Effects of Water Temperature on Growth, Hematological Measurements and Stress-Related Gene Expression of Atlantic Salmon (Salmo salar) Parr Reared in a Recirculating Aquaculture System
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Rearing Conditions
2.2. Experimental Diet and Feeding
2.3. Growth Performance and Sampling
- Survival rate (SR, %) = (final number of fish/initial number of fish) × 100.
- Weight gain (WG, %) = (final weight (g) − initial weight (g))/initial weight (g) × 100.
- Specific growth rate (SGR, %/day) = (ln final weight (g) − ln initial weight (g))/days × 100.
- Daily feed intake (DFI, %) = feed intake/[(initial fish weight (g) + final fish weight (g) + dead fish weight (g)) × days reared/2] × 100.
- Feed efficiency (FE, %) = (final weight (g) − initial weight (g))/feed consumed (g) × 100.
- Protein efficiency ratio (PER) = (final weight (g) − initial weight (g))/protein intake (g).
- Condition factor (CF) = (final weight (g)/total length (cm)3) × 100.
- Hepatosomatic index (HSI, %) = liver weight (g)/body weight (g) × 100.
- Viscerosomatic index (VSI, %) = viscera weight (g)/body weight (g) × 100.
2.4. Whole-Body Proximate Composition
2.5. Plasma Biochemical Analysis
2.6. Antioxidant Enzyme Activity and Cortisol
2.7. Gene Expression Analysis
2.8. Statistical Analysis
3. Results
3.1. Water Quality
3.2. Growth Performance
3.3. Whole-Body Proximate Composition
3.4. Plasma Biochemical Parameters
3.5. Antioxidant Enzyme Activity and Cortisol
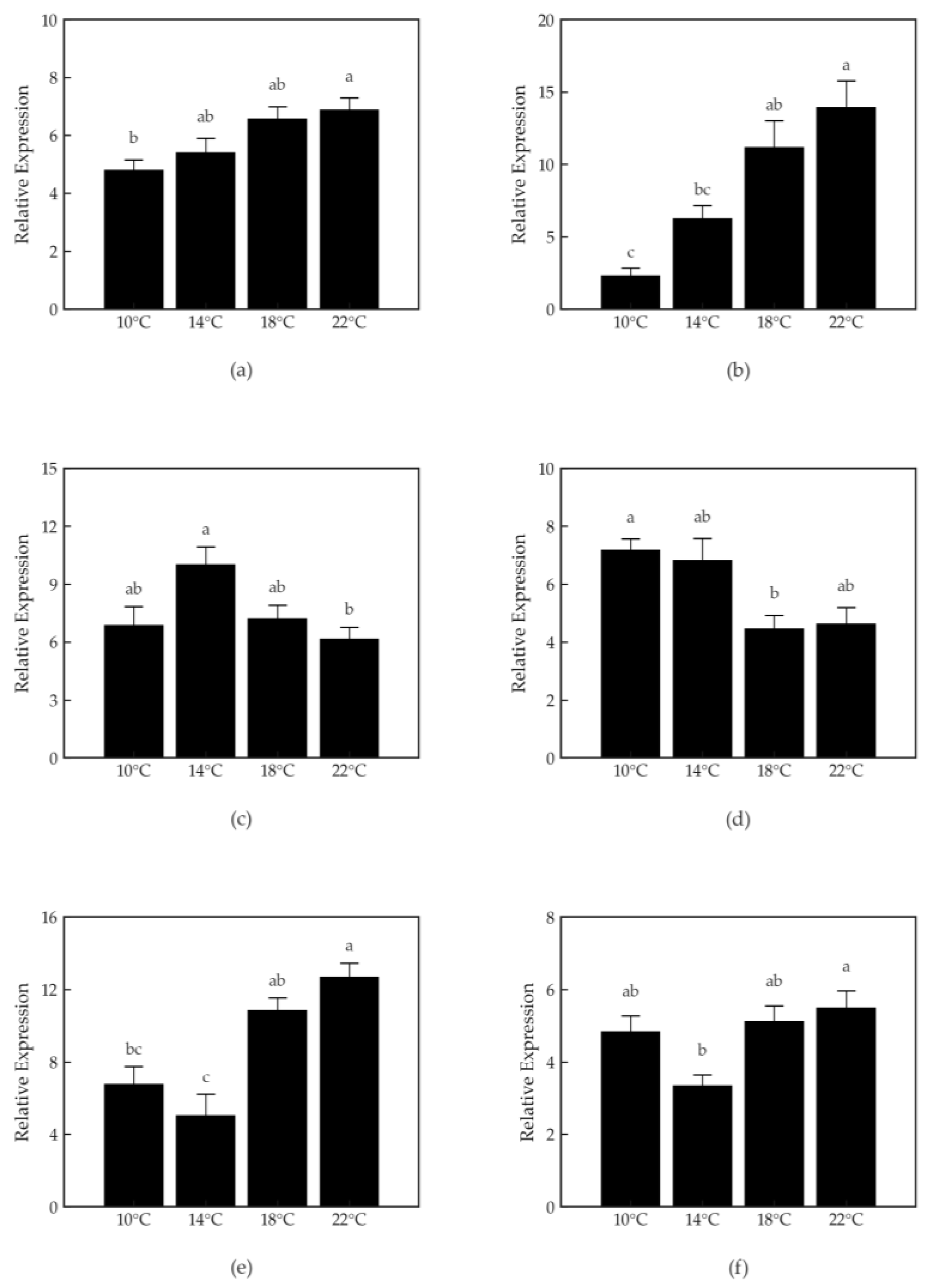
3.6. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Kim, B.T. An analysis of the impact of FTA tariff elimination on the export price of Norwegian fresh and chilled salmon to Korea. J. Fish. Bus. Adm. 2018, 49, 37–48. [Google Scholar] [CrossRef]
- MOMAF (Ministry of Marine Affairs and Fisheries). Statistic Database for Fisheries Production. Available online: https://www.fips.go.kr/p/S020305/ (accessed on 8 July 2024).
- KMI (Korea Maritime Institute). Analysis of KMI Trends; Monthly Report 51; KMI: Busan, Republic of Korea, 2017; pp. 1–16. [Google Scholar]
- KMI (Korea Maritime Institute). Analysis of KMI Trends; Monthly Report 127; KMI: Busan, Republic of Korea, 2019; pp. 1–14. [Google Scholar]
- Beitinger, T.L.; Fitzpatrick, L.C. Physiological and ecological correlates of preferred temperature in fish. Am. Zool. 1979, 19, 319–329. [Google Scholar] [CrossRef]
- Brett, J.R. Environmental factors and growth. In Fish Physiology; Hoar, W.S., Randall, D.J., Brett, J.R., Eds.; Academic Press: London, UK, 1979; Volume 8, pp. 599–675. [Google Scholar]
