Differential Metabolomic Signatures in Boar Sperm with Varying Liquid Preservation Capacities at 17 °C
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Semen Collection and Processing
2.3. Assessment of Sperm Parameters and Classification of Semen Doses
2.4. Mass Spectrometric Analysis
2.4.1. Metabolite Extraction
2.4.2. UPLC/MS Analysis
2.5. Data Analysis
3. Results
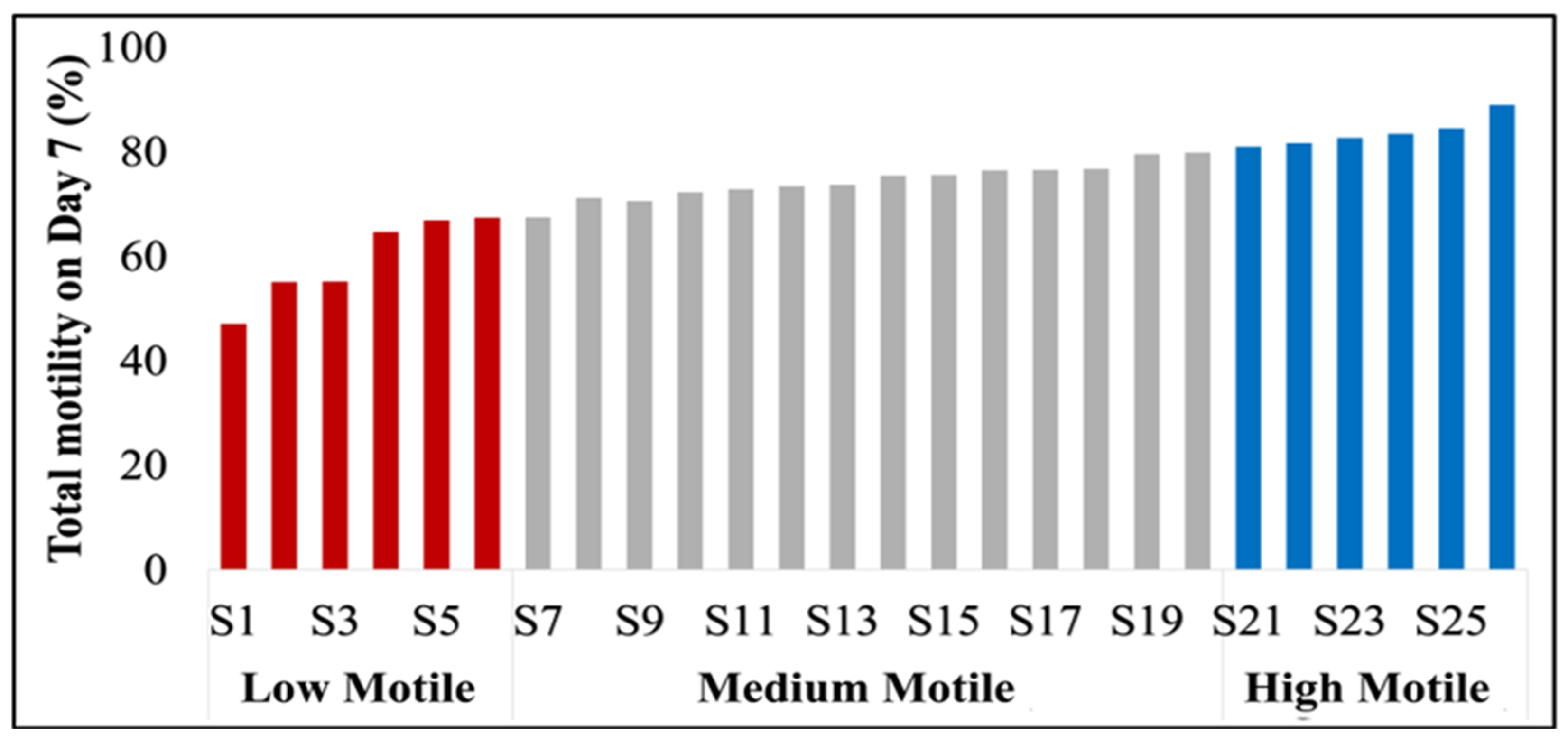
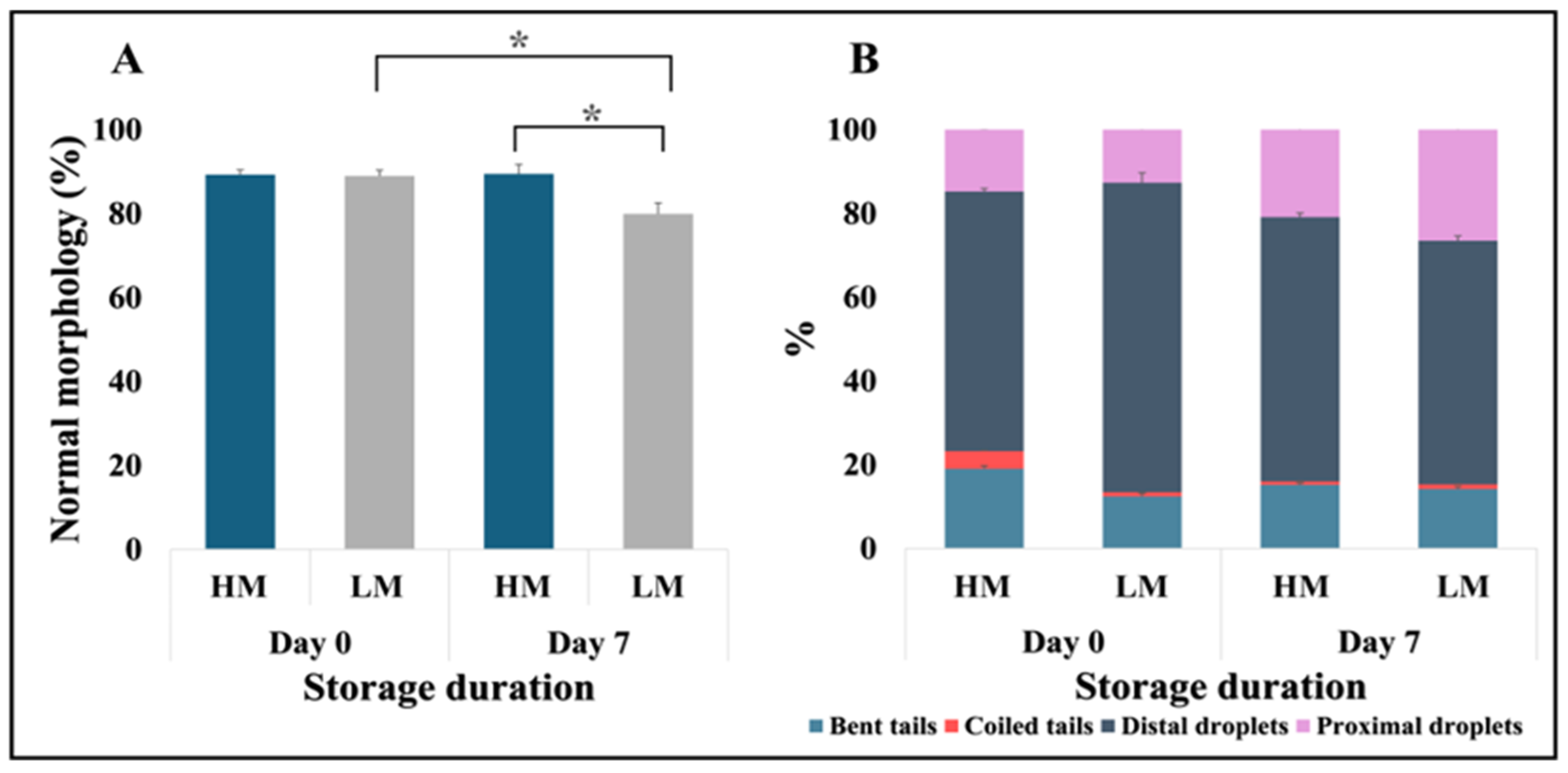
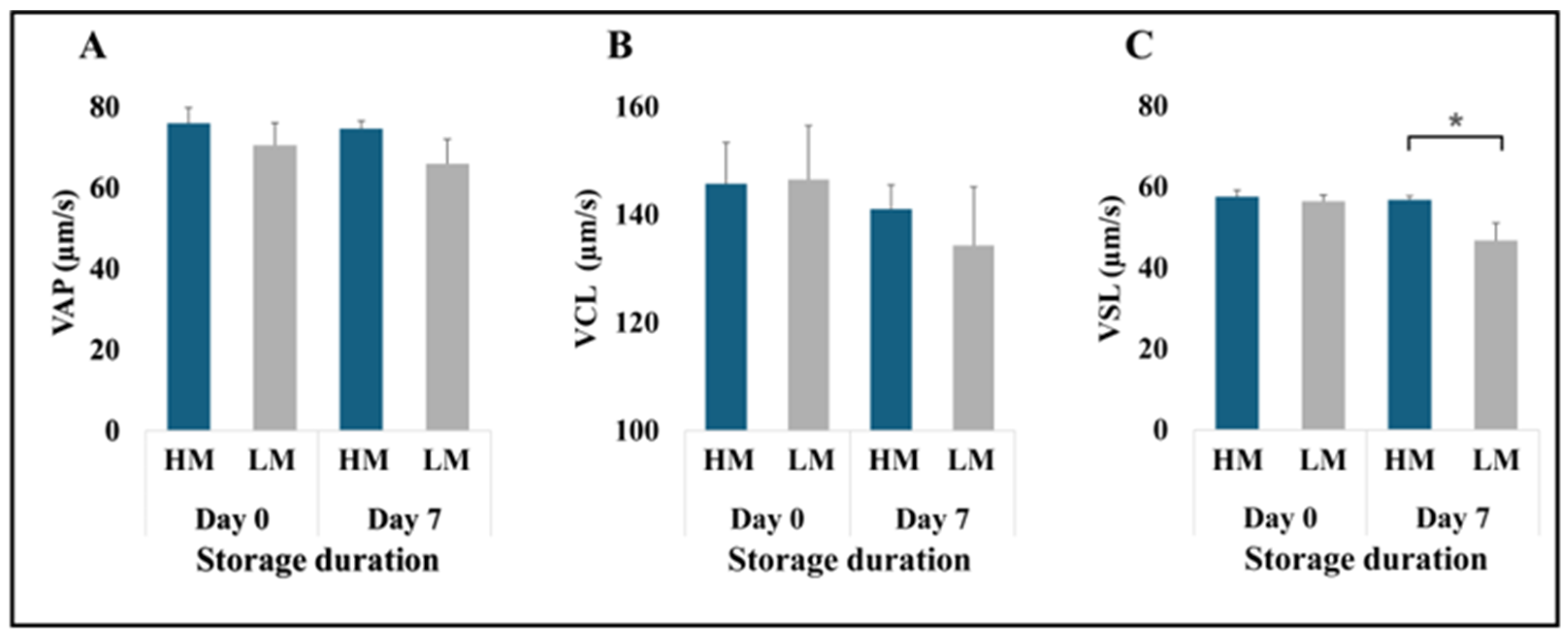
3.1. Identification and Characterization of High- and Low-Motile Semen
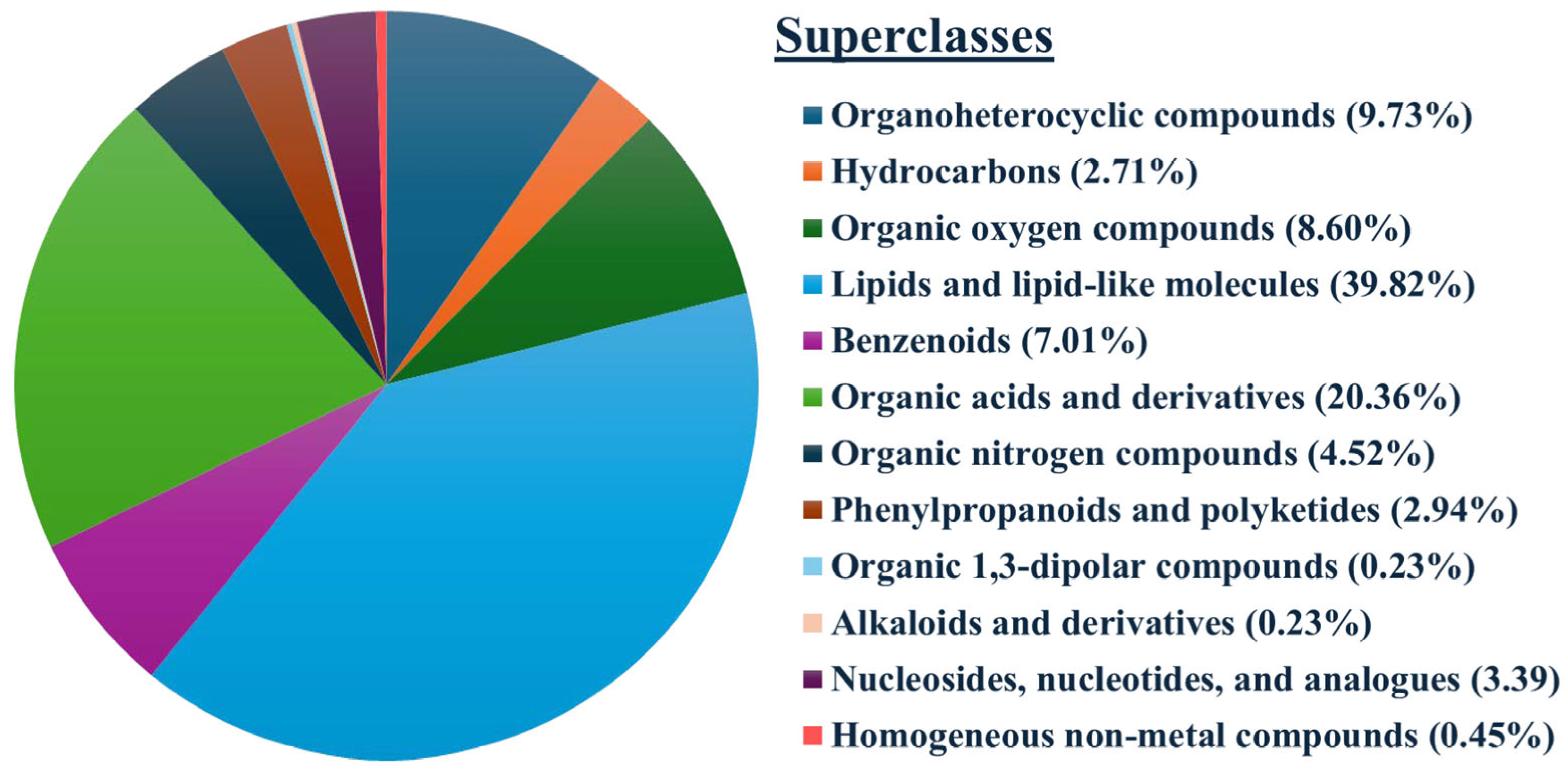
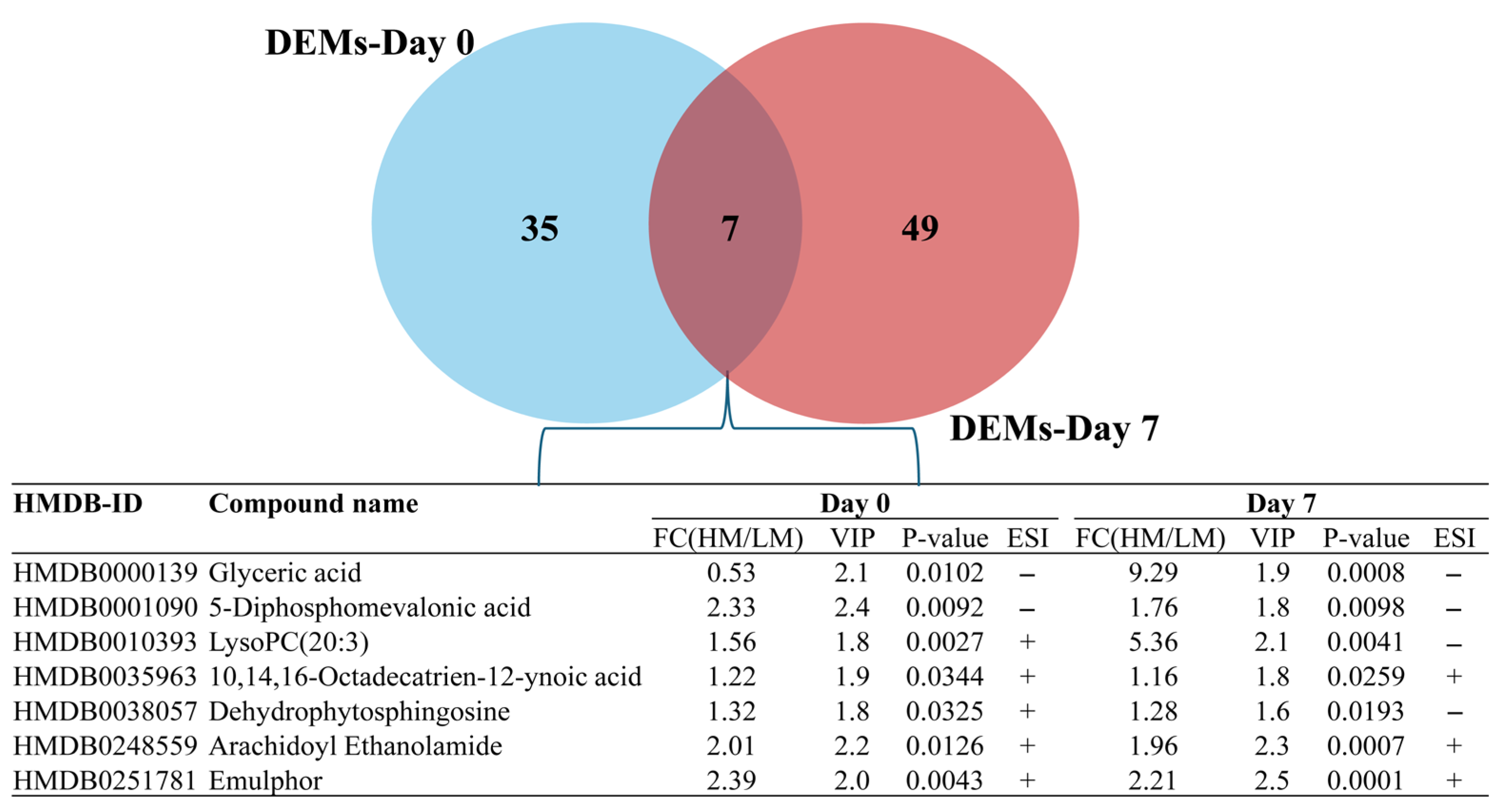
3.2. Total and Differentially Expressed Metabolites (DEMs) in Spermatozoa Between Low- and High-Motile Semen
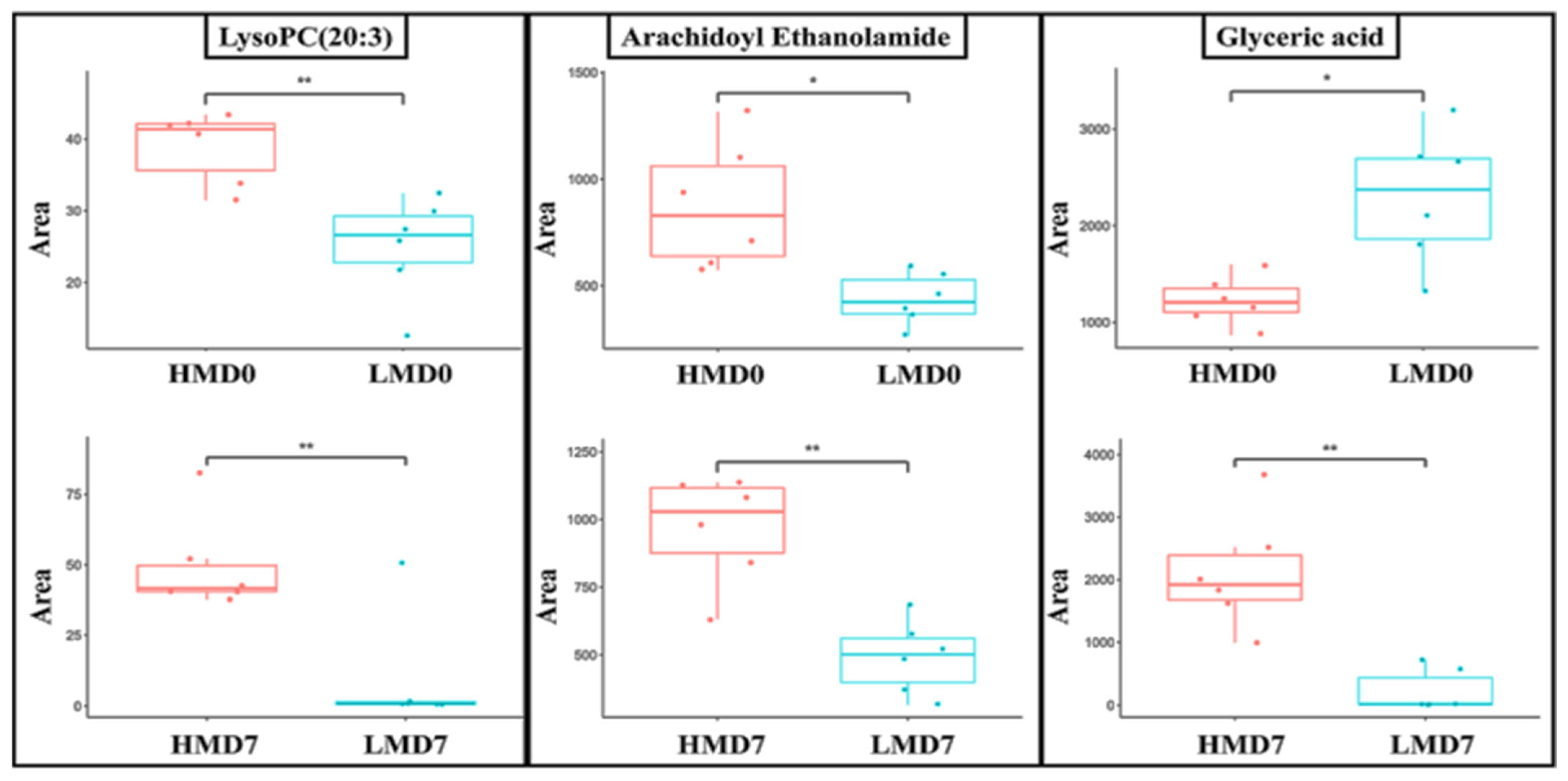
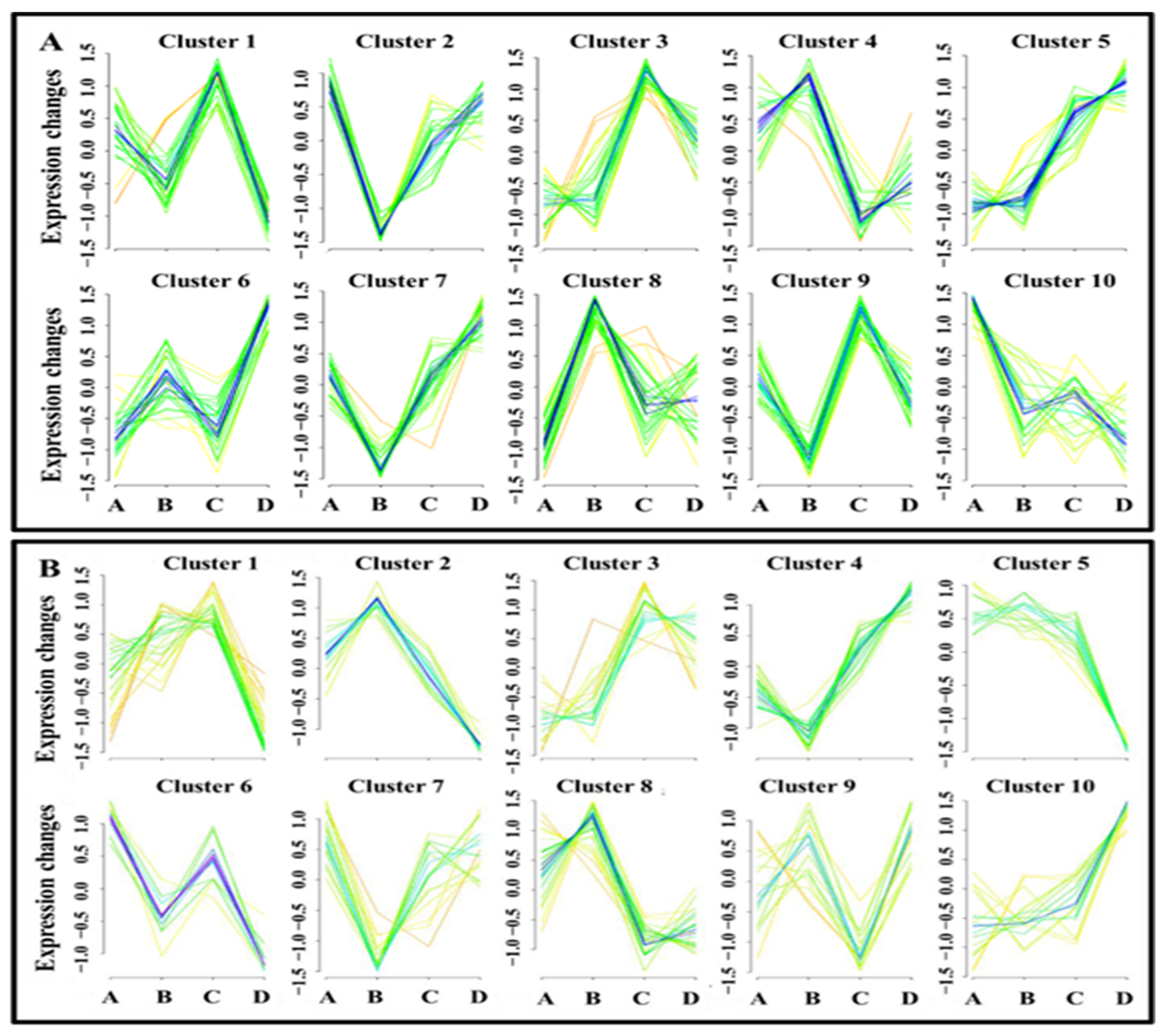
3.3. Cluster Analysis of Differentially Expressed Metabolites and Identification of Potential Biomarkers of Low- and High-Motile Semen Phenotypes
3.4. Pathway Analysis of Differentially Expressed Metabolites Between Low- and High-Motile Semen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial insemination |
| DEMs | Differentially expressed metabolites |
| HM | High motile |
| LM | Low motile |
| MM | Medium motile |
| MOT | Total motility |
| PM | Progressive motility |
References
- Bolarin, A.; Berndtson, J.; Tejerina, F.; Cobos, S.; Pomarino, C.; D’Alessio, F.; Blackburn, H.; Kaeoket, K. Boar Semen Cryopreservation: State of the Art, and International Trade Vision. Anim. Reprod. Sci. 2024, 269, 107496. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M. State-of-the-Art of Boar Sperm Preservation in Liquid and Frozen State. Anim. Reprod. 2017, 14, 69–81. [Google Scholar] [CrossRef]
- Kameni, S.L.; Meutchieye, F.; Ngoula, F. Liquid Storage of Ram Semen: Associated Damages and Improvement. Open J. Anim. Sci. 2021, 11, 473–500. [Google Scholar] [CrossRef]
- Henning, H.; Nguyen, Q.T.; Wallner, U.; Luther, A.-M.; Waberski, D. Liquid Preservation of Boar Semen: Insights into the Sperm’s Energy Budget. Anim. Reprod. Sci. 2022, 247, 107109. [Google Scholar] [CrossRef]
- Calderón-Calderón, J.; Sevilla, F.; Roldan, E.R.S.; Barquero, V.; Valverde, A. Influence of Fat-Soluble Vitamin Intramuscular Supplementation on Kinematic and Morphometric Sperm Parameters of Boar Ejaculates. Front. Vet. Sci. 2022, 9, 908763. [Google Scholar] [CrossRef] [PubMed]
