Factors Affecting the Incidence of Double Ovulations in Lactating Dairy Cows: Estrous Cycle Length
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cows and Herd Management
2.2. Ultrasound Exams and Study Population
2.3. Genetic Merit Assessment
2.4. Data Collection and Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajakoski, E. The ovarian follicular system in sexually mature heifers with special reference to seasonal, cyclical, end left-right variations. Acta Endocrinol. 1960, 34 (Suppl. S52), 1–68. [Google Scholar]
- Bane, A.; Rajakoski, E. The bovine estrous cycle. Cornell Vet. 1961, 51, 77–95. [Google Scholar] [PubMed]
- Sirois, J.; Fortune, J.E. Ovarian follicular dynamics during the estrous cycle in heifers monitored by real-time ultrasonography. Biol. Reprod. 1988, 39, 308–317. [Google Scholar] [CrossRef]
- Savio, J.D.; Keenan, L.; Boland, M.P.; Roche, J.F. Pattern of growth of dominant follicles during the oestrous cycle of heifers. J. Reprod. Fertil. 1988, 83, 663–671. [Google Scholar] [CrossRef]
- Ginther, O.J.; Knopf, L.; Kastelic, J.P. Temporal associations among ovarian events in cattle during oestrous cycles with two and three follicular waves. J. Reprod. Fertil. 1989, 87, 223–230. [Google Scholar] [CrossRef]
- Knopf, L.; Kastelic, J.P.; Schallenberger, E.; Ginther, O.J. Ovarian follicular dynamics in heifers: Test of two-wave hypothesis by ultrasonically monitoring individual follicles. Domest. Anim. Endocrinol. 1989, 6, 111–119. [Google Scholar] [CrossRef]
- Savio, J.D.; Boland, M.P.; Roche, J.F. Development of dominant follicles and length of ovarian cycles in post-partum dairy cows. J. Reprod. Fertil. 1990, 88, 581–591. [Google Scholar] [CrossRef]
- Sartori, R.; Haughian, J.M.; Shaver, R.D.; Rosa, G.J.; Wiltbank, M.C. Comparison of ovarian function and circulating steroids in estrous cycles of Holstein heifers and lactating cows. J. Dairy Sci. 2004, 87, 905–920. [Google Scholar] [CrossRef]
- Wolfenson, D.; Inbar, G.; Roth, Z.; Kaim, M.; Bloch, A.; Braw-Tal, R. Follicular dynamics and concentrations of steroids and gonadotropins in lactating cows and nulliparous heifers. Theriogenology 2004, 62, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Bleach, E.C.; Glencross, R.G.; Knight, P.G. Association between ovarian follicle development and pregnancy rates in dairy cows undergoing spontaneous oestrous cycles. Reproduction 2004, 127, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.T. Factors regulating the growth of the preovulatory follicle in the sheep and human. J. Reprod. Fertil. 1983, 69, 343–352. [Google Scholar] [CrossRef]
- Zeleznik, A.J. The physiology of follicle selection. Reprod. Biol. Endocrinol. 2004, 2, 31. [Google Scholar] [CrossRef]
- Ginther, O.J. Intraovarian spatial and vascular harmony between follicles and corpus luteum in monovulatory heifers, mares, and women. Theriogenology 2019, 128, 31–39. [Google Scholar] [CrossRef]
- Kirillova, A.; Martazanova, B.; Mishieva, N.; Semenova, M. Follicular waves in ontogenesis and female fertility. Biosystems 2021, 210, 104558. [Google Scholar] [CrossRef]
- Adams, G.P.; Jaiswal, R.; Singh, J.; Malhi, P. Progress in understanding ovarian follicular dynamics in cattle. Theriogenology 2008, 69, 72–80. [Google Scholar] [CrossRef]