- Jobling, M. The influence of environmental temperature on growth and conversion efficiency in fish. In Proceedings of the International Council for the Exploration of the Sea (ICES) Annual Science Conference, Aalborg, Denmark, 21 September 1995. [Google Scholar]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef]
- Scharsack, J.P.; Franke, F. Temperature effects on teleost immunity in the light of climate change. J. Fish Biol. 2022, 101, 780–796. [Google Scholar] [CrossRef]
- Stickland, N.C.; White, R.N.; Mescall, P.E.; Crook, A.R.; Thorpe, J.E. The effect of temperature on myogenesis in embryonic development of the Atlantic salmon (Salmo salar L.). Anat. Embryol. 1988, 178, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, W.P.; Piper, R.G. Atlantic salmon growth efficiency as affected by temperature. Prog. Fish-Cult. 1987, 49, 57–59. [Google Scholar] [CrossRef]
- Vikingstad, E.; Andersson, E.; Hansen, T.J.; Norberg, B.; Mayer, I.; Stefansson, S.O.; Rjelldal, P.G.; Taranger, G.L. Effects of temperature on the final stages of sexual maturation in Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 2016, 42, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Handeland, S.O.; Berge, Å.; Björnsson, B.T.; Stefansson, S.O. Effects of temperature and salinity on osmoregulation and growth of Atlantic salmon (Salmo salar L.) smolts in seawater. Aquaculture 1998, 168, 289–302. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Slater, M.J. Responses of aquaculture fish to climate change-induced extreme temperatures: A review. J. World Aquac. Soc. 2022, 53, 314–366. [Google Scholar] [CrossRef]
- Beemelmanns, A.; Zanuzzo, F.S.; Sandrelli, R.M.; Rise, M.L.; Gamperl, A.K. The Atlantic salmon’s stress-and immune-related transcriptional responses to moderate hypoxia, an incremental temperature increase, and these challenges combined. G3 2021, 11, jkab102. [Google Scholar] [CrossRef]
- Forseth, T.; Hurley, M.A.; Jensen, A.J.; Elliott, J.M. Functional Models for Growth and Food Consumption of Atlantic Salmon Parr, Salmo salar, from a Norwegian River. Freshw. Biol. 2001, 46, 173–186. [Google Scholar] [CrossRef]
- Atkins, M.E.; Benfey, T.J. Effect of Acclimation Temperature on Routine Metabolic Rate in Triploid Salmonids. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 149, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.J.; Olsen, R.E.; Stien, L.; Oppedal, F.; Torgersen, T.; Breck, O.; Remen, M.; Vågseth, T.; Fjelldal, P.G. Effect of Water Oxygen Level on Performance of Diploid and Triploid Atlantic Salmon Post-Smolt Reared at High Temperature. Aquaculture 2015, 435, 354–360. [Google Scholar] [CrossRef]
- Sambraus, F.; Olsen, R.E.; Remen, M.; Hansen, T.J.; Torgersen, T.; Fjelldal, P.G. Water Temperature and Oxygen: The Effect of Triploidy on Performance and Metabolism in Farmed Atlantic Salmon (Salmo salar L.) Post-Smolt. Aquaculture 2017, 473, 1–12. [Google Scholar] [CrossRef]