- Hensel, B.; Pieper, L.; Jung, M.; Schulze, M. Influence of Age, Breed, and Season on the Quality of Boar Semen Stored at Low-Temperature. Theriogenology 2023, 208, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Szymanowicz, J.; Schwarz, T.; Murawski, M.; Małopolska, M.; Oszczęda, Z.; Tuz, R.; Nowicki, J.; Mieczyslaw Bartlewski, P. Storage of Boar Semen at 16–18 °C in the Long-Term Commercial Extender Prepared with Deionized Water or Nanowater. Anim. Reprod. 2019, 16, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, H.; Scheinpflug, K.; Mühldorfer, K.; Gianluppi, R.; Lucca, M.S.; Mellagi, A.P.G.; Bortolozzo, F.P.; Waberski, D. In Vitro Performance and In Vivo Fertility of Antibiotic-Free Preserved Boar Semen Stored at 5 °C. J. Anim. Sci. Biotechnol. 2021, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- De Lazari, F.L.; Sontag, R.; Schneider, A.; Alencar, A.; Moura, A.; Vasconcelos, R.; Nagano, C.S.; Ferrari Dalberto, P.; Valim Bizarro, C.; Mattos, R.C.; et al. Proteomic Identification of Boar Seminal Plasma Proteins Related to Sperm Resistance to Cooling at 17 °C. Theriogenology 2020, 147, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.M.; Kameni, S.L.; Dlamini, N.H.; Liao, S.F.; Feugang, J.M. Discriminating Highly Resilient Spermatozoa During Long-Term Chilled Storage. J. Anim. Sci. 2024, 102, 100–101. [Google Scholar] [CrossRef]
- Song, C.; Zhang, Z.; Wei, Y.; Dou, Y.; Qi, K.; Li, X.; Yang, F.; Li, X.; Wang, K.; Qiao, R.; et al. Proteomic Analysis of Boar Sperm with Differential Ability of Liquid Preservation at 17 °C. Theriogenology 2024, 215, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Lopez Rodriguez, A.; Van Soom, A.; Arsenakis, I.; Maes, D. Boar management and semen handling factors affect the quality of boar extended semen. Porc. Health Manag. 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, Y.; Ren, C.; Cheng, X.; Chen, J.; Ge, Z. Identification of Proteomic Markers for Ram Spermatozoa Motility Using a Tandem Mass Tag (TMT) Approach. J. Proteom. 2020, 210, 103438. [Google Scholar] [CrossRef] [PubMed]
- Gòdia, M.; Castelló, A.; Rocco, M.; Cabrera, B.; Enric Rodríguez-Gil, J.; Balasch, S.; Lewis, C.; Sánchez, A.; Clop, A. Identification of Circular RNAs in Porcine Sperm and Evaluation of Their Relation to Sperm Motility. Sci. Rep. 2020, 10, 7985. [Google Scholar] [CrossRef] [PubMed]
