L-Glutamine Supplementation Improves the In Vitro Qualitative Parameters of Cryopreserved Qinchuan Bull Sperm
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Semen Collection and Quality Assessment
2.2. Semen Freezing and Grouping
2.3. Metabolomic Analysis
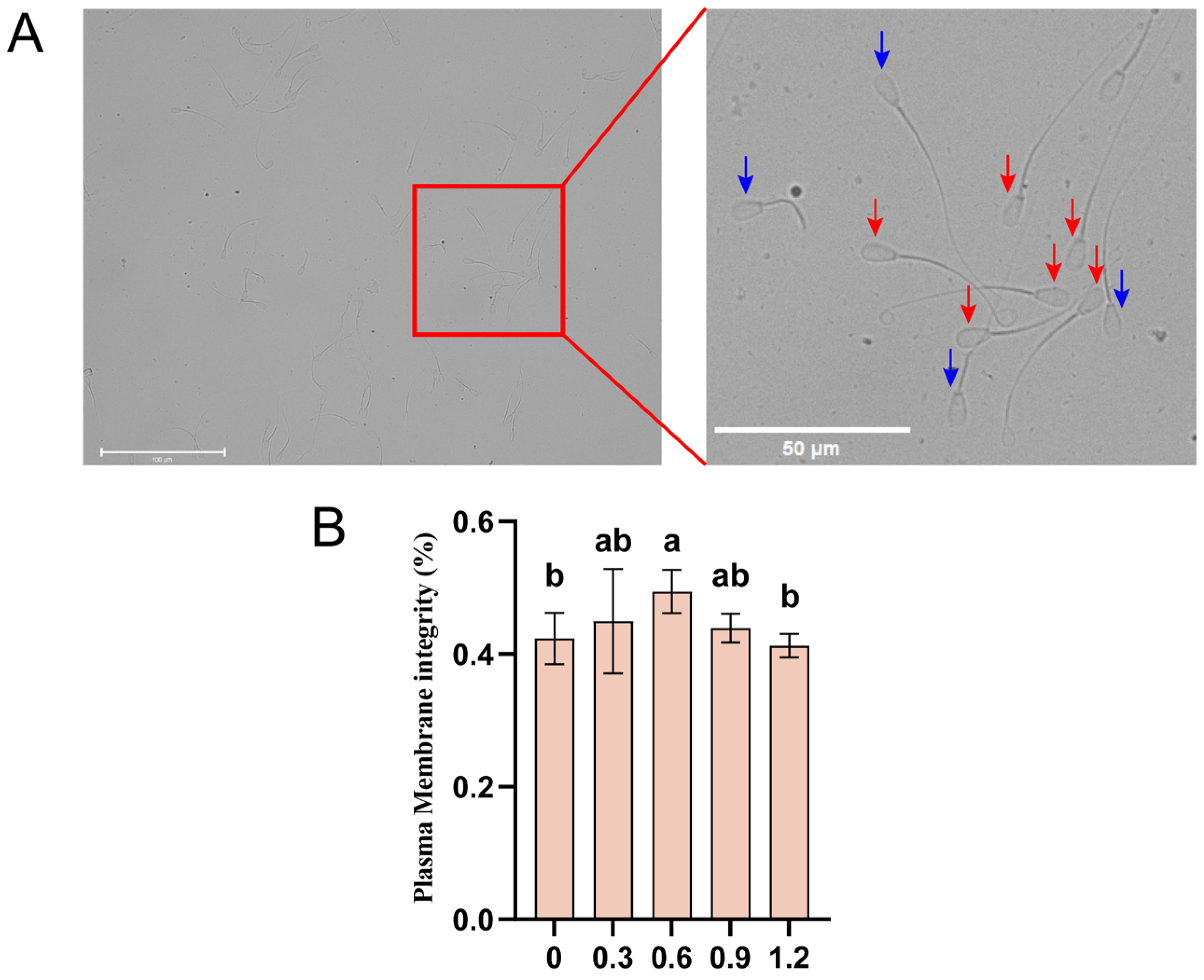
2.4. Detection of Sperm Membrane Integrity
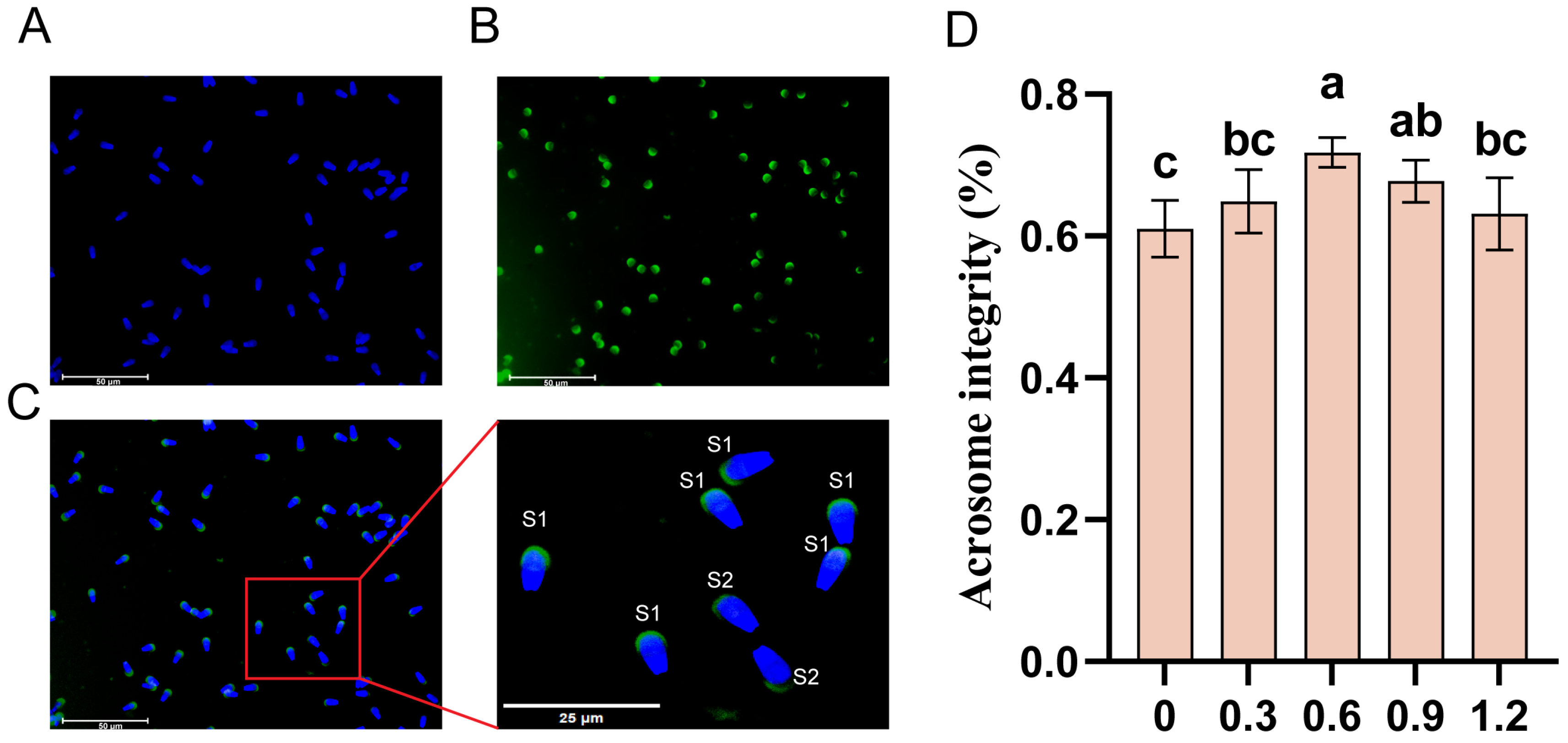
2.5. Detection of Sperm Acrosome Integrity
2.6. Detection of Semen Antioxidant Capacity
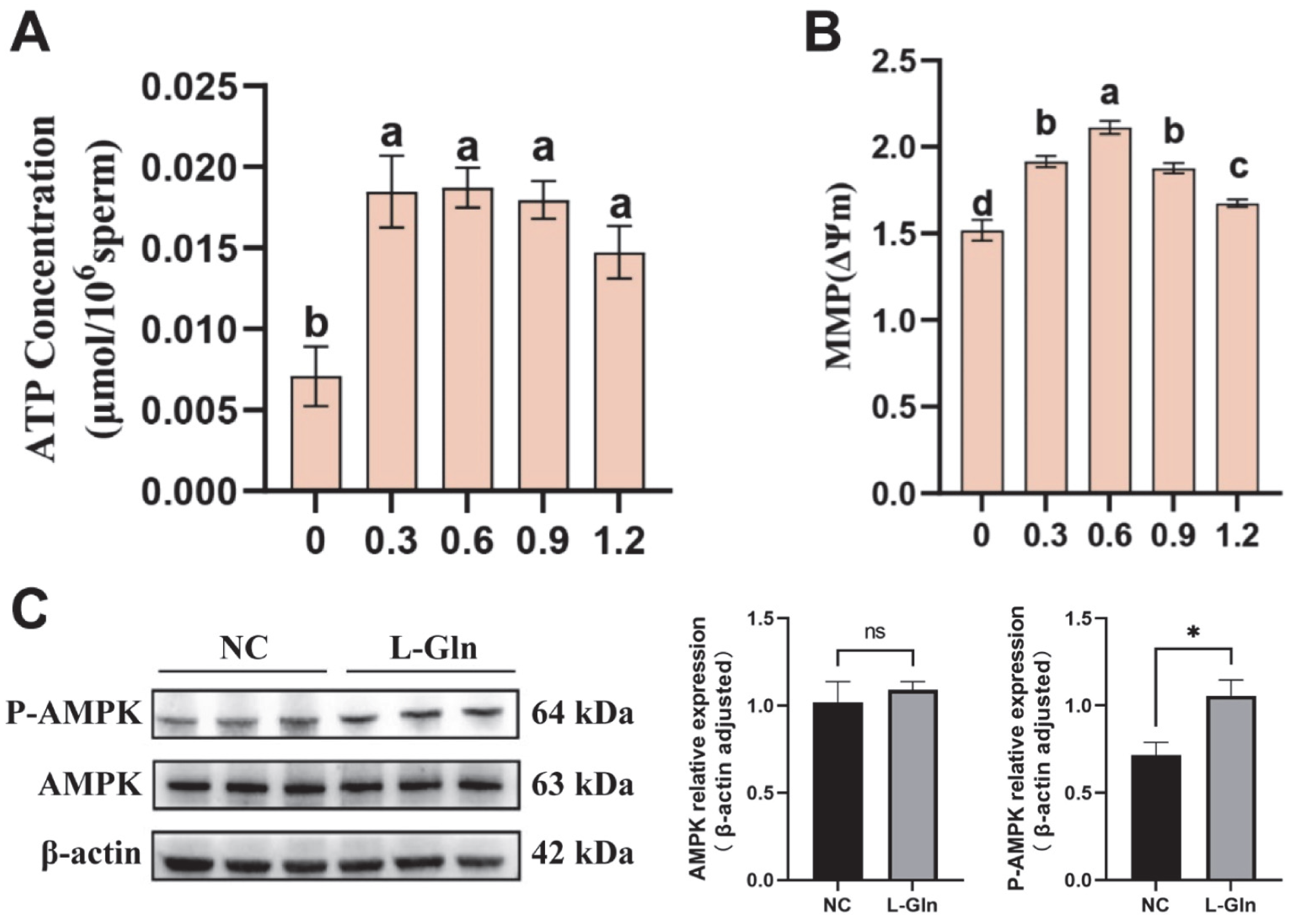
2.7. Detection of ATP Content and Mitochondrial Membrane Potential in Semen
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
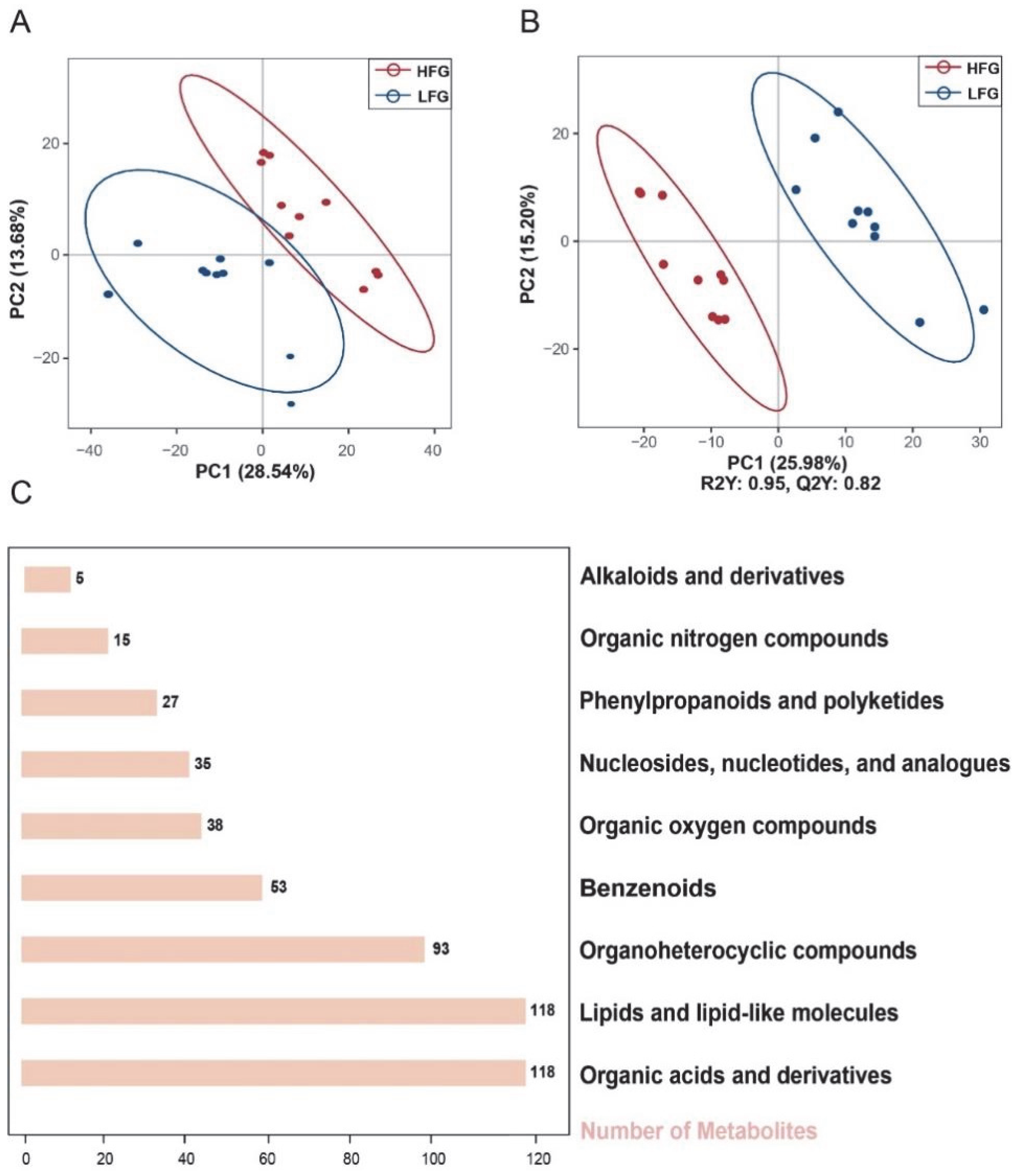
3.1. Classification of Semen into HFG and LFG
3.2. Classification of Metabolites Between HFG and LFG
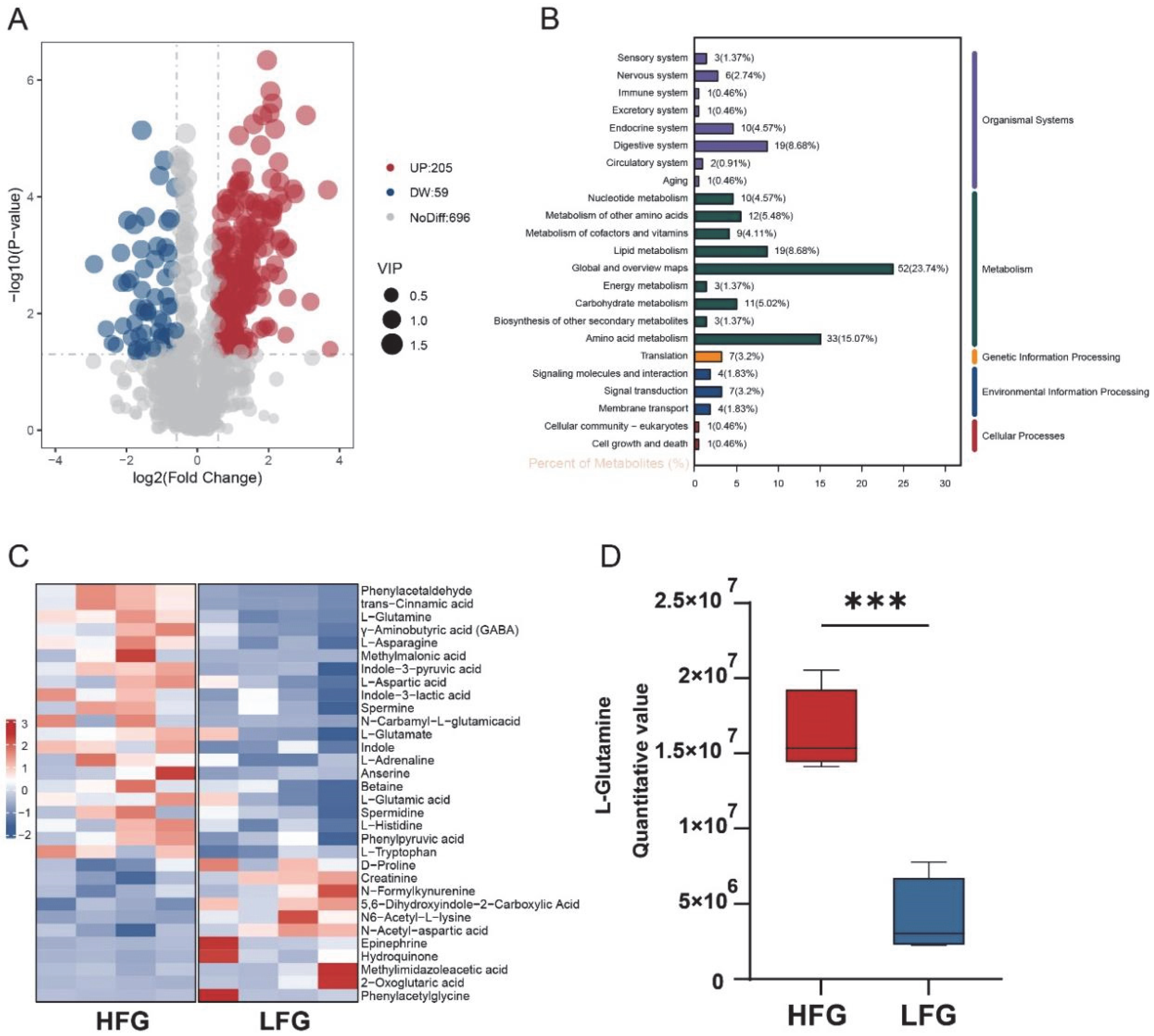
3.3. Screening of Differential Metabolites Between HFG and LFG
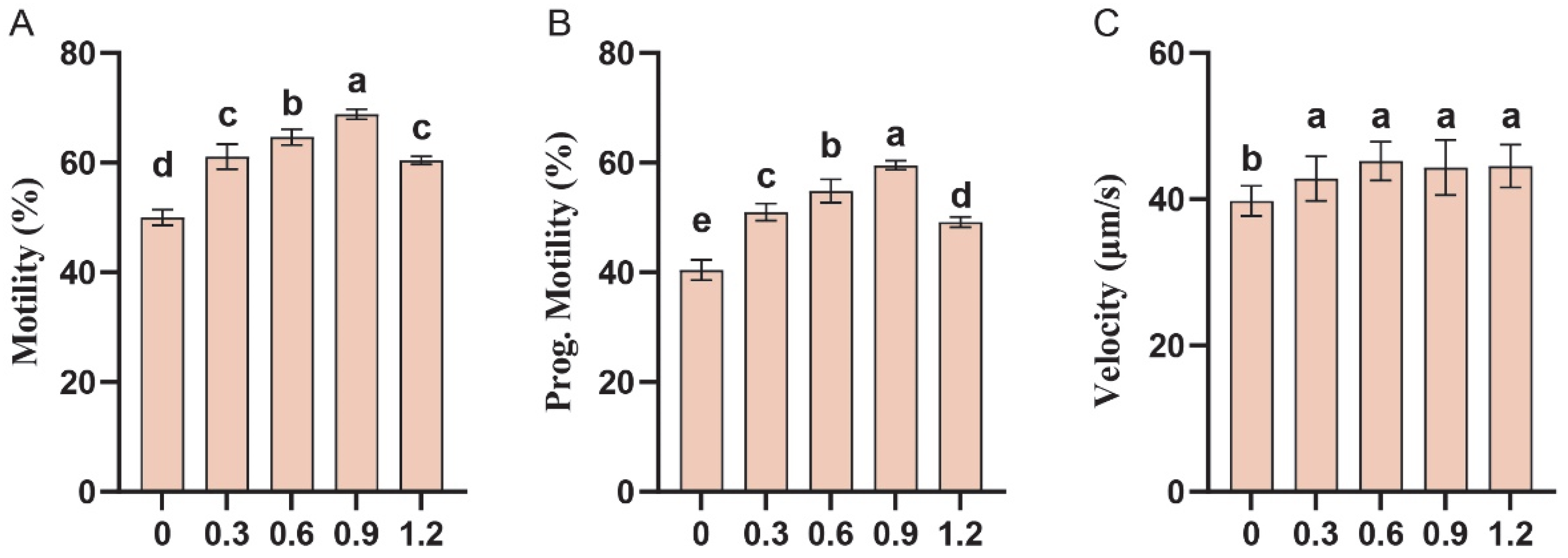
3.4. Effect of L-Glutamine on Sperm Motility in Frozen–Thawed Sperm
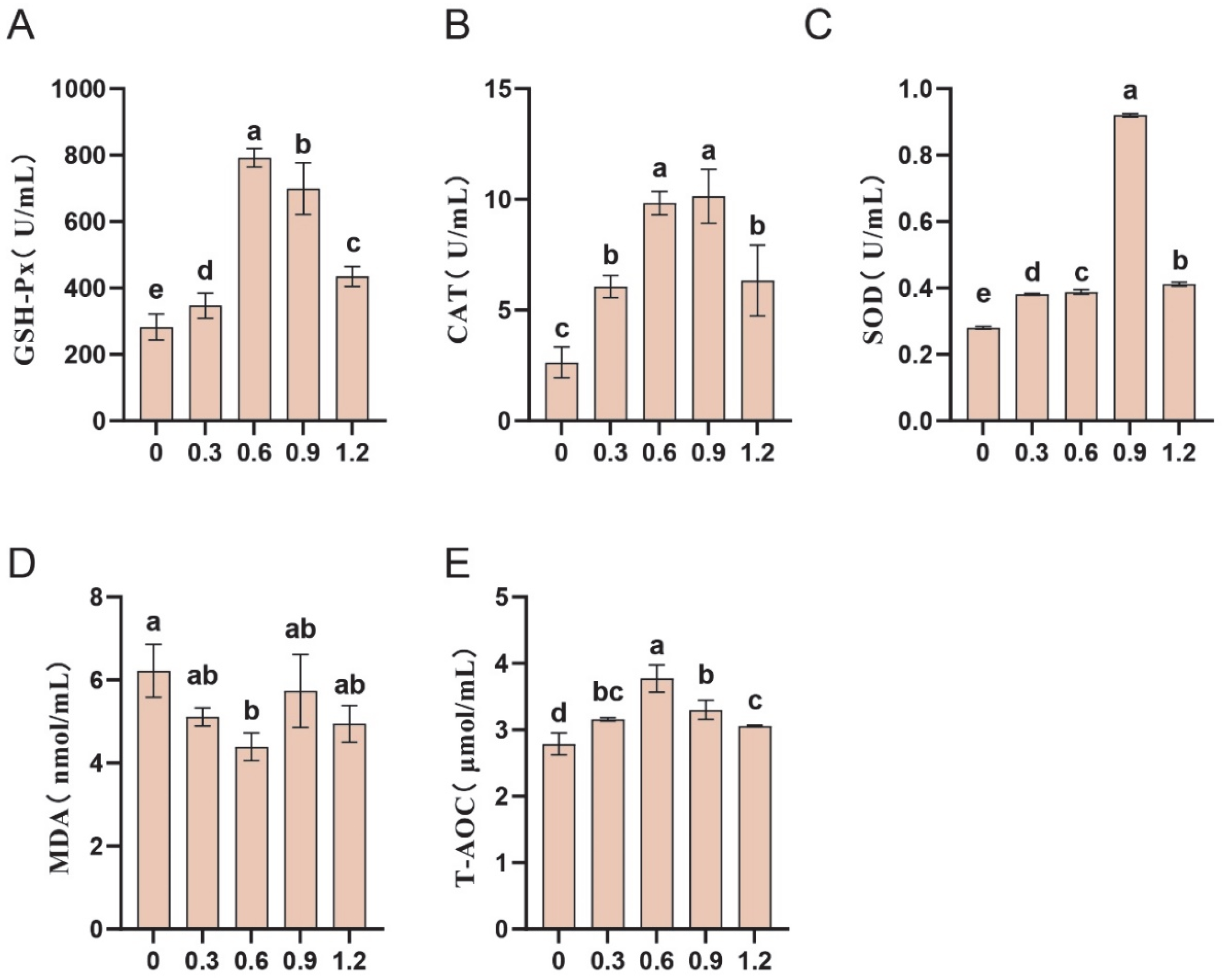
3.5. Effect of L-Glutamine on Antioxidant Capacity in Frozen–Thawed Sperm
3.6. Effect of L-Glutamine on Membrane Integrity in Frozen–Thawed Sperm
3.7. Effect of L-Glutamine on Acrosomal Integrity in Frozen–Thawed Sperm
3.8. Effect of L-Glutamine on Energy Metabolism and AMPK Phosphorylation in Frozen–Thawed Sperm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| L-Gln | L-glutamine |
| HFG | high-freezing group |
| LFG | low-freezing group |
| ROS | reactive oxygen species |
| LC-MS | liquid chromatography–mass spectrometry |