- Ginther, O.J. The theory of follicle selection in cattle. Domest. Anim. Endocrinol. 2016, 57, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Miura, R. Physiological characteristics and effects on fertility of the first follicular wave dominant follicle in cattle. J. Reprod. Dev. 2019, 65, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.; Schwieger, R.; Plöntzke, J.; Schäfer, S.; Röblitz, S. Follicular competition in cows: The selection of dominant follicles as a synergistic effect. J. Math. Biol. 2019, 78, 579–606. [Google Scholar] [CrossRef]
- Noseir, W.M. Ovarian follicular activity and hormonal profile during estrous cycle in cows: The development of 2 versus 3 waves. Reprod. Biol. Endocrinol. 2003, 1, 50. [Google Scholar] [CrossRef]
- Jaiswal, R.S.; Singh, J.; Marshall, L.; Adams, G.P. Repeatability of 2-wave and 3-wave patterns of ovarian follicular development during the bovine estrous cycle. Theriogenology 2009, 72, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.N.; VanDemark, N.L. The relationship of the interval between inseminations to bovine fertility. J. Anim. Sci. 1951, 10, 988–992. [Google Scholar] [CrossRef]
- Heersche, G., Jr.; Nebel, R.L. Measuring efficiency and accuracy of detection of estrus. J. Dairy Sci. 1994, 77, 2754–2761. [Google Scholar] [CrossRef] [PubMed]
- Echternkamp, S.E.; Roberts, A.J.; Lunstra, D.D.; Wise, T.; Spicer, L.J. Ovarian follicular development in cattle selected for twin ovulations and births. J. Anim. Sci. 2004, 82, 459–471. [Google Scholar] [CrossRef]
- Dahlen, C.R.; DiCostanzo, A.; Spell, A.R.; Lamb, G.C. Use of embryo transfer seven days after artificial insemination or transferring identical demi-embryos to increase twinning in beef cattle. J. Anim. Sci. 2012, 90, 4823–4832. [Google Scholar] [CrossRef]
- Hashiyada, Y. The contribution of efficient production of monozygotic twins to beef cattle breeding. J. Reprod. Dev. 2017, 63, 527–538. [Google Scholar] [CrossRef]
- López-Gatius, F.; Andreu-Vázquez, C.; Mur-Novales, R.; Cabrera, V.E.; Hunter, R.H.F. The dilemma of twin pregnancies in dairy cattle. A review of practical prospects. Live Sci. 2017, 197, 121–126. [Google Scholar] [CrossRef]
- Szenci, O. Recent Possibilities for the diagnosis of early pregnancy and embryonic mortality in dairy cows. Animals 2021, 11, 1666. [Google Scholar] [CrossRef]
- Nielen, M.; Schukken, Y.H.; Scholl, D.T.; Wilbrink, H.J.; Brand, A. Twinning in dairy cattle: A study of risk factors and effects. Theriogenology 1989, 32, 845–862. [Google Scholar] [CrossRef]
- Echternkamp, S.E.; Gregory, K.E. Effects of twinning on gestation length, retained placenta, and dystocia. J. Anim. Sci. 1999, 77, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Cuttance, E.; Laven, R. Perinatal mortality risk factors in dairy calves. Vet. J. 2019, 253, 105394. [Google Scholar] [CrossRef] [PubMed]
- Eddy, R.G.; Davies, O.; David, C. An economic assessment of twin births in British dairy herds. Vet. Rec. 1991, 129, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Beerepoot, G.M.; Dykhuizen, A.A.; Nielen, M.; Schukken, Y.H. The economics of naturally occurring twinning in dairy cattle. J. Dairy Sci. 1992, 75, 1044–1051. [Google Scholar] [CrossRef]