- Menezes, T.d.A.; Bustamante-Filho, I.C.; Paschoal, A.F.L.; Dalberto, P.F.; Bizarro, C.V.; Bernardi, M.L.; Ulguim, R.d.R.; Bortolozzo, F.P.; Mellagi, A.P.G. Differential Seminal Plasma Proteome Signatures of Boars with High and Low Resistance to Hypothermic Semen Preservation at 5 °C. Andrology 2020, 8, 1907–1922. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, N.H.; Nguyen, T.; Gad, A.; Tesfaye, D.; Liao, S.F.; Willard, S.T.; Ryan, P.L.; Feugang, J.M. Characterization of Extracellular Vesicle-Coupled MiRNA Profiles in Seminal Plasma of Boars with Divergent Semen Quality Status. Int. J. Mol. Sci. 2023, 24, 3194. [Google Scholar] [CrossRef] [PubMed]
- Gòdia, M.; Estill, M.; Castelló, A.; Balasch, S.; Rodríguez-Gil, J.E.; Krawetz, S.A.; Sánchez, A.; Clop, A. A RNA-Seq Analysis to Describe the Boar Sperm Transcriptome and Its Seasonal Changes. Front. Genet. 2019, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hidalgo, D.; Macías-García, B.; García-Marín, L.J.; Bragado, M.J.; González-Fernández, L. Boar Spermatozoa Proteomic Profile Varies in Sperm Collected During the Summer and Winter. Anim. Reprod. Sci. 2020, 219, 106513. [Google Scholar] [CrossRef] [PubMed]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics Activity Screening for Identifying Metabolites That Modulate Phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Otero, Y.; Madrid-Gambin, F.; Llavanera, M.; Gomez-Gomez, A.; Haro, N.; Pozo, O.J.; Yeste, M. Sperm Physiology and In Vitro Fertilising Ability Rely on Basal Metabolic Activity: Insights from the Pig Model. Commun. Biol. 2023, 6, 344. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Pedrosa, A.C.; Novais, F.J.; Alkmin, D.V.; Cooper, B.R.; Yasui, G.S.; Fukumasu, H.; Machaty, Z.; Andrade, A.F.C.D. Metabolomic Signature of Spermatozoa Established During Holding Time Is Responsible for Differences in Boar Sperm Freezability. Biol. Reprod. 2022, 106, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, W.; Liu, Y.; Liu, Y.; Liang, H.; Xu, Q.; Liu, Z.; Weng, X. Plasma Membrane Lipid Composition and Metabolomics Analysis of Yorkshire Boar Sperms with High and Low Resistance to Cryopreservation. Theriogenology 2023, 206, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, H.; Liu, Y.; Zhao, M.; Xu, Q.; Liu, Z.; Weng, X. Metabolomic Analysis and Identification of Sperm Freezability-Related Metabolites in Boar Seminal Plasma. Animals 2021, 11, 1939. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Chang, L.; Wang, B.; Zhang, Z.; Wei, Y.; Dou, Y.; Qi, K.; Yang, F.; Li, X.; Li, X.; et al. Seminal Plasma Metabolomics Analysis of Differences in Liquid Preservation Ability of Boar Sperm. J. Anim. Sci. 2023, 101, skad392. