Psittacine Beak and Feather Disease: Global Spread, International Trade, and Conservation Challenges
Abstract
Simple Summary
Abstract
1. Introduction
2. Literature Search
2.1. Publised Date Range
2.2. Search Parameters and Limitation
3. Taxonomy and Nomenclature
3.1. Taxonomy
3.2. Nomenclature of PBFD and BFDV
PBFD (Disease Terminology)
4. Virus Structure and Replication
4.1. Virus Structure
4.2. Genome Structure and Gene Function
4.3. Replication Cycle
5. Host Range, Virulence and Pathogenesis
5.1. Host Range
5.1.1. Captive Psittacine Populations
5.1.2. Wild Psittacine Populations
5.1.3. Infections in Non-Psittacine Species
5.2. Pathogenesis and Virulence
6. Epidemiology and Transmission
6.1. Epidemiology
6.2. Transmission
6.2.1. Vertical Transmission
6.2.2. Horizontal Transmission
6.3. Role of Asymptomatic Carriers in Viral Transmission
7. Clinical Signs and Histopathology
7.1. Clinical Manifestations in Psittacines
7.2. Experimental Infections and Disease in Non-Psittacines
8. Diagnosis
8.1. Molecular Diagnostics
- -
- -
- Feather pulp samples are widely used due to ease of collection and relatively high viral loads, particularly in actively growing feathers. However, their reliability decreases during non-molting periods or in samples exposed to environmental contamination [62].
- -
- Cloacal and buccal swabs provide practical non-invasive options, though sensitivity varies based on viral shedding dynamics and sample quality [63].
- -
- Fecal samples are primarily suited for flock-level surveillance; however, they are prone to PCR inhibition and environmental degradation of viral nucleic acids [36].
- -
- Tissue biopsies (either ante-mortem or post-mortem) offer conclusive diagnosis when molecular results are correlated with characteristic histopathological findings. In advanced disease, the virus is typically distributed across multiple organs including liver, spleen, and feather follicles [36].
8.2. Advanced Molecular Techniques
8.2.1. Rolling Circle Amplification (RCA)
8.2.2. Real-Time Quantitative PCR (RT-qPCR)
8.2.3. High-Resolution Melting Analysis (HRM)
8.3. Integrated Diagnostic Strategies
- -
- Repeat testing with a different sample type (e.g., from blood to feather pulp);
- -
- Employ alternative PCR primers or confirmatory tests;
- -
- Implement quantitative PCR (qPCR) to monitor viral dynamics over time.
9. Management: Therapeutics, Prevention, and Vaccination
9.1. Therapeutics
9.1.1. Core Supportive Measures
9.1.2. Experimental and Supportive Therapeutic Strategies
9.2. Experimental Antiviral Agents
- -
- -
- L-742001: A Rep endonuclease inhibitor conceptually derived from circovirus models. Although not tested in psittacines, it may interfere with critical replication domains [28].
- -
- Ribavirin: A broad-spectrum nucleoside analog that impairs RNA and DNA virus replication. In vitro studies in related viruses (e.g., porcine nidovirus) show suppression, but avian safety and efficacy remain unvalidated [80].
9.3. Immunomodulatory Treatments
- -
- Avian interferon-gamma (IFN-γ): A key cytokine in antiviral defense. Anecdotal improvements have been reported in African grey parrots, but some studies caution that IFNs may paradoxically exacerbate circoviral replication [72].
- -
- β-(1,3/1,6)-D-glucan: A fungal polysaccharide known to enhance innate immunity. Its use in cockatoos and horned parakeets (Eunymphicus cornutus) correlated with reduced viral DNA loads and improved feather condition, though data remain uncontrolled [81].
- -
- Experimental hypothermia: Hypothesized to suppress systemic inflammation but remains speculative and untested in birds.
- -
- Natural recovery: Documented in certain species, notably lorikeets and Eclectus parrots, some birds are able to clear infection or suppress clinical signs—likely via robust immune responses or antibody production [14].
9.4. Hematopoietic Support
- -
- Filgrastim (G-CSF): A human granulocyte colony-stimulating factor used in mammals for neutropenia. Its use in PBFD remains anecdotal, with some reports of transient leukocyte count improvement [82].
- -
- Combined off-label regimens: A non-peer-reviewed case series reported a 70% BFDV PCR clearance rate using avian IFN-γ (106 IU IM daily for 90 days) and nebulized F10® disinfectant (1:125 dilution, 15 min daily). While intriguing, these results lack formal clinical validation, and the safety of nebulized disinfectant exposure in birds remains unproven [83].
9.5. Cross-Species Antivirals (Insights from PCV2 Models)
9.6. Prevention and Biosecurity
- -
- Disinfection: Beak and feather disease virus (BFDV) is notably resilient, persisting in the environment for extended periods. However, peroxygen-based disinfectants such as Virkon® S (1%) have demonstrated efficacy against circoviruses and are recommended in biosecurity protocols, particularly by Australian wildlife agencies [84]. Quaternary ammonium compounds (e.g., Virex®) have also been successfully used in field settings, including nest site sanitation. Regular disinfection of all surfaces—including cages, perches, bowls, and equipment—is critical in multi-bird environments.
- -
- Quarantine and Testing: Quarantine is foundational for PBFD prevention. New birds should be isolated for 30–45 days and tested for BFDV using multiple diagnostics: PCR on blood and feathers, and where possible, hemagglutination (HA) and hemagglutination inhibition (HI) assays, which enhance detection sensitivity, especially in early or subclinical infections [14,20]. Physical separation and the use of dedicated clothing/equipment within quarantine zones are essential.
- -
- Routine Screening: Because PBFD can be asymptomatic, regular flock surveillance (every 6–12 months) is vital to detect latent infections before transmission occurs. Combining feather and blood PCR increases diagnostic reliability [59].
- -
- Strict Access Control: Facilities should enforce biosecurity protocols, including footbaths, hand hygiene, and controlled visitor access. Equipment and gloves must be changed between enclosures to avoid cross-contamination. Segregating susceptible species like cockatoos and African grey parrots from potentially infected birds is strongly advised [84].
- -
- Environmental Hygiene: PBFD virus is shed in feather dust, feces, and crop secretions [20]. To reduce airborne spread, HEPA-filter vacuums or damp-cleaning methods (wet mopping) are preferred over dry sweeping. Proper waste disposal and ventilation systems that prevent recirculation of contaminated air are also critical.
- -
- Strengthening Host Health: A strong immune system improves resistance to PBFD. Stress minimization, nutritional support, and control of concurrent infections play a protective role. Birds with high HI antibody titers may resist disease development after exposure [20].
9.6.1. Practical Guidelines for Breeders and Pet Owners
- -
- Quarantine New Arrivals: Establish dedicated quarantine areas for incoming birds. New birds must be isolated for a minimum of 30 days and undergo BFDV testing (PCR on blood and feathers, supplemented by serological tests if available). Birds should only join the main collection after obtaining consecutive negative test results at both the start and end of quarantine [74,78].
- -
- Protective Gear: When handling quarantined birds, caretakers must use disposable gloves, coveralls, and shoe covers. This gear should be replaced before entering resident bird areas. Ideally, separate personnel should handle isolated and resident populations to avoid fomite transmission.
- -
- Routine Surveillance: Implement routine BFDV screening every 6–12 months, as asymptomatic carriers can persist undetected. Immediate testing is also essential when clinical signs arise, such as feather dystrophy or beak abnormalities [39].