- Esmaeili, N. Blood performance: A new formula for fish growth and health. Biology 2021, 10, 1236. [Google Scholar] [CrossRef]
- Leaver, M.J.; Diab, A.; Boukouvala, E.; Williams, T.D.; Chipman, J.K.; Moffat, C.F.; Robinson, C.D.; George, S.G. Hepatic gene expression in flounder chronically exposed to multiply polluted estuarine sediment: Absence of classical exposure ‘biomarker’ signals and induction of inflammatory, innate immune and apoptotic pathways. Aquat. Toxicol. 2010, 96, 234–245. [Google Scholar] [CrossRef]
- Kroon, F.; Streten, C.; Harries, S. A protocol for identifying suitable biomarkers to assess fish health: A systematic review. PLoS ONE 2017, 12, e0174762. [Google Scholar] [CrossRef]
- Kang, H.W.; Kim, K.I.; Lim, H.J.; Kang, H.S. Effect of Water Temperature on the Expression of Stress Related Genes in Atlantic Salmon (Salmo salar) Fry. Korean J. Environ. Biol. 2018, 36, 131–139. [Google Scholar] [CrossRef]
- Jang, S.W.; Kang, H.S.; Kang, D.Y.; Cho, K.S. Effect of Rearing Water Temperature on Growth and Physiological Response of Juvenile Chum Salmon (Oncorhynchus keta). Korean J. Environ. Biol. 2022, 40, 651–665. [Google Scholar] [CrossRef]
- Blaxter, K.L. Energy Metabolism in Animals and Man; CUP Archive: Cambridge, UK, 1989. [Google Scholar]
- Animal Care and Use Committee. Guidelines for the Preparation and Use of MS-222; East Carolina University: Greenville, NC, USA, 2024. [Google Scholar]
- Søderstrøm, S.; Søfteland, L.; Sele, V.; Lundebye, A.K.; Berntssen, M.H.; Lie, K.K. Enniatin B and beauvericin affect intestinal cell function and hematological processes in Atlantic salmon (Salmo salar) after acute exposure. Food Chem. Toxicol. 2023, 172, 113557. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Weihe, R.; Dessen, J.E.; Arge, R.; Thomassen, M.S.; Hatlen, B.; Rørvik, K.A. Improving production efficiency of farmed Atlantic salmon (Salmo salar L.) by isoenergetic diets with increased dietary protein-to-lipid ratio. Aquac. Res. 2018, 49, 1441–1453. [Google Scholar] [CrossRef]
- Kellogg, R.L.; Gift, J.J. Relationship between optimum temperatures for growth and preferred temperatures for the young of four fish species. Trans. Am. Fish. Soc. 1983, 112, 424–430. [Google Scholar] [CrossRef]
- Edsall, T.A.; Cleland, J. Optimum temperature for growth and preferred temperatures of age-0 lake trout. N. Am. J. Fish. Manag. 2000, 20, 804–809. [Google Scholar] [CrossRef]
- Forseth, T.; Jonsson, B. The growth and food ration of piscivorous brown trout (Salmo trutta). Funct. Ecol. 1994, 8, 171–177. [Google Scholar] [CrossRef]
- Richter, A.; Kolmes, S.A. Maximum temperature limits for Chinook, coho, and chum salmon, and steelhead trout in the Pacific Northwest. Rev. Fish. Sci. 2005, 13, 23–49. [Google Scholar] [CrossRef]