- Cabrera, V.E.; Fricke, P.M. Economics of twin pregnancies in dairy cattle. Animals 2021, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Baba, T.; Suzuki, M. Genetic analysis of twinning rate and milk yield using a threshold-linear model in Japanese Holsteins. Anim. Sci. J. 2015, 86, 31–36. [Google Scholar] [CrossRef] [PubMed]
- McGovern, S.P.; Weigel, D.J.; Fessenden, B.C.; Gonzalez-Peña, D.; Vukasinovic, N.; McNeel, A.K.; Di Croce, F.A. Genomic prediction for twin pregnancies. Animals 2021, 11, 843. [Google Scholar] [CrossRef]
- Kirkpatrick, B.W.; Berry, D.P. A genomic analysis of twinning rate and its relationship with other reproductive traits in Holstein-Friesian cattle. J. Dairy Sci. 2025, 108, 1686–1698. [Google Scholar] [CrossRef]
- Katende, J.S.; Costa, A.; Santinello, M.; Galluzzo, F.; Marusi, M.; Finocchiaro, R.; Cassandro, M.; Penasa, M. Genetics of twinning rate in Italian Holsteins. J. Dairy Sci. 2025, 108, 5103–5113. [Google Scholar] [CrossRef]
- Hüneke, L.; Schmidtmann, C.; Alkhoder, H.; Liu, Z.; Segelke, D.; Rensing, S.; Heise, J.; Thaller, G. Genetics of twin birth rate in German Holstein and options for breeding. J. Dairy Sci. 2025, 108, 6161–6173. [Google Scholar] [CrossRef]
- Kidder, H.E.; Barrett, G.R.; Casida, L.E. A study of ovulations in six families of Holstein-friesians. J. Dairy Sci. 1952, 35, 436–444. [Google Scholar] [CrossRef]
- López-Gatius, F. Negative photoperiod induces an increase in the number of ovulations in dairy cattle. J. Reprod. Dev. 2024, 70, 35–41. [Google Scholar] [CrossRef]
- Cady, R.A.; Van Vleck, L.D. Factors affecting twinning and effects of twinning in Holstein dairy cattle. J. Anim. Sci. 1978, 46, 950–956. [Google Scholar] [CrossRef]
- Ryan, D.P.; Boland, M.P. Frequency of twin births among Holstein-Friesian cows in a warm dry climate. Theriogenology 1991, 36, 1–10. [Google Scholar] [CrossRef]
- Silva-del-Río, N.; Kirkpatrick, B.W.; Fricke, P.M. Observed frequency of monozygotic twinning in Holstein dairy cattle. Theriogenology 2006, 66, 1292–1299. [Google Scholar] [CrossRef]
- López-Gatius, F.; Garcia-Ispierto, I.; Hunter, R.H.F. Twin pregnancies in dairy cattle: Observations in a large herd of Holstein-Friesian dairy cows. Animals 2020, 10, 2165. [Google Scholar] [CrossRef]
- López-Gatius, F.; Garcia-Ispierto, I.; Ganau, S.; Wijma, R.; Weigel, D.J.; Di Croce, F.A. Effect of genetic and environmental factors on twin pregnancy in primiparous dairy cows. Animals 2023, 13, 2008. [Google Scholar] [CrossRef]
- Garcia-Ispierto, I.; Pal, P.; Ganau, S.; López-Gatius, F. Association of genomic prediction for twin pregnancies with the incidence of double ovulation in primiparous dairy cows. Reprod. Domest. Anim. 2024, 59, e14687. [Google Scholar] [CrossRef]
- Miura, R.; Haneda, S.; Lee, H.H.; Miyamoto, A.; Shimizu, T.; Miyahara, K.; Miyake, Y.; Matsui, M. Evidence that the dominant follicle of the first wave is more active than that of the second wave in terms of its growth rate, blood flow supply and steroidogenic capacity in cows. Anim. Reprod. Sci. 2014, 145, 114–122. [Google Scholar] [CrossRef]