- -
- Dedicated Equipment: Maintain separate cleaning tools, feeding utensils, and housing items for each aviary group. Equipment must not be shared between enclosures unless thoroughly disinfected using proven virucidal solutions [78].
- -
- Biosecure Facility Design: Limit facility access to essential staff. Require footbaths, handwashing, and protective clothing on entry. Use “all-in, all-out” bird movement protocols when possible. Visitors should be strictly limited or monitored, particularly around high-risk groups [39].
- -
- Regular Disinfection: Weekly cleaning of cages, perches, bowls, and toys using bird-safe disinfectants like 1% Virkon S. Ensure complete drying before reuse.
- -
- -
- Avoid Sharing Items: Never share toys, bowls, or grooming tools between birds without thorough disinfection. Feather and fecal dust may contaminate porous materials.
- -
- Observation and Prompt Action: Be alert to signs like feather loss, overgrown beak, or behavioral changes. If suspected, isolate the bird immediately and consult a veterinarian for PCR testing [77].
- -
- Educate and Network: Stay informed about PBFD. Ensure pet shops, boarding services, and bird clubs enforce biosecurity. Encourage others to adopt safe practices [78].
9.6.2. Prevention in Conservation and Restoration Programs
- -
- Artificial Rearing Protocols: If breeding stock poses a BFDV risk, artificial incubation and hand-rearing of chicks under sterile conditions may be necessary to disrupt vertical or early postnatal transmission. This approach has been implemented successfully in species such as the Mauritius parakeet (Psittacula eques) and Hawaiian forest birds, especially where intensive disease management is required [74,85].
- -
- Maximized Biosecurity: Facilities managing endangered parrots should enforce strict biosafety, including controlled human access, facility-wide disinfection protocols, and the physical separation of cohorts. Segregation of family lines in independent enclosures with separate airflow systems can mitigate intra-facility transmission risk [40].
- -
- Pre-release Screening: All individuals destined for release must undergo comprehensive health assessments, including BFDV PCR testing. Releasing positive individuals into wild habitats may result in irreversible spillover events, as documented in the orange-bellied parrot (Neophema chrysogaster) where novel BFDV genotypes were introduced into critically endangered populations [13].
- -
- Vaccination (Future Outlook): Although no commercial PBFD vaccine currently exists, experimental strategies using recombinant capsid proteins have shown promise in preliminary trials [7]. Conservation breeding programs may contribute to vaccine development by providing biological samples and participating in future field trials.
9.7. Vaccination Efforts
9.7.1. Inactivated Virus Vaccines
- -
- Viral Harvesting Requirements: Production of inactivated vaccines depends on harvesting BFDV from naturally infected birds, raising significant ethical concerns and biosafety risks.
- -
- Carrier Risk and Shedding: While vaccinated birds may be protected from clinical disease, inactivated vaccines may not prevent viral replication or shedding. There is concern that vaccinated birds could still act as asymptomatic carriers [87].
- -
- Scalability and Contamination: Vaccine production using field-derived viral isolates introduces the risk of contamination with other pathogens and presents challenges in scalability and standardization.
9.7.2. Recombinant Subunit Vaccines
9.7.3. Inactivated Adjuvanted Vaccines (Oil Emulsion)
- -
- Scaling up vaccine production remains resource intensive.
- -
- Sourcing BFDV from infected birds raises biosafety and ethical concerns.
- -
- Oil-adjuvanted formulations may cause injection-site reactions, particularly problematic in small-bodied species.
9.7.4. DNA Vaccines
- -
- Efficient gene delivery into avian cells is technically challenging.
- -
- Avian-specific promoters and vector optimization are needed.
- -
- Immunogenicity varies depending on expression levels and adjuvant use.
9.7.5. Viral Vector Vaccines
9.7.6. Current Status and Challenges
- -
- Efficacy: Although recombinant vaccines significantly reduce clinical signs and viral load, they do not achieve complete sterilizing immunity. Transient, low-level viral replication has been detected even in vaccinated birds [88]. This is likely due to BFDV’s capacity for immune evasion and persistent infection, a common feature of circoviruses.
- -
- Duration of Immunity: Given that parrots can live for decades, vaccines must ideally confer long-term immunity. Most experimental vaccines, such as recombinant Cap subunits, show protection over a few months but lack long-term data on antibody durability or memory T-cell responses [90].
- -
- Safety: No live-attenuated vaccines are currently under development due to concerns of reversion to virulence and interspecies transmission. Experimental DNA and subunit vaccines have shown good safety profiles in psittacines, including neonates [88].
- -
- Genetic Diversity: BFDV displays significant global genetic variation, including recombinant strains, yet the Cap gene remains relatively conserved across genotypes, supporting its utility as a universal vaccine target [42].
- -
- -
- Improve antigen yield and particle assembly using plant or yeast expression systems.
- -
- Evaluate novel adjuvants, including CpG oligonucleotides and nano emulsions, for better immune stimulation [90].
- -
- Develop combinatorial immunization strategies (e.g., DNA prime–protein boost) to enhance immune breadth and longevity.
10. Conservation and Policy Implications
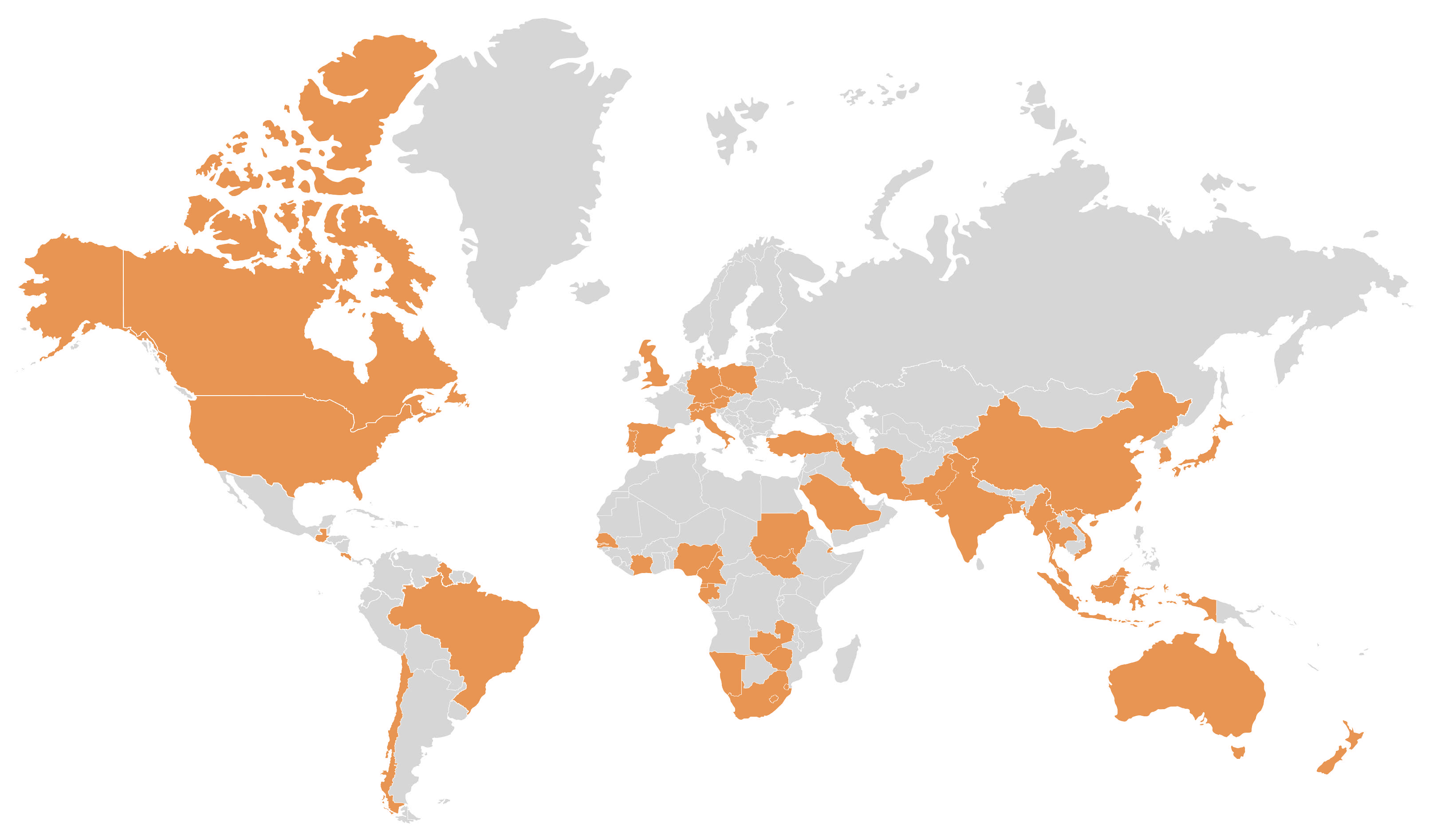
10.1. Global Spread and Surveillance in Wild Populations
- -
- South Korea: The first officially confirmed PBFD case in South Korea was reported in a blue-and-yellow macaw in 2014 using PCR and sequencing-based diagnostics [45].