| PR.MOT | Progressive motility |
| MDA | Malondialdehyde |
| CAT | catalase |
| GSH-Px | glutathione peroxidase |
| SOD | superoxide dismutase |
| T-AOC | total antioxidant capacity |
| MMP | Mitochondrial Membrane Potential |
| ATP | Adenosine triphosphate |
| AMPK | Adenosine 5′-monophosphate-activated protein kinase |
| Nrf2 | Nuclear factor e2-related factor 2 |
| TCA | Tricarboxylic acid |
| GABA | γ-aminobutyric acid |
References
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martín-Maestro, A.; Sánchez-Ajofrín, I.; Medina-Chávez, D.A.; Fernández-Santos, M.R.; García-Álvarez, O.; Maroto-Morales, A.; Montoro, V.; et al. Sperm Cryodamage in Ruminants: Understanding the Molecular Changes Induced by the Cryopreservation Process to Optimize Sperm Quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.C.; Castro, A.C.S.E.; Silva, R.R.; Chaves, J.E.V.; Silva, V.A.O.; Capobianco, N.E.; Queiroz, P.J.B.; Melo, L.d.F.E.; Barbosa, E.A.; Dode, M.A.N.; et al. Comparison of Post-Thaw Motility and In Vitro Fertility Between Ejaculated and Epididymal Semen, and Seminal cfDNA Characterization in Pantaneiro Bulls. Biology 2025, 14, 465. [Google Scholar] [CrossRef]
- Hai, E.; Li, B.; Song, Y.; Zhang, J.; Zhang, J. Ferroptosis Emerges as the Predominant Form of Regulated Cell Death in Goat Sperm Cryopreservation. J. Anim. Sci. Biotechnol. 2025, 16, 26. [Google Scholar] [CrossRef]
