Simple Summary
The present study was conducted to analyze the effects of milk fat content on the rumen microbiome and metabolomics of Binglangjiang buffaloes (river buffaloes). The results showed that there are differences in the rumen microbial composition and host metabolites of Bingangjiang buffaloes with high and low milk fat contents. These changes in the microbiota may lead to alterations in fatty acid biosynthesis precursors in the rumen and plasma, ultimately affecting milk fat content. Our research revealed a significant impact on these processes, including Quienlla, Stilbella, Cyrenella, Microsphaeropsis, and Paraphaeosphaeria, which are associated with the following metabolites in the rumen: lucidenic acid J, secoeremopetasitolide B, and erinacine P, as well as the plasma metabolite corchorifatty acid F, PC (18:3 (6Z, 9Z, 12Z)/18:3 (9Z, 12Z, 15Z)). The roles of the rumen microbiota and metabolites can be further validated in the future.
Abstract
The present study was conducted to analyze the correlation between the milk fat content of Binglangjiang buffaloes and their microbial and host metabolites. The 10 buffaloes with the highest milk fat content (HF, 5.60 ± 0.61%) and the 10 with the lowest milk fat content (LF, 1.49 ± 0.13%) were selected. Their rumen fluid and plasma were collected for rumen microbiota and metabolome analysis. The results showed that the rumen bacteria abundance of Synergistota, Quinella, Selenomonas, and Fretibacterium was significantly higher in the HF buffaloes. The abundance of 14 rumen fungi, including Candida, Talaromyces, Cyrenella, and Stilbella, was significantly higher in the HF buffaloes. The analysis of the metabolites in the rumen and plasma showed that several metabolites differed between the HF and LF buffaloes. A total of 68 and 42 differential metabolites were identified in the rumen and plasma, respectively. By clustering these differential metabolites, most of those clustered in the HF group were lipid and lipid-like molecules such as secoeremopetasitolide B, lucidenic acid J LysoPE (0:0/18:2 (9Z, 12Z)), and 5-tetradecenoic acid. Spearman’s rank correlations showed that Quinella, Fretibacterium, Selenomonas, Cyrenella, and Stilbella were significantly positively correlated with the metabolites of the lipids and lipid-like molecules in the rumen and plasma. The results suggest that rumen microbiota such as Quinella, Fretibacterium, Selenomonas, and Cyrenella may regulate milk fat synthesis by influencing the lipid metabolites in the rumen and plasma. In addition, the combined analysis of the rumen microbiota and host metabolites may provide a fundamental understanding of the role of the microbiota and host in regulating milk fat synthesis.
1. Introduction
Milk is rich in a variety of essential nutrients, including fat, protein, and various trace elements that are important for healthy human development, especially many kinds of unsaturated fatty acids. Milk fat is a milk component with high nutritional value, which is the most important determinant of milk quality. A high fat content is an important characteristic of buffalo milk []. In addition, milk fat is an important source of essential fatty acids, phospholipids, and fat-soluble vitamins, providing the body with a large amount of nutrients and energy [,]. Buffalo milk, as a best-selling dairy raw material in domestic and international markets, is milky and thick, with a high content of nutrients. Its content of milk fat, milk protein, total solids, and minerals is higher than that of Holstein milk; therefore, the development of buffalo milk is particularly important [,].
Ruminants have been domesticated for more than 10,000 years [] and rely on fermentation by the rumen microbial community to convert complex polysaccharides, which make up a major portion of the plant, into available nutritive substances [,]. Furthermore, the rumen microbiota is one of the key factors affecting the formation of milk fat precursors. Changes in milk fat production and milk fatty acid composition are usually caused by alterations in rumen fermentation and biohydrogenation pathways []. Previous research has indicated that there are interactions between rumen microbes and the mammary gland. Gastrointestinal microbial metabolites can enter the bloodstream through the intestinal epithelium into the mammary gland, which then influences the composition of milk secreted by the mammary gland []. It seems that rumen metabolites, as important precursors for milk fat production, have a direct impact on milk fat percentage in dairy cows []. Several studies have shown that the difference in milk fat percentage may be caused by the interactions between gut microorganisms and their metabolites, especially Firmicutes myristic acid and Proteus cholin []. However, the levels of metabolites in rumen fluid do not directly reflect the levels of relevant metabolites in the blood [].