- -
- United Arab Emirates (UAE): A 2016 study documented BFDV detection in the UAE in multiple psittacine birds using molecular testing methods [92].
- -
- Chile: BFDV prevalence was confirmed in a 2019 study that detected infection in wild and captive parrots in Chile using PCR methods [33].
- -
- Turkey: A 2020 study conducted in Turkey confirmed BFDV presence through sequence analysis of affected parrots [93].
- -
- Mexico: The first peer-reviewed confirmation of PBFD in Mexico appeared in 2020, identifying BFDV in psittacines via PCR [94].
- -
- Bangladesh: A 2022 study reported a 37% BFDV positivity rate among psittacine birds sampled from the pet trade, confirming active circulation of the virus in the region [32].
- -
- Namibia: A 2023 molecular study confirmed BFDV infections in Namibian parrots, with sequencing results contributing to regional genetic data [95].
- -
- Sequencing newly identified strains and mapping their phylogenetic relationships to global lineages [44].
- -
- Assessing PBFD prevalence in under-surveyed regions and species, especially those previously thought to be disease-free [98].
- -
- Detecting genotypic shifts that suggest viral adaptation to novel hosts or environments [41].
- -
- Advancing diagnostic technology, such as portable PCR systems, for rapid field deployment [99].
10.2. Parrot Trade and Invasive Species: Spillover Dynamics
10.3. PBFD-Related Policies and Management Programs
10.3.1. Orange-Bellied Parrot (OBP) Recovery Program
10.3.2. Mauritius Parakeet Recovery Program
11. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Latimer, K.S.; Rakich, P.M.; Steffens, W.L.; Kircher, I.M.; Ritchie, B.W.; Niagro, F.D.; Lukert, P.D. A Novel DNA Virus Associated with Feather Inclusions in Psittacine Beak and Feather Disease. Vet. Pathol. 1991, 28, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Ashby, E. Parrakeets Moulting. Emu-Austral Ornithol. 1907, 6, 193–194. [Google Scholar] [CrossRef]
- Perry, R.A. A Psittacine Combined Beak and Feather Disease Syndrome with Particular Reference to the Australian Cockatoos Cacatua galerita (Sulphur-Crested Cockatoo), Cacatua leadbeateri (Major Mitchell or Pink Cockatoo) and Cacatua sanguinea (Little Corella). Proceedings 1981, 55, 81–108. [Google Scholar]
- Pass, D.A.; Perry, R.A. The Pathology of Psittacine Beak and Feather Disease. Aust. Vet. J. 1984, 61, 69–74. [Google Scholar] [CrossRef]
- Todd, D. Circoviruses: Immunosuppressive Threats to Avian Species: A Review. Avian Pathol. 2000, 29, 373–394. [Google Scholar] [CrossRef]
- Ritchie, B.W.; Niagro, F.D.; Lukert, P.D.; Steffens, W.L.; Latimer, K.S. Characterization of a New Virus from Cockatoos with Psittacine Beak and Feather Disease. Virology 1989, 171, 83–88. [Google Scholar] [CrossRef]
- Raidal, S.; Sarker, S.; Peters, A. Review of Psittacine Beak and Feather Disease and Its Effect on Australian Endangered Species. Aust. Vet. J. 2015, 93, 466–470. [Google Scholar] [CrossRef]
- Sarker, S.; Ghorashi, S.A.; Forwood, J.K.; Bent, S.J.; Peters, A.; Raidal, S.R. Phylogeny of Beak and Feather Disease Virus in Cockatoos Demonstrates Host Generalism and Multiple-Variant Infections within Psittaciformes. Virology 2014, 460–461, 72–82. [Google Scholar] [CrossRef]
- Sarker, S.; Moylan, K.G.; Ghorashi, S.A.; Forwood, J.K.; Peters, A.; Raidal, S.R. Evidence of a Deep Viral Host Switch Event with Beak and Feather Disease Virus Infection in Rainbow Bee-Eaters (Merops ornatus). Sci. Rep. 2015, 5, 14511. [Google Scholar] [CrossRef]
- Sarker, S.; Lloyd, C.; Forwood, J.; Raidal, S.R. Forensic Genetic Evidence of Beak and Feather Disease Virus Infection in a Powerful Owl, Ninox strenua. Emu-Austral Ornithol. 2016, 116, 71–74. [Google Scholar] [CrossRef]
- Circella, E.; Legretto, M.; Pugliese, N.; Caroli, A.; Bozzo, G.; Accogli, G.; Lavazza, A.; Camarda, A. Psittacine Beak and Feather Disease–like Illness in Gouldian Finches (Chloebia gouldiae). Avian Dis. 2014, 58, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Smith, K.; Sarker, S.; Peters, A.; Adriaanse, K.; Eden, P.; Ghorashi, S.A.; Forwood, J.K.; Raidal, S.R. Repeat Spillover of Beak and Feather Disease Virus into an Endangered Parrot Highlights the Risk Associated with Endemic Pathogen Loss in Endangered Species. J. Wildl. Dis. 2020, 56, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Patterson, E.I.; Baker, B.G.B.; Holdsworth, M.; Sarker, S.; Ghorashi, S.A.; Raidal, S.R. Evidence of Psittacine Beak and Feather Disease Virus Spillover into Wild Critically Endangered Orange-bellied Parrots (Neophema chrysogaster). J. Wildl. Dis. 2014, 50, 288. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, B.; Bonne, N.; Stewart, M.; Sharp, M.; Raidal, S. A Comparison of Haemagglutination, Haemagglutination Inhibition and PCR for the Detection of Psittacine Beak and Feather Disease Virus Infection and a Comparison of Isolates Obtained from Loriids. J. Gen. Virol. 2005, 86, 3039–3046. [Google Scholar] [CrossRef]
- Shearer, P.L.; Bonne, N.; Clark, P.; Sharp, M.; Raidal, S.R. Beak and Feather Disease Virus Infection in Cockatiels (Nymphicus hollandicus). Avian Pathol. 2008, 37, 75–81. [Google Scholar] [CrossRef]
- Raidal, S. Psittacine Beak and Feather Disease. In Current Therapy in Avian Medicine and Surgery; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016; pp. 51–57. ISBN 978-1-4557-4671-2. [Google Scholar]
- Rinder, M.; Schmitz, A.; Peschel, A.; Wörle, B.; Gerlach, H.; Korbel, R. Molecular Characterization of a Recently Identified Circovirus in Zebra Finches (Taeniopygia guttata) Associated with Immunosuppression and Opportunistic Infections. Avian Pathol. 2017, 46, 106–116. [Google Scholar] [CrossRef]