- Handeland, S.O.; Arnesen, A.M.; Stefansson, S.O. Seawater adaptation and growth of post-smolt Atlantic salmon (Salmo salar) of wild and farmed strains. Aquaculture 2003, 220, 367–384. [Google Scholar] [CrossRef]
- Elliott, J.; Elliott, J.A. Temperature requirements of Atlantic salmon Salmo salar, brown trout Salmo trutta and Arctic charr Salvelinus alpinus: Predicting the effects of climate change. J. Fish Biol. 2010, 77, 1793–1817. [Google Scholar] [CrossRef]
- Clarke, A.; Johnston, N.M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999, 68, 893–905. [Google Scholar] [CrossRef]
- Handeland, S.O.; Imsland, A.K.; Stefansson, S.O. The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 2008, 283, 36–42. [Google Scholar] [CrossRef]
- Ruyter, B.; Moya-Falcón, C.; Rosenlund, G.; Vegusdal, A. Fat content and morphology of liver and intestine of Atlantic salmon (Salmo salar): Effects of temperature and dietary soybean oil. Aquaculture 2006, 252, 441–452. [Google Scholar] [CrossRef]
- Hosseinpour, F.; Vazirzadeh, A.; Farhadi, A.; Sajjadi, S.H. Acclimation to higher temperature and antioxidant supplemented diets improved rainbow trout (Oncorhynchus mykiss) resilience to heatwaves. Sci. Rep. 2024, 14, 11375. [Google Scholar] [CrossRef] [PubMed]
- Burel, C.; Person-Le Ruyet, J.; Gaumet, F.; Le Roux, A.; Severe, A.; Boeuf, G. Effects of temperature on growth and metabolism in juvenile turbot. J. Fish Biol. 1996, 49, 678–692. [Google Scholar] [CrossRef]
- Tromp, J.J.; Jones, P.L.; Brown, M.S.; Donald, J.A.; Biro, P.A.; Afonso, L.O. Chronic exposure to increased water temperature reveals few impacts on stress physiology and growth responses in juvenile Atlantic salmon. Aquaculture 2018, 495, 196–204. [Google Scholar] [CrossRef]
- Raposo de Magalhães, C.; Schrama, D.; Farinha, A.P.; Revets, D.; Kuehn, A.; Planchon, S.; Rodrigues, P.M.; Cerqueira, M. Protein changes as robust signatures of fish chronic stress: A proteomics approach to fish welfare research. BMC Genom. 2020, 21, 309. [Google Scholar] [CrossRef]
- Klein, R.D.; Borges, V.D.; Rosa, C.E.; Colares, E.P.; Robaldo, R.B.; Martinez, P.E.; Bianchini, A. Effects of increasing temperature on antioxidant defense system and oxidative stress parameters in the Antarctic fish Notothenia coriiceps and Notothenia rossii. J. Therm. Biol. 2017, 68, 110–118. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants—Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Dawood, M.A.; Alkafafy, M.; Sewilam, H. The antioxidant responses of gills, intestines and livers and blood immunity of common carp (Cyprinus carpio) exposed to salinity and temperature stressors. Fish Physiol. Biochem. 2022, 48, 397–408. [Google Scholar] [CrossRef]