- Miura, R.; Haneda, S.; Matsui, M. Ovulation of the preovulatory follicle originating from the first-wave dominant follicle leads to formation of an active corpus luteum. J. Reprod. Dev. 2015, 61, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Kulick, L.J.; Bergfelt, D.R.; Kot, K.; Ginther, O.J. Follicle selection in cattle: Follicle deviation and codominance within sequential waves. Biol. Reprod. 2001, 65, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.; Fricke, P.M.; Ferreira, J.C.; Ginther, O.J.; Wiltbank, M.C. Follicular deviation and acquisition of ovulatory capacity in bovine follicles. Biol. Reprod. 2001, 65, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.A.; Meira, C.; Bergfelt, D.R.; Ginther, O.J. Role of oestradiol in growth of follicles and follicle deviation in heifers. Reproduction 2003, 125, 847–854. [Google Scholar] [CrossRef]
- Lopez, H.; Sartori, R.; Wiltbank, M.C. Reproductive hormones and follicular growth during development of one or multiple dominant follicles in cattle. Biol. Reprod. 2005, 72, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Miura, R.; Matsumoto, N.; Haneda, S.; Matsui, M. Double ovulation rate of the first follicular wave follicles is higher in the first follicular wave dominant follicle in the ovary contralateral to the corpus luteum treated with human chorionic gonadotropin five days after estrus in lactating dairy cows. J. Vet. Med. Sci. 2019, 81, 1685–1687. [Google Scholar] [CrossRef]
- López-Gatius, F. Can thermoregulatory response to heat stress be improved in lactating dairy cows? Insights from counter-current heat transfer systems impacting reproduction. J. Reprod. Dev. 2025, 71, 68–70. [Google Scholar] [CrossRef]
- Jones, S.V.H.; Rouse, J.E. The relation of age of dam to observed fecundity in domesticated animals: I. Multiple births in cattle and sheep. J. Dairy Sci. 1920, 3, 260–290. [Google Scholar] [CrossRef]
- Andreu-Vázquez, C.; Garcia-Ispierto, I.; López-Gatius, F. Photoperiod length and the estrus synchronization protocol used before AI affect the twin pregnancy rate in dairy cattle. Theriogenology 2012, 78, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- López-Gatius, F.; López-Béjar, M.; Fenech, M.; Hunter, R.H.F. Ovulation failure and double ovulation in dairy cattle: Risk factors and effects. Theriogenology 2005, 63, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, A.J.; Lean, I.J.; Weaver, C.O.; Farver, T.; Webster, G. A body condition scoring chart for Holstein dairy cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Townson, D.H.; Ginther, O.J. Ultrasonic echogenicity of developing corpora lutea in pony mares. Anim. Reprod. Sci. 1989, 20, 143–153. [Google Scholar] [CrossRef]
- Singh, J.; Pierson, R.A.; Adams, G.P. Ultrasound image attributes of the bovine corpus luteum: Structural and functional correlates. J. Reprod. Fert. 1997, 109, 35–44. [Google Scholar] [CrossRef]
- Kito, S.; Okuda, K.; Miyazawa, K.; Sato, K. Study on the appearance of the cavity in the corpus luteum of cows by using ultrasonic scanning. Theriogenology 1986, 25, 325–333. [Google Scholar] [CrossRef]
- Kastelic, J.P.; Pierson, R.A.; Ginther, O.J. Ultrasonic morphology of corpora lutea and central luteal cavities during the estrous cycle and early pregnancy in heifers. Theriogenology 1990, 34, 487–498. [Google Scholar] [CrossRef]
- Perez-Marin, C. Formation of corpora lutea and central luteal cavities and their relationship with plasma progesterone levels and other metabolic parameters in dairy cattle. Reprod. Domest. Anim. 2009, 44, 384–389. [Google Scholar] [CrossRef]