- Niagro, F.D.; Forsthoefel, A.N.; Lawther, R.P.; Kamalanathan, L.; Ritchie, B.W.; Latimer, K.S.; Lukert, P.D. Beak and Feather Disease Virus and Porcine Circovirus Genomes: Intermediates Between the Geminiviruses and Plant Circoviruses. Arch. Virol. 1998, 143, 1723–1744. [Google Scholar] [CrossRef]
- Bassami, M.R.; Berryman, D.; Wilcox, G.E.; Raidal, S.R. Psittacine Beak and Feather Disease Virus Nucleotide Sequence Analysis and Its Relationship to Porcine Circovirus, Plant Circoviruses, and Chicken Anaemia Virus. Virology 1998, 249, 453–459. [Google Scholar] [CrossRef]
- Ritchie, B.W.; Niagro, F.D.; Latimer, K.S.; Steffens, W.L.; Pesti, D.; Ancona, J.; Lukert, P.D. Routes and Prevalence of Shedding of Psittacine Beak and Feather Disease Virus. Am. J. Vet. Res. 1991, 52, 1804–1809. [Google Scholar] [CrossRef]
- Sarker, S.; Terrón, M.C.; Khandokar, Y.; Aragão, D.; Hardy, J.M.; Radjainia, M.; Jiménez-Zaragoza, M.; de Pablo, P.J.; Coulibaly, F.; Luque, D.; et al. Structural Insights into the Assembly and Regulation of Distinct Viral Capsid Complexes. Nat. Commun. 2016, 7, 13014. [Google Scholar] [CrossRef]
- Stewart, M.E.; Bonne, N.; Shearer, P.; Khalesi, B.; Sharp, M.; Raidal, S. Baculovirus Expression of Beak and Feather Disease Virus (BFDV) Capsid Protein Capable of Self-Assembly and Haemagglutination. J. Virol. Methods 2007, 141, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Ghorashi, S.A.; Swarbrick, C.M.D.; Khandokar, Y.B.; Himiari, Z.; Forwood, J.K.; Raidal, S.R. An Efficient Approach for Recombinant Expression and Purification of the Viral Capsid Protein from Beak and Feather Disease Virus (BFDV) in Escherichia coli. J. Virol. Methods 2015, 215–216, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nath, B.; Das, S.; Roby, J.A.; Sarker, S.; Luque, D.; Raidal, S.; Forwood, J. Structural Perspectives of Beak and Feather Disease Virus and Porcine Circovirus Proteins. Viral Immunol. 2021, 34, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Heath, L.; Williamson, A.; Rybicki, E. The Capsid Protein of Beak and Feather Disease Virus Binds to the Viral DNA and is Responsible for Transporting the Replication-Associated Protein into the Nucleus. J. Virol. 2006, 80, 7219–7225. [Google Scholar] [CrossRef]
- Bonnamy, M.; Blanc, S.; Michalakis, Y. Replication Mechanisms of Circular ssDNA Plant Viruses and Their Potential Implication in Viral Gene Expression Regulation. mBio 2023, 14, e01692-23. [Google Scholar] [CrossRef]
- Lefebvre, D.J.; Meerts, P.; Costers, S.; Misinzo, G.; Barbé, F.; Van Reeth, K.; Nauwynck, H.J. Increased Porcine Circovirus Type 2 Replication in Porcine Leukocytes In Vitro and In Vivo by Concanavalin A Stimulation. Vet. Microbiol. 2008, 132, 74–86. [Google Scholar] [CrossRef]
- Chen, J.K.; Hsiao, C.; Wu, J.S.; Lin, S.Y.; Wang, C.Y. Characterization of the Endonuclease Activity of the Replication-Associated Protein of Beak and Feather Disease Virus. Arch. Virol. 2019, 164, 2091–2106. [Google Scholar] [CrossRef]
- Huang, S.W.; Liu, H.P.; Chen, J.K.; Shien, Y.W.; Wong, M.L.; Wang, C.Y. Dual ATPase and GTPase Activity of the Replication-Associated Protein (Rep) of Beak and Feather Disease Virus. Virus Res. 2016, 213, 149–161. [Google Scholar] [CrossRef]
- Amery-Gale, J.; Marenda, M.S.; Owens, J.; Eden, P.A.; Browning, G.F.; Devlin, J.M. A High Prevalence of Beak and Feather Disease Virus in Non-Psittacine Australian Birds. J. Med. Microbiol. 2017, 66, 1005–1013. [Google Scholar] [CrossRef]
- Rahaus, M.; Wolff, M. Psittacine Beak and Feather Disease: A First Survey of the Distribution of Beak and Feather Disease Virus inside the Population of Captive Psittacine Birds in Germany. J. Vet. Med. B Infect. Dis. Vet. Public Health 2003, 50, 368–371. [Google Scholar] [CrossRef]
- Ahaduzzaman, M.; Nath, C.; Hossain, M.S. Evidence of Circulation of Beak and Feather Disease Virus in Captive Psittacine and Non-Psittacine Birds in Bangladesh. Arch. Virol. 2022, 167, 2567–2575. [Google Scholar] [CrossRef]
- González-Hein, G.; Gil, I.A.; Sanchez, R.; Huaracan, B. Prevalence of Aves Polyomavirus 1 and Beak and Feather Disease Virus from Exotic Captive Psittacine Birds in Chile. J. Avian Med. Surg. 2019, 33, 141. [Google Scholar] [CrossRef]
- Martens, J.M.; Stokes, H.S.; Berg, M.L.; Walder, K.; Segal, Y.; Magrath, M.J.L.; Bennett, A.T.D. Beak and Feather Disease Virus and Chlamydiales Infections in Wild Australian Psittacines: No Statistical Evidence for Dependence. Emu-Austral Ornithol. 2021, 121, 333–339. [Google Scholar] [CrossRef]
- MacColl, C.; Watson, J.E.M.; Leseberg, N.P.; Seaton, R.; Das, T.; Das, S.; Raidal, S.R. Beak and Feather Disease Virus Detected in the Endangered Red Goshawk (Erythrotriorchis radiatus). Sci. Rep. 2024, 14, 10263. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Forwood, J.K.; Raidal, S.R. Beak and Feather Disease Virus: Biology and Resultant Disease. WikiJ. Sci. 2020, 3, 7. [Google Scholar] [CrossRef]
- Robino, P.; Grego, E.; Rossi, G.; Bert, E.; Tramuta, C.; Stella, M.C.; Bertoni, P.; Nebbia, P. Molecular Analysis and Associated Pathology of Beak and Feather Disease Virus Isolated in Italy from Young Congo African Grey Parrots (Psittacus erithacus) with an “Atypical Peracute Form” of the Disease. Avian Pathol. 2014, 43, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, I.; Kwang, J. Characterization of a Previously Unidentified Viral Protein in Porcine Circovirus Type 2-Infected Cells and Its Role in Virus-Induced Apoptosis. J. Virol. 2005, 79, 8262–8274. [Google Scholar] [CrossRef]