- Cao, B.; Qin, J.; Pan, B.; Qazi, I.H.; Ye, J.; Fang, Y.; Zhou, G. Oxidative Stress and Oocyte Cryopreservation: Recent Advances in Mitigation Strategies Involving Antioxidants. Cells 2022, 11, 3573. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, J.K.; Srivastava, N.; Ghosh, S.K. Strategies to Minimize Various Stress-Related Freeze-Thaw Damages During Conventional Cryopreservation of Mammalian Spermatozoa. Biopreserv. Biobank. 2019, 17, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Viudes de Castro, M.P.; Marco-Jimenez, F.; Vicente, J.S. Rabbit Sperm Cryopreservation. Methods Mol. Biol. 2025, 2897, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.S.; Tahir, M.Z.; Zahoor, M.Y.; Hifz-Ul-Rahman, null; Riaz, A. Effect of Naringenin on Post-Thaw Quality, Fertility-Associated Gene Expression and Fertilization Potential of Buffalo (Bubalus Bubalis) Bull Sperm. Cryobiology 2024, 116, 104953. [Google Scholar] [CrossRef]
- Selvaraju, S.; Gowda, N.K.S.; Krishnappa, B.; Binsila, B.K.; Athira, T.; Heena, H.S.; Manjunatha, A.T.; Sahoo, A. Boron Protects Sperm Functional Attributes During Cryopreservation of Bull Semen. Biol. Trace Elem. Res. 2025. [Google Scholar] [CrossRef]
- Sharafi, M.; Blondin, P.; Vincent, P.; Anzar, M.; Benson, J.D. Hydroxytyrosol and Resveratrol Improves Kinetic and Flow Cytometric Parameters of Cryopreserved Bull Semen with Low Cryotolerance. Anim. Reprod. Sci. 2022, 245, 107065. [Google Scholar] [CrossRef]
- Wang, M.; Wu, S.; Yang, B.; Ye, M.; Tan, J.; Zan, L.; Yang, W. Grape Seed Proanthocyanidins Improve the Quality of Fresh and Cryopreserved Semen in Bulls. Animals 2023, 13, 2781. [Google Scholar] [CrossRef]
- Ciftci, H.B.; Aygun, A. Poultry Semen Cryopreservation Technologies. Worlds Poult. Sci. J. 2018, 74, 699–709. [Google Scholar] [CrossRef]