- McNeel, A.K.; Reiter, B.C.; Weigel, D.; Osterstock, J.; Di Croce, F.A. Validation of genomic predictions for wellness traits in US Holstein cows. J. Dairy Sci. 2017, 100, 9115–9124. [Google Scholar] [CrossRef] [PubMed]
- Fessenden, B.; Weigel, D.J.; Osterstock, J.; Galligan, D.T.; Di Croce, F. Validation of genomic predictions for a lifetime merit selection index for the US dairy industry. J. Dairy Sci. 2020, 103, 10414–10428. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Miura, R.; Haneda, S.; Kayano, M.; Matsui, M. Short communication: Development of the first follicular wave dominant follicle on the ovary ipsilateral to the corpus luteum is associated with decreased conception rate in dairy cattle. J. Dairy Sci. 2015, 98, 318–321. [Google Scholar] [CrossRef]
- Miura, R.; Matsumoto, N.; Izumi, T.; Kayano, M.; Haneda, S.; Matsui, M. Effects of human chorionic gonadotropin treatment after artificial inseminations on conception rate with the first follicular wave dominant follicle in the ovary ipsilateral to the corpus luteum in lactating dairy cows. J. Reprod. Dev. 2018, 64, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Miura, R.; Matsumoto, N.; Haneda, S.; Matsui, M. Influence of ipsilateral coexistence of the first wave dominant follicle and corpus luteum on ovarian dynamics and plasma sex steroid hormone concentrations in lactating dairy cows treated with human chorionic gonadotropin. J. Reprod. Dev. 2020, 66, 265–269. [Google Scholar] [CrossRef]
- Fricke, P.M.; Wiltbank, M.C. Effect of milk production on the incidence of double ovulation in dairy cows. Theriogenology 1999, 52, 1133–1143. [Google Scholar] [CrossRef]
- Stevenson, J.S.; Pursley, J.R.; Garverick, H.A.; Fricke, P.M.; Kesler, D.J.; Ottobre, J.S.; Wiltbank, M.C. Treatment of cycling and noncycling lactating dairy cows with progesterone during Ovsynch. J. Dairy Sci. 2006, 89, 2567–2578. [Google Scholar] [CrossRef]
- Garcia-Ispierto, I.; López-Helguera, I.; Martino, A.; López-Gatius, F. Reproductive performance of anoestrous high-producing dairy cows improved by adding equine chorionic gonadotrophin to a progesterone-based oestrous synchronizing protocol. Reprod. Domest. Anim. 2012, 47, 752–758. [Google Scholar] [CrossRef] [PubMed]
- López-Gatius, F.; Garcia-Ispierto, I.; Serrano-Pérez, B.; Hunter, R.H.F. The presence of two ovulatory follicles at timed artificial insemination influences the ovulatory response to GnRH in high-producing dairy cows. Theriogenology 2018, 120, 91–97. [Google Scholar] [CrossRef]
- Palacín-Chauri, R.J.; Garcia-Ispierto, I. Cow and management factors and their association with estimates of uterine size and position at the time of insemination of dairy cattle. Reprod. Domest. Anim. 2023, 58, 1338–1341. [Google Scholar] [CrossRef]
- Morrow, D.A.; Roberts, S.J.; McEntee, K.A. Pospartum ovarian activity and involution of the uterus and cervix in dairy cattle. II. Involution of uterus and cervix. Cornell Vet. 1969, 59, 190–198. [Google Scholar] [PubMed]
- Miettinen, P.V. Uterine involution in Finnish dairy cows. Acta Vet. Scan. 1990, 31, 181–185. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, H.; Ahmad, M.J.; Yang, Y.; Yang, W.; Riaz, H.; Abulaiti, A.; Zhang, S.; Yang, L.; Hua, G. Postpartum uterine involution and embryonic development pattern in Chinese Holstein dairy cows. Front. Vet. Sci. 2021, 7, 604729. [Google Scholar] [CrossRef]