- Fogell, D.J.; Martin, R.O.; Groombridge, J.J. Beak and Feather Disease Virus in Wild and Captive Parrots: An Analysis of Geographic and Taxonomic Distribution and Methodological Trends. Arch. Virol. 2016, 161, 2059–2074. [Google Scholar] [CrossRef]
- Fogell, D.J.; Martin, R.O.; Bunbury, N.; Lawson, B.; Sells, J.; McKeand, A.M.; Tatayah, V.; Trung, C.T.; Groombridge, J.J. Trade and Conservation Implications of New Beak and Feather Disease Virus Detection in Native and Introduced Parrots. Conserv. Biol. 2018, 32, 1325–1335. [Google Scholar] [CrossRef]
- Franzo, G.; Dundon, W.G.; De Villiers, M.; De Villiers, L.; Coetzee, L.M.; Khaiseb, S.; Cattoli, G.; Molini, U. Phylodynamic and Phylogeographic Reconstruction of Beak and Feather Disease Virus Epidemiology and Its Implications for the International Exotic Bird Trade. Transbound. Emerg. Dis. 2022, 69, e2677–e2687. [Google Scholar] [CrossRef]
- Varsani, A.; Regnard, G.L.; Bragg, R.; Hitzeroth, I.I.; Rybicki, E.P. Global Genetic Diversity and Geographical and Host-Species Distribution of Beak and Feather Disease Virus Isolates. J. Gen. Virol. 2011, 92, 752–767. [Google Scholar] [CrossRef]
- Julian, L.; Lorenzo, A.; Chenuet, J.-P.; Bonzon, M.; Marchal, C.; Vignon, L.; Collings, D.A.; Walters, M.; Jackson, B.; Varsani, A. Evidence of Multiple Introductions of Beak and Feather Disease Virus into the Pacific Islands of Nouvelle-Calédonie (New Caledonia). J. Gen. Virol. 2012, 93, 2466–2472. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.T.; Wang, J.; Liu, Y.; Hussain, B.; Ma, Z.-H.; Wu, C.; Xing, L. The Phylogenetic and Phylogeographic Landscape of the Beak and Feather Disease Virus, 1996-2022. Infect. Genet. Evol. 2023, 112, 105442. [Google Scholar] [CrossRef]
- Kim, H.J.; Kang, D.Y.; Kim, E.M.; Kim, E.G.; Lee, B.H.; Yeo, S.G.; Park, C.K. Detection of Psittacine Beak and Feather Disease Virus from a Caged Blue and Yellow Macaw (Ara ararauna) in Korea. Korean J. Vet. Serv. 2014, 37, 219–224. [Google Scholar] [CrossRef]
- Regnard, G.L.; Boyes, R.S.; Martin, R.O.; Hitzeroth, I.; Rybicki, E. Beak and Feather Disease Viruses Circulating in Cape Parrots (Poicepahlus robustus) in South Africa. Arch. Virol. 2014, 160, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Catedral, L.; Wallace, C.J.; Heinsohn, R.; Krebs, E.A.; Langmore, N.E.; Vukelic, D.; Bucher, E.H.; Varsani, A.; Masello, J.F. A PCR-Based Retrospective Study for Beak and Feather Disease Virus (BFDV) in Five Wild Populations of Parrots from Australia, Argentina and New Zealand. Diversity 2022, 14, 148. [Google Scholar] [CrossRef]
- Rahaus, M.; Desloges, N.; Probst, S.; Loebbert, B.; Lantermann, W.; Wolff, M.H. Detection of Beak and Feather Disease Virus DNA in Embryonated Eggs of Psittacine Birds. Vet. Med. 2008, 53, 53–58. [Google Scholar] [CrossRef]
- Martens, J.M.; Stokes, H.S.; Berg, M.L.; Walder, K.; Raidal, S.R.; Magrath, M.J.L.; Bennett, A.T.D. A Non-Invasive Method to Assess Environmental Contamination with Avian Pathogens: Beak and Feather Disease Virus (BFDV) Detection in Nest Boxes. PeerJ 2020, 8, e9211. [Google Scholar] [CrossRef]
- Portas, T.; Jackson, B.; Das, S.; Shamsi, S.; Raidal, S. Beak and Feather Disease Virus Carriage by Knemidocoptes PILAE in a Sulphur-crested Cockatoo (Cacatua galerita). Aust. Vet. J. 2017, 95, 486–489. [Google Scholar] [CrossRef]
- Blanch-Lázaro, B.; Chamings, A.; Ribot, R.F.H.; Bhatta, T.R.; Berg, M.L.; Alexandersen, S.; Bennett, A.T.D. Beak and Feather Disease Virus (BFDV) Persists in Tissues of Asymptomatic Wild Crimson Rosellas. Commun. Biol. 2024, 7, 1017. [Google Scholar] [CrossRef]
- Doneley, R. Acute Beak and Feather Disease in Juvenile African Grey Parrots--an Uncommon Presentation of a Common Disease. Aust. Vet. J. 2003, 81, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Mao, Y.; Li, Q.; Xie, S.; Xia, Y.; Sun, H.; Niu, K.; Sun, S.; Li, J.; Feng, Y.; et al. Complete Genome Sequence of Genotype Psittacine Beak and Feather Disease Virus, a Strain Identified from Budgerigars in China. Microbiol. Resour. Announc. 2019, 8, e00040-19. [Google Scholar] [CrossRef] [PubMed]
- Olivares, R.W.I.; Bass, L.G.; Sáenz-Bräutigam, A.; Sandí-Carmiol, J.; Villada-Rosales, A.M.; Dolz, G.; Solórzano-Morales, A.; Zúniga-Moya, M.J.; Granados-Solano, R.; McHale, B.; et al. Psittacine Beak and Feather Disease in 2 Free-Living Great Green Macaws: A Case Report and Literature Review. J. Vet. Diagn. Investig. 2025, 37, 10406387251333410. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; Fowler, M.E. Fowler’s Zoo and Wild Animal Medicine Current Therapy; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011; Volume 7, ISBN 978-1-4377-1985-7. [Google Scholar]
- Ramis, A.; Latimer, K.S.; Niagro, F.D.; Campagnoli, R.P.; Ritchie, B.W.; Pesti, D. Diagnosis of Psittacine Beak and Feather Disease (PBFD) Viral Infection, Avian Polyomavirus Infection, Adenovirus Infection and Herpesvirus Infection in Psittacine Tissues Using DNA in Situ Hybridization. Avian Pathol. 1994, 23, 643–657. [Google Scholar] [CrossRef]
- Shearer, P.L.; Bonne, N.; Clark, P.; Sharp, M.; Raidal, S.R. Development and Applications of a Monoclonal Antibody to a Recombinant Beak and Feather Disease Virus (BFDV) Capsid Protein. J. Virol. Methods 2008, 147, 206–212. [Google Scholar] [CrossRef]
- Sarker, S.; Athukorala, A.; Phalen, D.N. Genome Sequence of a Beak and Feather Disease Virus from an Unusual Novel Host, Australian Boobook Owl (Ninox boobook). Microbiol. Resour. Announc. 2022, 11, e00172-22. [Google Scholar] [CrossRef]