- Tian, X.; Li, D.; He, Y.; Zhang, W.; He, H.; Du, R.; Pang, W.; Yang, G.; Yu, T. Supplementation of Salvianic Acid A to Boar Semen Extender to Improve Seminal Quality and Antioxidant Capacity. Anim. Sci. J. 2019, 90, 1142–1148. [Google Scholar] [CrossRef]
- Mateo-Otero, Y.; Fernández-López, P.; Delgado-Bermúdez, A.; Nolis, P.; Roca, J.; Miró, J.; Barranco, I.; Yeste, M. Metabolomic Fingerprinting of Pig Seminal Plasma Identifies in Vivo Fertility Biomarkers. J. Anim. Sci. Biotechnol. 2021, 12, 113. [Google Scholar] [CrossRef]
- Ugur, M.R.; Dinh, T.; Hitit, M.; Kaya, A.; Topper, E.; Didion, B.; Memili, E. Amino Acids of Seminal Plasma Associated With Freezability of Bull Sperm. Front. Cell Dev. Biol. 2019, 7, 347. [Google Scholar] [CrossRef]
- Longobardi, V.; Kosior, M.A.; Pagano, N.; Fatone, G.; Staropoli, A.; Vassetti, A.; Vinale, F.; Campanile, G.; Gasparrini, B. Changes in Bull Semen Metabolome in Relation to Cryopreservation and Fertility. Animals 2020, 10, 1065. [Google Scholar] [CrossRef]
- Velho, A.L.C.; Menezes, E.; Dinh, T.; Kaya, A.; Topper, E.; Moura, A.A.; Memili, E. Metabolomic Markers of Fertility in Bull Seminal Plasma. PLoS ONE 2018, 13, e0195279. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; McLellan, L.I. Glutathione and Glutathione-Dependent Enzymes Represent a Co-Ordinately Regulated Defence against Oxidative Stress. Free Radic. Res. 1999, 31, 273–300. [Google Scholar] [CrossRef] [PubMed]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motohashi, H. Nrf2 Redirects Glucose and Glutamine into Anabolic Pathways