Stage of Vibrio parahaemolyticus and WSSV. Animals 2021, 11, 2160. [Google Scholar] [CrossRef]
- Nagoshi, H.; Inagawa, H.; Morii, K.; Harada, H.; Kohchi, C.; Nishizawa, T.; Taniguchi, Y.; Uenobe, M.; Honda, T.; Kondoh, M.; et al. Cloning and characterization of a LPS-regulatory gene having an LPS binding domain in kuruma prawn Marsupenaeus japonicus. Mol. Immunol. 2006, 43, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Matos, G.M.; Schmitt, P.; Barreto, C.; Farias, N.D.; Toledo-Silva, G.; Guzmán, F.; Destoumieux-Garzón, D.; Perazzolo, L.M.; Rosa, R.D. Massive gene expansion and sequence diversification is associated with diverse tissue distribution, regulation and antimicrobial properties of Anti-Lipopolysaccharide Factors in Shrimp. Mar. Drugs. 2018, 16, 381. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Y.; Li, F.; Zhang, X.; Xiang, J. A new anti-lipopolysaccharide factor (ALF) gene with its SNP polymorphisms related to WSSV-resistance of Litopenaeus vannamei. Fish Shellfish Immunol. 2014, 39, 24–33. [Google Scholar] [CrossRef]
- Shockey, J.E.; O’Leary, N.A.; de la Vega, E.; Browdy, C.L.; Baatz, J.E.; Gross, P.S. The role of crustins in Litopenaeus vannamei in response to infection with shrimp pathogens: An in vivo approach. Dev. Comp. Immunol. 2009, 33, 668–673. [Google Scholar] [CrossRef]
- Pushpanathan, M.; Gunasekaran, P.; Rajendhran, J. Antimicrobial peptides: Versatile biological properties. Int. J. Pept. 2013, 2013, 675391. [Google Scholar] [CrossRef]
- Pavlick, K.P.; Laroux, F.S.; Fuseler, J.; Wolf, R.E.; Gray, L.; Hoffman, J.; Grisham, M.B. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic. Biol. Med. 2002, 33, 311–322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).