- Raidal, S.; Khalesi, B.; Bonne, N.J.; Stewart, M.E.; Sharp, M. Sensitivity and Specificity of HA, HI and PCR for Detecting Psittacine Beak and Feather Disease Virus (BFDV) Testing. In Proceedings of the Australian Committee Association of Avian Veterinarians Annual Conference (AVA 2006), Tasmania, Australia, 21–26 May 2006; pp. 103–112. [Google Scholar]
- Fungwitaya, P.; Bunlertcharoensuk, A.; Uttamaburana, W.; Sariya, L.; Chaichoune, K.; Ratanakorn, P.; Boonyarittichaikij, R. Prevalence of Psittacine Beak and Feather Disease and Avian Polyomavirus Disease Infection in Captive Psittacines in the Central Part of Thailand by Multiplex Polymerase Chain Reaction. J. Appl. Anim. Sci. 2009, 2, 33–41. [Google Scholar]
- Morales, A.; Sibrián, X.; Porras, F.D. Survey of Beak and Feather Disease Virus (BFDV) in Guatemalan Neotropical Psittacine Birds. J. Avian Med. Surg. 2021, 35, 325–332. [Google Scholar] [CrossRef]
- Hess, M.; Scope, A.; Heincz, U. Comparitive Sensitivity of Polymerase Chain Reaction Diagnosis of Psittacine Beak and Feather Disease on Feather Samples, Cloacal Swabs and Blood from Budgerigars (Melopsittacus undulates, Shaw 18005). Avian Pathol. 2004, 33, 477–481. [Google Scholar] [CrossRef]
- Das, S.; Sarker, S.; Ghorashi, S.A.; Forwood, J.K.; Raidal, S.R. A Comparison of PCR Assays for Beak and Feather Disease Virus and High Resolution Melt (HRM) Curve Analysis of Replicase Associated Protein and Capsid Genes. J. Virol. Methods 2016, 237, 47–57. [Google Scholar] [CrossRef]
- Olsen, G.; Speer, B. Laboratory Reporting Accuracy of Polymerase Chain Reaction Testing for Psittacine Beak and Feather Disease Virus. J. Avian Med. Surg. 2009, 23, 194–198. [Google Scholar] [CrossRef]
- Regnard, G.L.; Rybicki, E.P.; Hitzeroth, I.I. Recombinant Expression of Beak and Feather Disease Virus Capsid Protein and Assembly of Virus-like Particles in Nicotiana benthamiana. Virol. J. 2017, 14, 174. [Google Scholar] [CrossRef]
- Shearer, P.L.; Sharp, M.; Bonne, N.; Clark, P.; Raidal, S.R. A Quantitative, Real-Time Polymerase Chain Reaction Assay for Beak and Feather Disease Virus. J. Virol. Methods 2009, 159, 98–104. [Google Scholar] [CrossRef]
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR Inhibitors—Occurrence, Properties and Removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef]
- Dolatyabi, S.; Peighambari, S.M.; Razmyar, J. Molecular Detection and Analysis of Beak and Feather Disease Viruses in Iran. Front. Vet. Sci. 2022, 9, 1053886. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Tian, Y.; Zhang, M.; Wang, W.; Li, Y.; Tian, F.; Cheng, Y.; Yan, Y.; Sun, J. Identification and Characterization of Novel Genotypes of Psittacine Beak and Feather Disease Virus from Budgerigar in China. Transbound. Emerg. Dis. 2019, 66, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Lee, W.; Lee, Y. Development of a Nucleic Acid Detection Method Based on the CRISPR-Cas13 for Point-of-Care Testing of Bovine Viral Diarrhea Virus-1b. J. Anim. Sci. Technol. 2024, 66, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Subir, S.; Forwood, J.K.; Ghorashi, S.A.; Raidal, S.R. Whole-Genome Sequence of a Beak and Feather Disease Virus Isolate from a Fledgling Red-Capped Parrot (Purpureicephalus spurius). Genome Announc. 2016, 4, e01108-16. [Google Scholar] [CrossRef]
- Stanford, M. Interferon Treatment of Circovirus Infection in Grey Parrots (Psittacus e erithacus). Vet. Rec. 2004, 154, 435–436. [Google Scholar] [CrossRef]
- Peters, A.; Meredith, A.; Skerratt, L.; Carver, S.; Raidal, S. Infectious Disease and Emergency Conservation Interventions. Conserv. Biol. 2020, 34, 784–785. [Google Scholar] [CrossRef]
- Fogell, D.J.; Groombridge, J.J.; Tollington, S.; Canessa, S.; Henshaw, S.; Zuel, N.; Jones, C.G.; Greenwood, A.; Ewen, J.G. Hygiene and Biosecurity Protocols Reduce Infection Prevalence but Do Not Improve Fledging Success in an Endangered Parrot. Sci. Rep. 2019, 9, 4779. [Google Scholar] [CrossRef]
- York, D. Psittacine Beak and Feather Disease (PBFD). Vet. Q. 2008, 30, 9. [Google Scholar] [CrossRef]
- Hoppes, S.M. Viral Diseases of Pet Birds—Exotic and Laboratory Animals. 2024. Available online: https://www.merckvetmanual.com/exotic-and-laboratory-animals/pet-birds/viral-diseases-of-pet-birds (accessed on 18 June 2025).
- Ortiz-Catedral, L.; McInnes, K.; Hauber, M.; Brunton, D. First Report of Beak and Feather Disease Virus (BFDV) in Wild Red-Fronted Parakeets (Cyanoramphus novaezelandiae) in New Zealand. Emu-Austral Ornithol. 2009, 109, 244–247. [Google Scholar] [CrossRef]
- Cross, G.M. Hygiene Protocols for the Prevention and Control of Diseases (Particularly Beak and Feather Disease) in Australian Birds. 2006. Available online: https://www.dcceew.gov.au/sites/default/files/documents/hygiene-protocols-all.pdf (accessed on 18 June 2025).
- Viñals, F.; McKenzie, F.R.; Pouysségur, J. Growth Factor-Stimulated Protein Synthesis Is Inhibited by Sodium Orthovanadate. Eur. J. Biochem. 2001, 268, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, C. Ribavirin Efficiently Suppresses Porcine Nidovirus Replication. Virus Res. 2012, 171, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, O.; Tukac, V. Psittacine Circovirus Infection in Parakeets of the Genus Eunymphicus and Treatment with β-(1,3/1,6)-D-Glucan. Avian Dis. 2007, 51, 989–991. [Google Scholar] [CrossRef]
- Pajkrt, D.; van Deventer, S.J.H. Is G-CSF Safe and Useful in the Treatment of Infectious Diseases in the Non-Neutropenic Host? Intensive Care Med. 1997, 23, 1–2. [Google Scholar] [CrossRef]
- Chitty, J. The Facts About F10. 2005. Available online: https://anyflip.com/rkfs/uiai/basic (accessed on 18 June 2025).