in Metabolic Reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Dewry, R.K.; Srivastava, R.; Nath, S.; Mohanty, T.K. Targeted Antioxidant Delivery Modulates Mitochondrial Functions, Ameliorates Oxidative Stress and Preserve Sperm Quality during Cryopreservation. Theriogenology 2022, 179, 22–31. [Google Scholar] [CrossRef]
- Correa, J.R.; Zavos, P.M. The Hypoosmotic Swelling Test: Its Employment as an Assay to Evaluate the Functional Integrity of the Frozen-Thawed Bovine Sperm Membrane. Theriogenology 1994, 42, 351–360. [Google Scholar] [CrossRef]
- Druart, X.; Rickard, J.P.; Tsikis, G.; de Graaf, S.P. Seminal Plasma Proteins as Markers of Sperm Fertility. Theriogenology 2019, 137, 30–35. [Google Scholar] [CrossRef]
- Teves, M.E.; Roldan, E.R.S. Sperm Bauplan and Function and Underlying Processes of Sperm Formation and Selection. Physiol. Rev. 2022, 102, 7–60. [Google Scholar] [CrossRef]
- Shan, S.; Xu, F.; Hirschfeld, M.; Brenig, B. Sperm Lipid Markers of Male Fertility in Mammals. Int. J. Mol. Sci. 2021, 22, 8767. [Google Scholar] [CrossRef]
- Ling, Z.-N.; Jiang, Y.-F.; Ru, J.-N.; Lu, J.-H.; Ding, B.; Wu, J. Amino Acid Metabolism in Health and Disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef]
- Raspa, M.; Paoletti, R.; Peltier, M.; Majjouti, M.; Protti, M.; Mercolini, L.; Mahabir, E.; Scavizzi, F. Oral D-Aspartate Treatment Improves Sperm Fertility in Both Young and Adult B6N Mice. Animals 2022, 12, 1350. [Google Scholar] [CrossRef] [PubMed]