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Sheng, M.; Luo, H.; Liu, G.; Meng, F.; Cao, Z.; Zhang, Y. Characterization of Freezability-Associated Metabolites in Boar Semen. Theriogenology 2023, 196, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Futschik, M. Mfuzz: A Software Package for Soft Clustering of Microarray Data. Bioinformation 2007, 2, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M.C. Storage of Boar Semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Wu, C.; Qiu, S.; Chen, X.; Cai, B.; Xie, H. Freeze-Thawing Impairs the Motility, Plasma Membrane Integrity and Mitochondria Function of Boar Spermatozoa Through Generating Excessive ROS. BMC Vet. Res. 2021, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.; Holt, W.V.; Moore, H.D.M.; Reed, H.C.B.; Curnock, R.M. Objectively Measured Boar Sperm Motility Parameters Correlate with the Outcomes of On-Farm Inseminations: Results of Two Fertility Trials. J. Androl. 1997, 18, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Elmi, A.; Banchelli, F.; Barone, F.; Fantinati, P.; Ventrella, D.; Forni, M.; Bacci, M.L. Semen Evaluation and In Vivo Fertility in a Northern Italian Pig Farm: Can Advanced Statistical Approaches Compensate for Low Sample Size? An Observational Study. Anim. Reprod. Sci. 2018, 192, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Odet, F.; Gabel, S.; London, R.E.; Goldberg, E.; Eddy, E.M. Glycolysis and Mitochondrial Respiration in Mouse LDHC-Null Sperm. Biol. Reprod. 2013, 88, 95. [Google Scholar] [CrossRef] [PubMed]
- Kameni, S.L.; Semon, B.; Chen, L.D.; Dlamini, N.H.; Ariunbold, G.O.; Vance-Kouba, C.K.; Feugang, J.M. Predicting Boar Sperm Survival During Liquid Storage Using Vibrational Spectroscopic Techniques. Biology 2024, 13, 763. [Google Scholar] [CrossRef] [PubMed]
- Karanwal, S.; Pal, A.; Chera, J.S.; Batra, V.; Kumaresan, A.; Datta, T.K.; Kumar, R. Identification of Protein Candidates in Spermatozoa of Water Buffalo (Bubalus bubalis) Bulls Helps in Predicting Their Fertility Status. Front. Cell Dev. Biol. 2023, 11, 1119220. [Google Scholar] [CrossRef] [PubMed]
- Cerolini, S.; Maldjian, F.; Pizzi, F.; Gliozzi, T.M. Changes in Sperm Quality and Lipid Composition during Cryopreservation of Boar Semen. Reproduction 2001, 121, 395–401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lucca, M.S.; Ulguim, R.d.R.; Bortolozzo, F.P.; Wentz, I.; Rocha, J.C.; Dias de Souza, M.C.; Escobar, R.V.; Calderam, K.; Filho, P.I.d.Q.; Marcos, R.A. Reproductive Performance of Sows Inseminated with Semen Doses Stored for up to Seven Days in Long-Term Extender in a Field Condition. Anim. Reprod. 2020, 17, e20190121. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, J.; Li, K.; Qin, J.; Sun, Y.; Zeng, J.; El-Ashram, S.; Zhao, Y. Metabolomic Analysis Reveals Spermatozoa and Seminal Plasma Differences Between Duroc and Liang Guang Small-Spotted Pig. Front. Vet. Sci. 2023, 9, 1078928. [Google Scholar] [CrossRef] [PubMed]