- Almroth, B.C.; Asker, N.; Wassmur, B.; Rosengren, M.; Jutfelt, F.; Gräns, A.; Sundell, K.; Axelsson, M.; Sturve, J. Warmer water temperature results in oxidative damage in an Antarctic fish, the bald notothen. J. Exp. Mar. Biol. Ecol. 2015, 468, 130–137. [Google Scholar] [CrossRef]
- Iwama, G.K.; Thomas, P.T.; Forsyth, R.B.; Vijayan, M.M. Heat shock protein expression in fish. Rev. Fish Biol. Fish. 1998, 8, 35–56. [Google Scholar] [CrossRef]
- Hevrøy, E.M.; Tipsmark, C.K.; Remø, S.C.; Hansen, T.; Fukuda, M.; Torgersen, T.; Vikeså, V.; Olsvik, P.A.; Waagbø, R.; Shimizu, M. Role of the GH-IGF-1 system in Atlantic salmon and rainbow trout postsmolts at elevated water temperature. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 188, 127–138. [Google Scholar] [CrossRef]
- Garcia de la Serrana, D.; Macqueen, D.J. Insulin-like growth factor-binding proteins of teleost fishes. Front. Endocrinol. 2018, 9, 80. [Google Scholar] [CrossRef]
- Robertsen, B. The interferon system of teleost fish. Fish Shellfish Immunol. 2006, 20, 172–191. [Google Scholar] [CrossRef]
- Pacitti, D.; Wang, T.; Martin, S.A.M.; Sweetman, J.; Secombes, C.J. Insights into the fish thioredoxin system: Expression profile of thioredoxin and thioredoxin reductase in rainbow trout (Oncorhynchus mykiss) during infection and in vitro stimulation. Dev. Comp. Immunol. 2014, 42, 261–277. [Google Scholar] [CrossRef]
- Liu, E.; Zhao, X.; Li, C.; Wang, Y.; Li, L.; Zhu, H.; Ling, Q. Effects of acute heat stress on liver damage, apoptosis and inflammation of pikeperch (Sander lucioperca). J. Therm. Biol. 2022, 106, 103251. [Google Scholar] [CrossRef] [PubMed]
- Myklatun, L.E.; Madaro, A.; Philip, A.J.P.; Pedersen, A.Ø.; Remø, S.; Hansen, T.J.; Fraser, T.W.K.; Sigholt, T.; Stefansson, S.; Fjelldal, P.G. Long term effects of smolt and post-smolt production strategy on mortality, growth, sexual maturation and melanized focal changes in farmed Atlantic salmon (Salmo salar L.). Aquaculture 2025, 602, 742371. [Google Scholar] [CrossRef]
- Crouse, C.; Davidson, J.; May, T.; Summerfelt, S.; Good, C. Production of market-size European strain Atlantic salmon (Salmo salar) in land-based freshwater closed containment aquaculture systems. Aquac. Eng. 2021, 92, 102138. [Google Scholar] [CrossRef]
| Ingredients | Value (%) |
|---|---|
| Fish meal | 50.00 |
| Black soldier fly meal | 5.00 |
| Soybean meal | 5.00 |
| Squid liver powder | 4.10 |
| Tankage meal | 2.50 |
| Wheat flour | 21.80 |
| Fish oil | 7.80 |
| Monocalcium phosphate | 1.00 |
| Mineral mix | 1.00 |
| Vitamin mix | 1.00 |
| Vitamin C | 0.20 |
| Choline | 0.60 |
| Total | 100 |
| Proximate analysis (% of dry matter basis) | |
| Moisture | 2.06 |