- Blancquaert, L.; Everaert, I.; Missinne, M.; Baguet, A.; Stegen, S.; Volkaert, A.; Petrovic, M.; Vervaet, C.; Achten, E.; DE Maeyer, M.; et al. Effects of Histidine and β-Alanine Supplementation on Human Muscle Carnosine Storage. Med. Sci. Sports Exerc. 2017, 49, 602–609. [Google Scholar] [CrossRef]
- Makris, A.; Alevra, A.I.; Exadactylos, A.; Papadopoulos, S. The Role of Melatonin to Ameliorate Oxidative Stress in Sperm Cells. Int. J. Mol. Sci. 2023, 24, 15056. [Google Scholar] [CrossRef]
- Xu, B.; Bai, X.; Zhang, J.; Li, B.; Zhang, Y.; Su, R.; Wang, R.; Wang, Z.; Lv, Q.; Zhang, J.; et al. Metabolomic Analysis of Seminal Plasma to Identify Goat Semen Freezability Markers. Front. Vet. Sci. 2023, 10, 1132373. [Google Scholar] [CrossRef]
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and Cancer: Cell Biology, Physiology, and Clinical Opportunities. J. Clin. Investig. 2013, 123, 3678–3684. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.J.; Ortiz-Rodríguez, J.M.; Gaitskell-Phillips, G.L.; Gil, M.C.; Ortega-Ferrusola, C.; Martín-Cano, F.E. An Integrated Overview on the Regulation of Sperm Metabolism (Glycolysis-Krebs Cycle-Oxidative Phosphorylation). Anim. Reprod. Sci. 2022, 246, 106805. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zeng, L.; Yao, K.; Kong, X.; Wu, G.; Yin, Y. The Glutamine-Alpha-Ketoglutarate (AKG) Metabolism and Its Nutritional Implications. Amino Acids 2016, 48, 2067–2080. [Google Scholar] [CrossRef]
- Ahmed, H.; Jahan, S.; Ullah, H.; Ullah, F.; Salman, M.M. The Addition of Resveratrol in Tris Citric Acid Extender Ameliorates Post-Thaw Quality Parameters, Antioxidant Enzymes Levels, and Fertilizing Capability of Buffalo (Bubalus Bubalis) Bull Spermatozoa. Theriogenology 2020, 152, 106–113. [Google Scholar] [CrossRef]