- Menezes, E.B.; Velho, A.L.C.; Santos, F.; Dinh, T.; Kaya, A.; Topper, E.; Moura, A.A.; Memili, E. Uncovering Sperm Metabolome to Discover Biomarkers for Bull Fertility. BMC Genom. 2019, 20, 714. [Google Scholar] [CrossRef] [PubMed]
- Abruzzese, G.A.; Sanchez-Rodriguez, A.; Roldan, E.R.S. Sperm Metabolism. Mol. Reprod. Dev. 2024, 91, e23772. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, G.; Tamanini, M.d.S.C.; Leal, L.A.; Wolf, L.M.; Christ, T.S.; Piton, Y.V.; Arbo, M.D.; Bernardi, M.L.; Ulguim, R.d.R.; Bortolozzo, F.P.; et al. L-Cysteine Improves Boar Semen Motility at 5 °C but Does Not Affect the Oxidative Status. Anim. Reprod. Sci. 2024, 260, 107384. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Ş.Ö.; Gür, S.; Kaya, E. Effect of L-Arginine Addition on Long-Term Storability of Ram Semen. Andrologia 2017, 50, e12945. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Feng, H.; Han, J.; Zhou, H.; Yuan, L.; Pan, H.; Wang, X.; Han, X.; Qiao, R.; Yang, F.; et al. Effect of L-Proline on Sperm Quality During Cryopreservation of Boar Semen. Anim. Reprod. Sci. 2023, 258, 107359. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; Jiang, H.; Zini, A.; Kodama, H.; Gagnon, C. Reactive Oxygen Species and Sperm Physiology. Rev. Reprod. 1997, 2, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.M.; Baumann, S.; Rolle-Kampczyk, U.; Schiller, J.; von Bergen, M.; Grunewald, S. Metabolomic Profiling Reveals Correlations Between Spermiogram Parameters and the Metabolites Present in Human Spermatozoa and Seminal Plasma. PLoS ONE 2019, 14, e0211679. [Google Scholar] [CrossRef] [PubMed]
- Rustamzadeh, A.; Zamani, S.; Heidarian, E.; Ahmadi, R.; Rahimi-madiseh, M.; Nouri, A. Effects of Licorice Hydroalcoholic Extract and Glyceric Acid on Sex Hormones, Malondialdehyde, and Testicular Histomorphometry in NMRI Adult Male Rats Exposed to Cyclophosphamide. Comp. Clin. Path 2023, 32, 651–659. [Google Scholar] [CrossRef]