- Peachey, M. Psittacine Beak and Feather Viral Disease in Parrots in the ACT. Canberra Bird Notes 2013, 38, 106–118. [Google Scholar]
- Raisin, C.; Frantz, A.C.; Kundu, S.; Greenwood, A.G.; Jones, C.G.; Zuel, N.; Groombridge, J.J. Genetic Consequences of Intensive Conservation Management for the Mauritius Parakeet. Conserv. Genet. 2012, 13, 707–715. [Google Scholar] [CrossRef]
- Ritchie, B.W.; Niagro, F.D.; Latimer, K.S.; Steffens, W.L.; Pesti, D.; Campagnoli, R.P.; Lukert, P.D. Antibody Response to and Maternal Immunity from an Experimental Psittacine Beak and Feather Disease Vaccine. Am. J. Vet. Res. 1992, 53, 1512–1518. [Google Scholar] [CrossRef]
- Raidal, S.; Firth, G.; Cross, G. Vaccination and Challenge Studies with Psittacine Beak and Feather Disease Virus. Aust. Vet. J. 1993, 70, 437–441. [Google Scholar] [CrossRef]
- Bonne, N.; Shearer, P.; Sharp, M.; Clark, P.; Raidal, S. Assessment of Recombinant Beak and Feather Disease Virus Capsid Protein as a Vaccine for Psittacine Beak and Feather Disease. J. Gen. Virol. 2009, 90, 640–647. [Google Scholar] [CrossRef]
- Raidal, S.; Cross, G. Control of Psittacine Beak and Feather Disease (PBFD) by Vaccination; Association of Avian Veterinarians, Australian Committee: Gold Coast, Australia, 1994. [Google Scholar]
- Shearer, P.L.; Sharp, M.; Bonne, N.; Clark, P.; Raidal, S.R. A Blocking ELISA for the Detection of Antibodies to Psittacine Beak and Feather Disease Virus (BFDV). J. Virol. Methods 2009, 158, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Bonne, N.; Shearer, P.L.; Sharp, M.; Clark, P.; Raidal, S.R. BFDV Vaccination Trial—An Update. In Proceedings of the Australian Committee Association of Avian Veterinarians Annual Conference (AVA 2007), Melbourne, Australia, 3–6 October 2007; pp. 223–228. [Google Scholar]
- Hakimuddin, F.; Abidi, F.; Jafer, O.; Li, C.; Wernery, U.; Hebel, C.; Khazanehdari, K. Incidence and Detection of Beak and Feather Disease Virus in Psittacine Birds in the UAE. Biomol. Detect. Quantif. 2016, 6, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Adiguzel, M.C.; Timurkan, M.O.; Cengiz, S. Investigation and Sequence Analysis of Avian Polyomavirus and Psittacine Beak and Feather Disease Virus from Companion Birds in Eastern Turkey. J. Vet. Res. 2020, 64, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Godoy, F.; Estrada-Arzate, D.; Torres-Torres, A.; Chávez-Maya, F.; Lima-Melo, A.; García-Espinosa, G. First Report of Psittacine Beak and Feather Disease in Imported Budgerigar (Melopsittacus undulatus) Chicks in Mexico. Braz. J. Vet. Pathol. 2020, 13, 549–554. [Google Scholar] [CrossRef]
- Molini, U.; De Villiers, M.; De Villiers, L.; Coetzee, L.M.; Hoebes, E.; Khaiseb, S.; Cattoli, G.; Dundon, W.G.; Franzo, G. Investigation and Sequence Analysis of Psittacine Beak and Feather Disease Virus and Avian Polyomavirus from Companion Birds in Windhoek, Namibia. Acta Trop. 2023, 238, 106739. [Google Scholar] [CrossRef]
- Fogell, D.J.; Tollington, S.; Tatayah, V.; Henshaw, S.; Naujeer, H.; Jones, C.; Raisin, C.; Greenwood, A.; Groombridge, J.J. Evolution of Beak and Feather Disease Virus across Three Decades of Conservation Intervention for Population Recovery of the Mauritius Parakeet. Diversity 2021, 13, 584. [Google Scholar] [CrossRef]
- Heath, L.; Martin, D.P.; Warburton, L.; Perrin, M.; Horsfield, W.; Kingsley, C.; Rybicki, E.P.; Williamson, A.L. Evidence of Unique Genotypes of Beak and Feather Disease Virus in Southern Africa. J. Virol. 2004, 78, 9277–9284. [Google Scholar] [CrossRef]
- Ko, J.C.K.; Choi, Y.W.Y.; Poon, E.S.K.; Wyre, N.; Sin, S.Y.W. Prevalence, Genotypes, and Infection Risk Factors of Psittacine Beak and Feather Disease Virus and Budgerigar Fledgling Disease Virus in Captive Birds in Hong Kong. Arch. Virol. 2024, 169, 91. [Google Scholar] [CrossRef]
- Sarker, S.; Ghorashi, S.A.; Forwood, J.K.; Raidal, S.R. Rapid Genotyping of Beak and Feather Disease Virus Using High-Resolution DNA Melt Curve Analysis. J. Virol. Methods 2014, 208, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Dalton, R. Ecologists Hit out at Plan to Export Argentinian Parrots. Nature 2004, 427, 4. [Google Scholar] [CrossRef] [PubMed]
- Cardador, L.; Abellán, P.; Anadón, J.; Carrete, M.; Tella, J. Naturalized Parrots of the World: Distribution, Ecology, and Impacts of the World’s Most Colorful Colonizers; Pruett-Jones, S., Ed.; Princeton University Press: Princeton, NJ, USA, 2021; pp. 13–21. ISBN 978-0691204413. [Google Scholar]
- Chan, D.T.C.; Poon, E.S.K.; Wong, A.T.C.; Sin, S.Y.W. Global Trade in Parrots—Influential Factors of Trade and Implications for Conservation. Glob. Ecol. Conserv. 2021, 30, e01784. [Google Scholar] [CrossRef]
- Romero-Vidal, P.; Carrete, M.; Hiraldo, F.; Blanco, G.; Tella, J. Confounding Rules Can Hinder Conservation: Disparities in Law Regulation on Domestic and International Parrot Trade Within and among Neotropical Countries. Animals 2022, 12, 1244. [Google Scholar] [CrossRef]