| Crude Protein | 49.08 |
| Crude Lipid | 14.56 |
| Crude Ash | 12.31 |
| Gross energy (MJ/kg) 1 | 23.74 |
| Non-protein energy (MJ/kg) 1 | 11.95 |
| Genes | Primer Sequences | Product Size (bp) | Accession Number | Primer Efficiency | R2 | Pearson’s Coefficient |
|---|---|---|---|---|---|---|
| hsp70 1 | F:CCCCTGTCCCTGGGTATTG R:CACCAGGCTGGTTGTCTGAGT | 121 | BG933934 | 96.1 | 0.997 | 0.995 |
| hsp90β 2 | F:CCACCATGGGCTACATGATG R:CCTTCACCGCCTTGTCATTC | 114 | NM_001123532 | 93.4 | 0.994 | 0.989 |
| igfbp1a 3 | F:GGTCCCTGTCATGTGGAGTT R:TTCCAGAAGGACACACACCA | 184 | KC122927 | 101.8 | 0.998 | 0.997 |
| igfbp1b 4 | F:GAGGACCAGGGACAAGAGAAAGT R:GCACCCTCATTTTTGGTGTCA | 101 | AY662657 | 89.7 | 0.987 | 0.978 |
| ifna1 5 | F:CACAGGCATGGGAGCTCATC R:TGACAGGGTCCCACGTGATT | 155 | AY216594 | 104.3 | 0.996 | 0.993 |
| trx 6 | F:GGATTCCTTCTTCAGTGCCC R:GATGTCACAGTGTTTGGCCA | 196 | BT049355 | 98.6 | 0.999 | 0.998 |
| β-act 7 | F:CCAAAGCCAACAGGGAGAA R:AGGGACAACACTGCCTGGAT | 102 | AF012125 | 95.2 | 0.995 | 0.992 |
| Parameters | Water Quality During Trial | |||
|---|---|---|---|---|
| 10 °C | 14 °C | 18 °C | 22 °C | |
| Temperature 2 (°C) | 10.31 ± 0.17 a | 14.38 ± 0.11 b | 18.31 ± 0.08 c | 22.23 ± 0.09 d |
| DO 2 (mg/L) | 8.50 ± 0.07 ns | 8.43 ± 0.08 | 8.33 ± 0.18 | 8.31 ± 0.09 |
| pH 3 | 7.79 ± 0.02 ns | 7.76 ± 0.05 | 7.78 ± 0.03 | 7.86 ± 0.02 |
| Ammonia (mg/L) | 0.00 ± 0.00 ns | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Nitrite (mg/L) | 0.16 ± 0.02 ns | 0.24 ± 0.01 | 0.19 ± 0.07 | 0.16 ± 0.06 |
| Nitrate (mg/L) | 2.91 ± 0.22 ns | 2.69 ± 0.18 | 2.93 ± 0.26 | 3.16 ± 0.23 |
| SS (mg/L) | 1.63 ± 0.35 ns | 1.77 ± 0.38 | 1.67 ± 0.41 | 1.32 ± 0.27 |
| Temperatures | ||||
|---|---|---|---|---|
| 10 °C | 14 °C | 18 °C | 22 °C | |
| Initial mean weight (g/fish) | 31.78 ± 0.07 ns | 30.98 ± 0.38 | 31.19 ± 0.24 | 31.15 ± 0.17 |
| Final mean weight (g/fish) | 74.69 ± 1.31 b | 97.68 ± 1.26 a | 84.18 ± 6.92 b | 60.91 ± 1.53 c |
| WG (%) 2 | 134.99 ± 4.20 c | 215.37 ± 3.88 a | 169.60 ± 5.78 b | 95.57 ± 5.85 d |
| SGR (%/day) 3 | 1.42 ± 0.03 c | 1.91 ± 0.02 a | 1.65 ± 0.04 b | 1.12 ± 0.05 d |
| DFI (%) 4 | 1.46 ± 0.02 b | 1.88 ± 0.02 a | 1.90 ± 0.10 a | 2.04 ± 0.03 a |
| FE (%) 5 | 92.36 ± 2.83 a | 91.84 ± 1.53 a | 80.82 ± 2.11 b | 52.82 ± 2.98 c |
| PER 6 | 1.88 ± 0.06 a | 1.87 ± 0.03 a | 1.65 ± 0.22 a | 1.08 ± 0.06 b |
| CF 7 | 0.88 ± 0.01 ns | 0.89 ± 0.00 | 0.93 ± 0.01 | 1.02 ± 0.11 |
| HSI (%) 8 | 1.33 ± 0.09 ns | 1.03 ± 0.09 | 1.33 ± 0.18 | 1.13 ± 0.09 |
| VSI (%) 9 | 11.13 ± 0.26 a | 9.73 ± 0.73 ab | 9.90 ± 0.38 ab | 8.70 ± 0.42 b |
| Survival (%) 10 | 95.38 ± 2.63 ns | 95.38 ± 2.63 | 96.83 ± 1.59 | 96.83 ± 1.59 |
| Temperatures | ||||
|---|---|---|---|---|
| 10 °C | 14 °C | 18 °C | 22 °C | |