- Li, Y.; Jin, L.; Li, Y.; Qian, J.; Wang, Z.; Zheng, X.; Xie, C.; Zhang, X.; Huang, H.; Zhou, Y. Lysophosphatidic Acid Improves Human Sperm Motility by Enhancing Glycolysis and Activating L-Type Calcium Channels. Front. Endocrinol. 2022, 13, 896558. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Estrada, E.; Del Álamo, M.-M.R.; Bonet, S.; Rigau, T. The Increase in Phosphorylation Levels of Serine Residues of Protein HSP70 During Holding Time at 17 °C Is Concomitant with a Higher Cryotolerance of Boar Spermatozoa. PLoS ONE 2014, 9, e90887. [Google Scholar] [CrossRef] [PubMed]
- Çoyan, K.; Başpınar, N.; Bucak, M.N.; Akalın, P.P. Effects of Cysteine and Ergothioneine on Post-Thawed Merino Ram Sperm and Biochemical Parameters. Cryobiology 2011, 63, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gungor, S.; Ozturk, C.; Omur, A.D. Positive Effects of Trehalose and Cysteine on Ram Sperm Parameters. Vet. Med. 2017, 62, 245–252. [Google Scholar] [CrossRef]
- Iqbal, S.; Riaz, A.; Andrabi, S.M.H.; Shahzad, Q.; Durrani, A.Z.; Ahmad, N. L-Cysteine Improves Antioxidant Enzyme Activity, Post-Thaw Quality and Fertility of Nili-Ravi Buffalo (Bubalus bubalis) Bull Spermatozoa. Andrologia 2016, 48, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yu, X.; Li, X.; Yu, B.; Peng, H. Protective Effects of L-Cysteine and N-Acetyl-L-Cysteine on Boar Sperm Quality during Hypothermic Liquid Storage with Bovine Serum Albumin as a Protectant. Theriogenology 2024, 216, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Lee, K.B.; Lee, J.H.; Kim, K.J.; Kim, E.Y.; Han, K.W.; Park, K.S.; Yu, J.; Kim, M.K. Glutathione and Cysteine Enhance Porcine Preimplantation Embryo Development In Vitro After Intracytoplasmic Sperm Injection. Theriogenology 2014, 81, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.H.; Li, Y.W.; Xie, H.L.; Li, Q.; Dong, H.B.; Sun, M.J.; Gao, W.Q.; Tan, J.H. Effects of Glucose Metabolism Pathways on Sperm Motility and Oxidative Status During Long-Term Liquid Storage of Goat Semen. Theriogenology 2016, 86, 839–849. [Google Scholar] [CrossRef] [PubMed]



| Replicates | Semen Doses | Day 0 (mean ± sem; %) | Day 7 (mean ± sem; %) | |||
|---|---|---|---|---|---|---|
| n | Status | Motility | Morphology | Motility | Morphology | |
| I | 4 | n.d. | 78.53 ± 4.07 | 86.8 ±3.53 | 70.08 ± 8.84 | 83.78 ± 1.44 |
| II | 4 | n.d. | 81.88 ± 2.04 | 91.25 ± 2.20 | 74.55 ± 2.47 | 84.95 ±2.33 |
| III | 4 | n.d. | 84.03 ± 2.35 | 90.73 ± 0.71 | 74.28 ±6.60 | 87.70 ± 4.96 |
| IV | 4 | n.d. | 78.18 ± 2.42 | 86.88 ± 3.46 | 75.58 ±2.53 | 85.08 ±1.93 |
| V | 5 | n.d. | 74.42 ± 4.86 | 81.42 ± 3.83 | 71.06 ±2.62 | 73.80 ± 2.39 |
| VI | 5 | n.d. | 78.53 ± 1.07 | 86.24 ± 1.72 | 76.22 ± 2.11 | 83.78 ± 1.44 |