- Dickinson, E.; Young, M.; Tanis, D.K.; Granatosky, M. Patterns and Factors Influencing Parrot (Order: Psittaciformes) Success in Establishing Thriving Naturalized Populations Within the Contiguous United States. Animals 2023, 13, 2101. [Google Scholar] [CrossRef]
- Martin, R. Grey Areas: Temporal and Geographical Dynamics of International Trade of Grey and Timneh Parrots (Psittacus erithacus and P. timneh) under CITES. Emu-Austral Ornithol. 2018, 118, 113–125. [Google Scholar] [CrossRef]
- Low, B.W. The Global Trade in Native Australian Parrots through Singapore Between 2005 and 2011: A Summary of Trends and Dynamics. Emu-Austral Ornithol. 2014, 114, 277–282. [Google Scholar] [CrossRef]
- Vall-Llosera, M.; Cassey, P. ‘Do You Come from a Land down under?’ Characteristics of the International Trade in Australian Endemic Parrots. Biol. Conserv. 2017, 207, 38–46. [Google Scholar] [CrossRef]
- Carrete, M.; Tella, J. Wild-Bird Trade and Exotic Invasions: A New Link of Conservation Concern? Front. Ecol. Environ. 2008, 6, 207–211. [Google Scholar] [CrossRef]
- Morinha, F.; Carrete, M.; Tella, J.L.; Blanco, G. High Prevalence of Novel Beak and Feather Disease Virus in Sympatric Invasive Parakeets Introduced to Spain From Asia and South America. Diversity 2020, 12, 192. [Google Scholar] [CrossRef]
- Valastanova, M.; Petrikova, M.; Kulikova, L.; Knotek, Z. Psittacine Beak and Feather Disease Virus and Avian Polyomavirus Detection Rate in Clinically Healthy Captive Birds in the Czech Republic. Vet. Med. 2021, 66, 72–75. [Google Scholar] [CrossRef]
- Blanco, G.; Morinha, F.; Carrete, M.; Tella, J.L. Apparent Lack of Circovirus Transmission from Invasive Parakeets to Native Birds. Int. J. Environ. Res. Public Health 2022, 19, 3196. [Google Scholar] [CrossRef]
- Massaro, M.; Ortiz-Catedral, L.; Julian, L.; Galbraith, J.A.; Kurenbach, B.; Kearvell, J.; Kemp, J.; van Hal, J.; Elkington, S.; Taylor, G.; et al. Molecular Characterisation of Beak and Feather Disease Virus (BFDV) in New Zealand and Its Implications for Managing an Infectious Disease. Arch. Virol. 2012, 157, 1651–1663. [Google Scholar] [CrossRef]
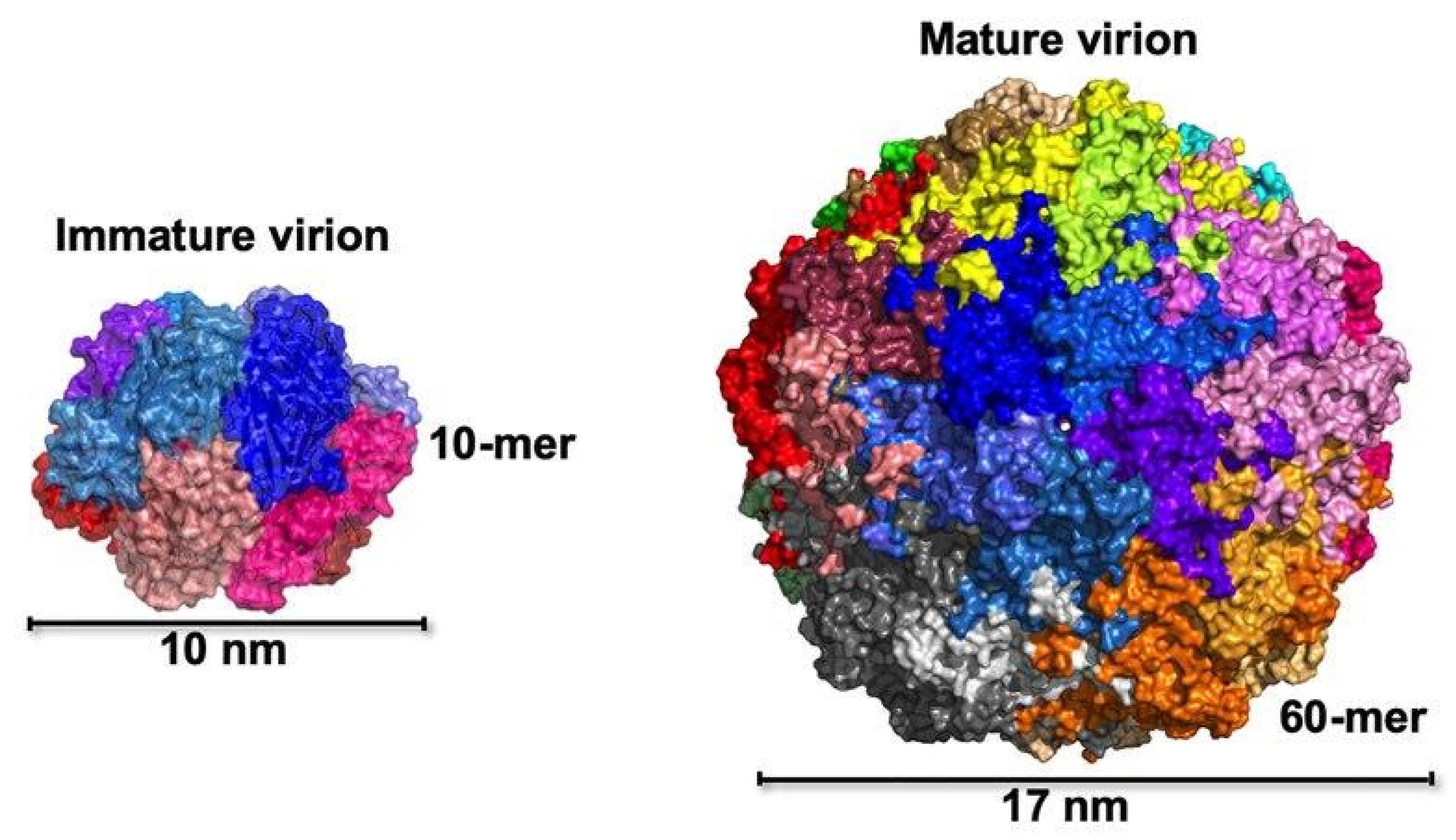

| Stage | Clinical Signs |
|---|---|
| Newly hatched chicks | Diarrhea, lethargy, crop stasis, anorexia; no feather signs observed; death within 1–2 weeks |
| Fledgling birds | Similar signs to newly hatched chicks; abnormal feathers observed; death within 1–2 weeks |
| Chronic cases (6 months to several years) | Feathers become twisted, split, and fall out; beak abnormalities; death within 6 months to 2 years |
| Methods | Description | Pros | Cons |
|---|---|---|---|
| HA | Hemagglutination assay (HA) is a method used to measure the ability of viruses or antibodies to cause red blood cells (erythrocytes) to clump or agglutinate. It is frequently employed to quantify viral particles or evaluate antigen–antibody interactions within diagnostic and research settings [14,59,63]. | Simplicity, Low cost, Quick Result | Specificity, Sensitivity, Variable Results |
| HI | Hemagglutination inhibition (HI) assay measures the presence of antibodies against viruses in serum by observing if they prevent the clumping of red blood cells [14,59,63]. | Specific Detection, Simplicity, Reliable | Limited Spectrum, Interference, Time Consuming |
| Histology | Histology in pathology involves the microscopic examination of tissue samples to diagnose disease. This analysis is critical for determining the nature, severity, and potential treatment of various medical conditions [56]. | Special Stains | Time consuming, Technique Sensitivity, Sampling Bias |
| Standard PCR | PCR (Polymerase Chain Reaction) is a technique used to amplify specific DNA sequences, enabling the detection and quantification of DNA samples [14,59,63]. | Specificity, Sensitivity, Established | Contamination, Size Limitation |
| WGS | Whole-genome sequencing (WGS) is a method that determines the complete DNA sequence of an organism’s genome at a single time [71]. | Comprehensive, High Resolution, Outbreak Tracking | High Cost, Data Overload |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, E.G.; Han, J.-H.; Shim, Y.J.; Lee, D.N.; Choi, K.-S.; Yeon, S.-C. Psittacine Beak and Feather Disease: Global Spread, International Trade, and Conservation Challenges. Animals 2025, 15, 2947. https://doi.org/10.3390/ani15202947
Kang EG, Han J-H, Shim YJ, Lee DN, Choi K-S, Yeon S-C. Psittacine Beak and Feather Disease: Global Spread, International Trade, and Conservation Challenges. Animals. 2025; 15(20):2947. https://doi.org/10.3390/ani15202947
Chicago/Turabian StyleKang, Eun Gu, Jang-Hee Han, Yong Ju Shim, Do Na Lee, Kang-Seuk Choi, and Seong-Chan Yeon. 2025. "Psittacine Beak and Feather Disease: Global Spread, International Trade, and Conservation Challenges" Animals 15, no. 20: 2947. https://doi.org/10.3390/ani15202947
APA StyleKang, E. G., Han, J.-H., Shim, Y. J., Lee, D. N., Choi, K.-S., & Yeon, S.-C. (2025). Psittacine Beak and Feather Disease: Global Spread, International Trade, and Conservation Challenges. Animals, 15(20), 2947. https://doi.org/10.3390/ani15202947