- Baez, G.M.; Barletta, R.V.; Guenther, J.N.; Gaska, J.M.; Wiltbank, M.C. Effect of uterine size on fertility of lactating dairy cows. Theriogenology 2016, 85, 1357–1366. [Google Scholar] [CrossRef]
- Young, C.D.; Schrick, F.N.; Pohler, K.G.; Saxton, A.M.; Di Croce, F.A.; Roper, D.A.; Wilkerson, J.B.; Edwards, J.L. Short communication: A reproductive tract scoring system to manage fertility in lactating dairy cows. J. Dairy Sci. 2017, 100, 5922–5927. [Google Scholar] [CrossRef]
- Madureira, A.; Poole, R.K.; Burnett, T.A.; Guida, T.G.; Edwards, J.L.; Schrick, F.N.; Vasconcelos, J.; Cerri, R.; Pohler, K.G. Size and position of the reproductive tract impacts fertility outcomes and pregnancy losses in lactating dairy cows. Theriogenology 2020, 158, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Epstein, H.; Mason, I.L. Cattle. In Evolution of Domesticated Animals; Mason, I.L., Ed.; Longman: London, UK, 1984; pp. 6–27. [Google Scholar]
- Buchenauer, D. Genetics of behaviour in cattle. In The Genetics of Cattle; Fries, R., Ruvinsky, A., Eds.; CAB International Wallingford: Wallingford, UK, 1999; pp. 365–390. [Google Scholar]
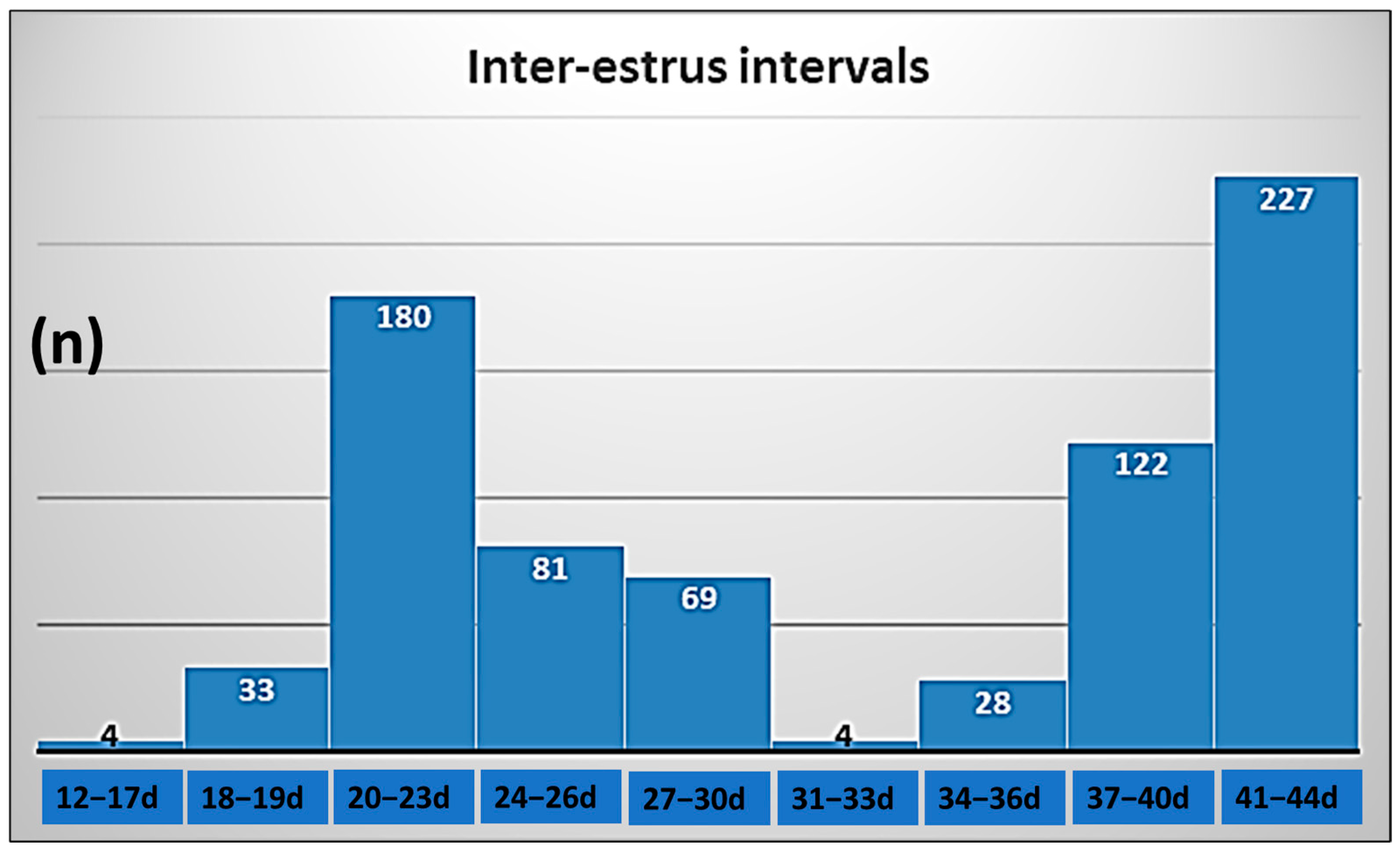
| Factor | Class | n | Double Ovulation | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|---|---|---|
| ZTWINS (a) | Continuous | 44/748 | 5.9% | 0.82 | 0.78–0.91 | <0.0001 |
| Photoperiod (b) | Positive | 14/343 | 4.1% | Reference | ||
| Negative | 30/405 | 7.4% | 2.3 | 1.4–3.7 | <0.0001 |
| Factor | Class | n | Double Ovulation | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|---|---|---|
| ZTWINS (a) | Continuous | 27/341 | 7.9% | 0.81 | 0.77–0.94 | 0.003 |
| Photoperiod (b) | Positive | 7/183 | 3.8% | Reference | ||
| Negative | 20/158 | 12.7% | 2.5 | 1.01–6.6 | 0.048 | |
| Inter-estrus interval | Normal (≤23 d) | 12/235 | 5.1% | Reference | ||
| Long (>23 d) | 15/106 | 14.2% | 2.8 | 1.1–7.0 | 0.02 |
| Factor | Class | n | Lengthened Interval | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|---|---|---|
| ZTWINS (a) | Continuous | 106/341 | 31.1% | 0.91 | 0.82–1.0 | 0.001 |
| Photoperiod (b) | Positive | 29/183 | 15.8% | Reference | ||
| Negative | 77/158 | 48.7% | 4.4 | 2.7–7.0 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Gatius, F.; Garcia-Ispierto, I. Factors Affecting the Incidence of Double Ovulations in Lactating Dairy Cows: Estrous Cycle Length. Animals 2025, 15, 3000. https://doi.org/10.3390/ani15203000
López-Gatius F, Garcia-Ispierto I. Factors Affecting the Incidence of Double Ovulations in Lactating Dairy Cows: Estrous Cycle Length. Animals. 2025; 15(20):3000. https://doi.org/10.3390/ani15203000
Chicago/Turabian StyleLópez-Gatius, Fernando, and Irina Garcia-Ispierto. 2025. "Factors Affecting the Incidence of Double Ovulations in Lactating Dairy Cows: Estrous Cycle Length" Animals 15, no. 20: 3000. https://doi.org/10.3390/ani15203000
APA StyleLópez-Gatius, F., & Garcia-Ispierto, I. (2025). Factors Affecting the Incidence of Double Ovulations in Lactating Dairy Cows: Estrous Cycle Length. Animals, 15(20), 3000. https://doi.org/10.3390/ani15203000