| Moisture | 69.73 ± 0.08 ab | 69.00 ± 0.13 c | 69.59 ± 0.16 b | 70.19 ± 0.15 a |
| Crude protein | 17.62 ± 0.03 ns | 18.81 ± 0.48 | 18.45 ± 0.86 | 18.15 ± 0.31 |
| Crude lipid | 10.07 ± 0.15 ns | 9.59 ± 0.59 | 9.09 ± 0.93 | 8.65 ± 0.40 |
| Crude ash | 2.38 ± 0.02 bc | 2.20 ± 0.01 c | 2.74 ± 0.09 a | 2.59 ± 0.05 ab |
| Temperatures | ||||
|---|---|---|---|---|
| 10 °C | 14 °C | 18 °C | 22 °C | |
| AST (U/l) 2 | 352.67 ± 31.06 ns | 319.00 ± 50.39 | 384.00 ± 108.45 | 358.00 ± 24.79 |
| ALT (U/l) 3 | 7.33 ± 0.33 ns | 6.33 ± 0.33 | 7.33 ± 0.33 | 7.00 ± 0.58 |
| GLU (mmol/dl) 4 | 5.44 ± 0.67 ns | 5.37 ± 0.58 | 6.04 ± 0.22 | 6.41 ± 0.16 |
| TCHO (mmol/dl) 5 | 22.61 ± 0.89 ns | 20.71 ± 1.22 | 22.75 ± 1.70 | 23.78 ± 0.43 |
| TP (g/dl) 6 | 6.00 ± 0.45 ab | 4.97 ± 0.32 b | 5.87 ± 0.28 ab | 6.80 ± 0.32 a |
| TG (mmol/dl) 7 | 20.74 ± 0.35 ns | 17.61 ± 3.40 | 14.22 ± 1.48 | 20.31 ± 0.93 |
| Na+ (mmol/l) | 159.33 ± 17.13 ns | 159.33 ± 11.55 | 162.00 ± 1.53 | 167.33 ± 6.64 |
| K+ (mmol/l) | 1.37 ± 0.15 ns | 1.37 ± 0.09 | 1.27 ± 0.15 | 1.47 ± 0.15 |
| Cl− (mmol/l) | 123.50 ± 3.67 ns | 142.67 ± 11.84 | 135.33 ± 4.33 | 142.67 ± 6.49 |
| OSM (mmol/kg) 8 | 318.67 ± 4.18 ns | 314.00 ± 2.52 | 323.67 ± 3.38 | 330.00 ± 9.07 |
| Temperatures | ||||
|---|---|---|---|---|
| 10 °C | 14 °C | 18 °C | 22 °C | |
| Cortisol (ng/mL) | 24.00 ± 2.44 ns | 21.83 ± 6.17 | 25.51 ± 2.53 | 17.16 ± 1.61 |
| GPx (mU/mL) 2 | 57.08 ± 14.85 b | 679.33 ± 90.58 a | 124.95 ± 39.20 b | 371.47 ± 128.58 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Cho, K.; Kim, H.; Jeon, H.; Lee, S. Effects of Water Temperature on Growth, Hematological Measurements and Stress-Related Gene Expression of Atlantic Salmon (Salmo salar) Parr Reared in a Recirculating Aquaculture System. Animals 2025, 15, 3048. https://doi.org/10.3390/ani15203048
Lee Y, Cho K, Kim H, Jeon H, Lee S. Effects of Water Temperature on Growth, Hematological Measurements and Stress-Related Gene Expression of Atlantic Salmon (Salmo salar) Parr Reared in a Recirculating Aquaculture System. Animals. 2025; 15(20):3048. https://doi.org/10.3390/ani15203048
Chicago/Turabian StyleLee, Yujin, Kyuseok Cho, Haham Kim, Hyuncheol Jeon, and Seunghyung Lee. 2025. "Effects of Water Temperature on Growth, Hematological Measurements and Stress-Related Gene Expression of Atlantic Salmon (Salmo salar) Parr Reared in a Recirculating Aquaculture System" Animals 15, no. 20: 3048. https://doi.org/10.3390/ani15203048
APA StyleLee, Y., Cho, K., Kim, H., Jeon, H., & Lee, S. (2025). Effects of Water Temperature on Growth, Hematological Measurements and Stress-Related Gene Expression of Atlantic Salmon (Salmo salar) Parr Reared in a Recirculating Aquaculture System. Animals, 15(20), 3048. https://doi.org/10.3390/ani15203048