| Total | 26 | 79.26 ± 1.36 | 87.22 ± 1.45 | 73.63 ± 1.02 | 83.18 ± 1.97 | |
| Semen Classification | 6 | LM | 79.92 ± 2.18 | 88.97 ± 1.41 | 59.40 ± 3.35 | 79.93 ± 2.61 |
| 14 | MM | 73.84 ± 2.15 | 83.44 ± 2.03 | 77.75 ± 0.82 | 80.16 ± 1.53 | |
| 6 | HM | 84.02 ± 1.84 | 89.25 ± 1.17 | 83.73 ± 1.17 | 89.45 ± 2.30 | |
| HMDB_ID | Compound Names | FC (HM/LM) | t-Test | VIP | ESI | Day |
|---|---|---|---|---|---|---|
| HMDB0059875 | Carvotanacetone | 1.8373 | 0.0009 | 2.2 | + | 0 |
| HMDB0255129 | N-Dodecylsarcosinate | 15.5784 | 0.0010 | 2.4 | − | 0 |
| HMDB0251781 | Emulphor | 2.3938 | 0.0043 | 2.0 | + | 0 |
| HMDB0037012 | 2-Hydroxy-p-mentha-1,8-dien-6-one | 1.2757 | 0.0053 | 2.0 | + | 0 |
| HMDB0013648 | Palmitoleoylethanolamde | 1.3352 | 0.0064 | 2.1 | + | 0 |
| HMDB0001090 | 5-Diphosphomevalonic acid | 2.3281 | 0.0092 | 2.4 | − | 0 |
| HMDB0000139 | Glyceric acid | 0.5340 | 0.0102 | 2.1 | − | 0 |
| HMDB0248559 | Arachidoyl ethanolamide | 2.0082 | 0.0126 | 2.2 | + | 0 |
| HMDB0341228 | 4-Indolecarbaldehyde | 2.0330 | 0.0152 | 2.1 | − | 0 |
| HMDB0029104 | Tyrosyl-glutamate | 0.6406 | 0.0168 | 2.0 | + | 0 |
| HMDB0243890 | 1-O-Hexadecyl-sn-glycero-3-phosphocholine | 2.5811 | 0.0169 | 2.0 | − | 0 |
| HMDB0254257 | Lykurim | 0.5465 | 0.0229 | 2.1 | + | 0 |
| HMDB0116636 | PG(a-13:0/a-13:0) | 9.4155 | 0.00003 | 2.1 | − | 7 |
| HMDB0028831 | Glutamyltyrosine | 7.9348 | 0.00003 | 2.1 | − | 7 |
| HMDB0011517 | LysoPE(20:4) | 8.1406 | 0.00008 | 2.1 | − | 7 |
| HMDB0251781 | Emulphor | 2.2117 | 0.00010 | 2.5 | + | 7 |
| HMDB0062549 | 2-Hydroxystearic acid | 26.8913 | 0.00014 | 2.2 | − | 7 |
| HMDB0041249 | 6-Hydroxyshogaol | 4.4944 | 0.00020 | 2.5 | + | 7 |
| HMDB0248559 | Arachidoyl ethanolamide | 1.9631 | 0.00067 | 2.3 | + | 7 |
| HMDB0010404 | LysoPC(22:6) | 5.2845 | 0.00119 | 2.1 | − | 7 |
| HMDB0000806 | Myristic acid | 5.0312 | 0.00250 | 2.1 | − | 7 |
| HMDB0010393 | LysoPC(20:3) | 5.3619 | 0.00407 | 2.1 | − | 7 |
| HMDB0000824 | Propionylcarnitine | 0.5699 | 0.00598 | 2.0 | + | 7 |
| HMDB0000791 | Octanoylcarnitine | 0.4283 | 0.00729 | 2.1 | + | 7 |
| HMDB0266619 | PA(8:0/PGJ2) | 3.1500 | 0.00888 | 2.0 | + | 7 |
| HMDB0266633 | PA(8:0/20:4+=O(5)) | 3.3804 | 0.00995 | 2.1 | + | 7 |
| HMDB0001406 | Niacinamide | 0.4928 | 0.0115 | 2.2 | + | 7 |
| HMDB0000651 | Decanoylcarnitine | 0.5544 | 0.0138 | 2.1 | + | 7 |
| HMDB0015353 | Budesonide | 106.9614 | 0.0269 | 2.0 | − | 7 |
| HMDB0032575 | Butylparaben | 0.2144 | 0.0296 | 2.1 | + | 7 |
| HMDB0029817 | Ethyl salicylate | 0.2226 | 0.0304 | 2.0 | + | 7 |