- Tulsiani, D.R.; Abou-Haila, A.; Loeser, C.R.; Pereira, B.M. The Biological and Functional Significance of the Sperm Acrosome and Acrosomal Enzymes in Mammalian Fertilization. Exp. Cell Res. 1998, 240, 151–164. [Google Scholar] [CrossRef]
- Sabeti, P.; Pourmasumi, S.; Rahiminia, T.; Akyash, F.; Talebi, A.R. Etiologies of Sperm Oxidative Stress. Int. J. Reprod. Biomed. 2016, 14, 231–240. [Google Scholar] [CrossRef]
- Takalani, N.B.; Monageng, E.M.; Mohlala, K.; Monsees, T.K.; Henkel, R.; Opuwari, C.S. Role of Oxidative Stress in Male Infertility. Reprod. Fertil. 2023, 4, e230024. [Google Scholar] [CrossRef]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP Synthesis and Storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef]
- Palma, F.R.; Gantner, B.N.; Sakiyama, M.J.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M.G. ROS Production by Mitochondria: Function or Dysfunction? Oncogene 2024, 43, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, H.; Pal, S.; Sabnam, S.; Pal, A. High Glucose Augments ROS Generation Regulates Mitochondrial Dysfunction and Apoptosis via Stress Signalling Cascades in Keratinocytes. Life Sci. 2020, 241, 117148. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Trefts, E.; Shaw, R.J. AMPK: Restoring Metabolic Homeostasis over Space and Time. Mol. Cell 2021, 81, 3677–3690. [Google Scholar] [CrossRef]