| HMDB0034240 | Styrene | 2.4391 | 0.0304 | 2.0 | + | 7 |
| HMDB0255266 | N,N-Dimethyloctadecylamine | 0.0204 | 0.0413 | 2.1 | + | 7 |
| HMDB0002833 | Testosterone sulfate | 8.8909 | 0.0453 | 2.0 | − | 7 |
| HMDB0030631 | Rubraflavone D | 2.6503 | 0.0491 | 2.0 | + | 7 |
| Storage Duration | Pathways | p-Value | Modes |
|---|---|---|---|
| Day 0 | Glycine, serine, and threonine metabolism | 5.525 × 10−4 | ESI− |
| Pentose phosphate pathway | 3.117 × 10−3 | ESI− | |
| Glycerolipid metabolism | 3.117 × 10−3 | ESI− | |
| Glyoxylate and dicarboxylate metabolism | 3.117 × 10−3 | ESI− | |
| Terpenoid backbone biosynthesis | 3.340 × 10−3 | ESI− | |
| Cysteine and methionine metabolism | 1.639 × 10−2 | ESI− | |
| Glycerolipid metabolism | 2.213 × 10−2 | ESI+ | |
| Purine metabolism | 6.147 × 10−2 | ESI+ | |
| Day 7 | Fatty acid biosynthesis | 7.849 × 10−4 | ESI− |
| Pentose phosphate pathway | 1.259 × 10−3 | ESI− | |
| Glycine, serine, and threonine metabolism | 1.259 × 10−3 | ESI− | |
| Glycerolipid metabolism | 1.259 × 10−3 | ESI− | |
| Glyoxylate and dicarboxylate metabolism | 1.259 × 10−3 | ESI− | |
| Pyrimidine metabolism | 1.289 × 10−3 | ESI− | |
| Purine metabolism | 2.214 × 10−3 | ESI− | |
| Glycerophospholipid metabolism | 2.395 × 10−3 | ESI− | |
| Terpenoid backbone biosynthesis | 4.938 × 10−3 | ESI− | |
| Glycerolipid metabolism | 8.543 × 10−3 | ESI+ | |
| Glycerophospholipid metabolism | 8.543 × 10−3 | ESI+ | |
| Nicotinate and nicotinamide metabolism | 9.484 × 10−3 | ESI+ | |
| Arginine and proline metabolism | 1.695 × 10−2 | ESI+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kameni, S.L.; Dlamini, N.H.; Feugang, J.M. Differential Metabolomic Signatures in Boar Sperm with Varying Liquid Preservation Capacities at 17 °C. Animals 2025, 15, 2163. https://doi.org/10.3390/ani15152163
Kameni SL, Dlamini NH, Feugang JM. Differential Metabolomic Signatures in Boar Sperm with Varying Liquid Preservation Capacities at 17 °C. Animals. 2025; 15(15):2163. https://doi.org/10.3390/ani15152163
Chicago/Turabian StyleKameni, Serge L., Notsile H. Dlamini, and Jean M. Feugang. 2025. "Differential Metabolomic Signatures in Boar Sperm with Varying Liquid Preservation Capacities at 17 °C" Animals 15, no. 15: 2163. https://doi.org/10.3390/ani15152163
APA StyleKameni, S. L., Dlamini, N. H., & Feugang, J. M. (2025). Differential Metabolomic Signatures in Boar Sperm with Varying Liquid Preservation Capacities at 17 °C. Animals, 15(15), 2163. https://doi.org/10.3390/ani15152163