- Gao, S.; Heng, N.; Liu, F.; Guo, Y.; Chen, Y.; Wang, L.; Ni, H.; Sheng, X.; Wang, X.; Xing, K.; et al. Natural Astaxanthin Enhanced Antioxidant Capacity and Improved Semen Quality through the MAPK/Nrf2 Pathway in Aging Layer Breeder Roosters. J. Anim. Sci. Biotechnol. 2021, 12, 112. [Google Scholar] [CrossRef] [PubMed]
| Group | Fresh Sperm Motility (%) | Fresh Sperm PR.MOT (%) | Frozen–Thawed Sperm Motility (%) | Frozen–Thawed Sperm PR.MOT (%) |
|---|---|---|---|---|
| LFG | 81.64 ± 4.10 a | 71.6 ± 3.33 a | 25.11 ± 1.29 a | 16.1 ± 1.45 a |
| HFG | 79.91 ± 4.07 a | 76.6 ± 3.33 a | 56.57 ± 1.29 b | 47.3 ± 1.45 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Liu, L.; Wang, N.; Zhou, Z.; Zhang, Z.; Li, Y.; Zan, L.; Yang, W. L-Glutamine Supplementation Improves the In Vitro Qualitative Parameters of Cryopreserved Qinchuan Bull Sperm. Animals 2025, 15, 3052. https://doi.org/10.3390/ani15203052
Yang B, Liu L, Wang N, Zhou Z, Zhang Z, Li Y, Zan L, Yang W. L-Glutamine Supplementation Improves the In Vitro Qualitative Parameters of Cryopreserved Qinchuan Bull Sperm. Animals. 2025; 15(20):3052. https://doi.org/10.3390/ani15203052
Chicago/Turabian StyleYang, Benshun, Li Liu, Nanfei Wang, Zhenghai Zhou, Zhipeng Zhang, Yuan Li, Linsen Zan, and Wucai Yang. 2025. "L-Glutamine Supplementation Improves the In Vitro Qualitative Parameters of Cryopreserved Qinchuan Bull Sperm" Animals 15, no. 20: 3052. https://doi.org/10.3390/ani15203052
APA StyleYang, B., Liu, L., Wang, N., Zhou, Z., Zhang, Z., Li, Y., Zan, L., & Yang, W. (2025). L-Glutamine Supplementation Improves the In Vitro Qualitative Parameters of Cryopreserved Qinchuan Bull Sperm. Animals, 15(20), 3052. https://doi.org/10.3390/ani15203052