Blood is a commonly used biofluid in metabolomics analyses, providing metabolites from all organs []. Blood metabolites can be used as physiological biomarkers to reflect metabolic performance and dysfunction []. Meanwhile, some typical metabolites are highly correlated with specific rumen microbiota, suggesting a functional correlation between the microbiome and its associated metabolites []. With the development of biotechnology, more researchers are using macro-genomics, macro-transcriptomics, and proteomics to explore the role of the rumen microbiota and their involvement in milk fat synthesis, milk production, and lipid metabolism [,]. However, this relationship has not yet been studied in Binglangjiang buffaloes. This buffalo species can be found across all continents and boasts abundant resources. It is classified into two subspecies: river buffaloes (50 chromosomes), primarily inhabiting India, Pakistan, and Mediterranean coastal countries and regions, and swamp buffaloes (48 chromosomes), which are mainly distributed in China and southeast Asian countries []. The Binglangjiang buffalo is the only species of river buffalo that has been discovered in China. A previous study found that the fat and protein contents in the milk of Binglangjiang buffaloes was about 2.1 and 1.2 times higher than that of Holstein milk, suggesting that their milk has excellent qualities []. However, the individual Binglangjiang buffalo vary greatly in terms of milk composition and reproductive performance, and their daily milk production is still at a low level compared to other countries. In this study, we speculate that the milk fat content of cows fed the same diet may be influenced by rumen microbiota composition and its association with the host metabolism. Therefore, we investigated the correlation between the rumen microbiota and the metabolomics of rumen and plasma in high fat content and low fat content Binglangjiang buffaloes.
2. Materials and Methods
2.1. Animals and Management
All the experimental protocols were approved by the Animal Care Committee at the Faculty of Animal Science and Technology, Yunnan Agricultural University (Kunming, P. R. China, approval No.: YNAU202205020). In this experiment, we randomly selected 75 healthy mid-lactation Binglangjiang buffaloes with similar lactation days (120–140 days) and similar body condition (body condition score from 2.5 to 3). Then, they were kept in a commercial buffalo farm in Tengchong City, Yunnan Province. The buffaloes were kept in individually tethered stalls in a barn with good ventilation and were fed 2 times daily at 08:00 h and 16:00 h. The buffaloes were fed the same diet (Tables S1 and S2) with a concentrate-to-forage ratio of 35:65 (DM basis) and had free access to water. Then, we continuously measured the composition of the milk for 6 weeks once a week, and the 10 highest milk fat content buffaloes (HF, 5.60 ± 0.61%) and 10 lowest milk fat content buffaloes (LF, 1.49 ± 0.13%) were selected accordingly (Figure S1).
2.2. Sample Collection and Measurement
Milk samples were collected at 06:30 with a bronopol tablet (milk preservative, D & F Control Systems, San Ramon, CA) and then stored at 4 °C before the infrared analysis of milk components and somatic cells using a spectrophotometer (Foss-4000, Foss, Hillerød, Denmark). Milk production was recorded for 7 consecutive days using a mobile milking machine, and feed intake was recorded prior to formal sample collection. Blood samples were collected from the jugular vein, and plasma was separated and then stored at −80 °C for metabolomic analysis. Rumen contents were sampled using oral stomach tubes and were used to measure microbiota and metabolites. The milk, blood and rumen samples were collected on the same day after milk production was recorded. The pretreatment and determination of rumen fluid VFAs were carried out with reference to Hu et al. [].
2.3. 16S rDNA and ITS Sequencing and Data Processing
Rumen microorganisms DNA were extracted from 2 mL rumen fluid by the kit method (Omega Bio-tek, Norcross, GA, USA). Before PCR amplification, we used 1.0% agarose gel electrophoresis and NanoDrop2000 to detect the purity and concentration of DNA, and we took an appropriate amount of sample from the centrifuge tube for dilution. Diluted DNA was used as a template. Specific primers were selected to amplify the V3 + V4 region of 16SrDNA. The fungus was subjected to ITS sequencing, and the amplified region was ITS1F-ITS2R. The primer sequences and PCR amplification conditions were referred to Yu et al. [].
We used FASTP (v0.20.0) software to control the quality of the original sequencing sequence [] and used FLASH (v1.2.7) software to splice []. Using UPARSE software (v7.1), OTU clustering was performed on sequences according to 97% similarity, and chimeras were eliminated []. RDP classifier (v2.2) was used to classify and annotate each sequence [], compared with the Silva 16S rDNA database (v138), and we set the comparison threshold to 70%. Then, we used Mothur software (v1.30.2) for alpha diversity analysis. Beta diversity was analyzed by principal component analysis (PCA) based on the Bray–Curtis distance in R language (v 3.3.1), while the 16S and ITS sequence data were compared between the two groups (HF vs. LF) using the Wilcoxon rank-sum test with the p-value < 0.05 considered significantly different.
2.4. Analysis of Rumen and Plasma Metabolome
Rumen and plasma metabolites were extracted according to Guo et al. []. First, 100 μL liquid sample was added to a 1.5 mL centrifuge tube with 400 μL solution (acetonitrile: methanol = 1:1 (v:v)) containing 0.02 mg/mL internal standard (L-2-chlorophenylalanine) to extract metabolites. The samples were mixed by vortex for 30 s and low-temperature sonicated for 30 min (5 °C, 40 KHz). The samples were placed at −20 °C for 30 min to precipitate the proteins. Then, the samples were centrifuged for 15 min (4 °C, 13,000× g). The supernatant was removed and blown dry under nitrogen. The sample was then re-solubilized with 100 µL solution (acetonitrile: water = 1:1) and extracted by low-temperature ultrasonication for 5 min (5 °C, 40 KHz), which was followed by centrifugation at 13,000× g and 4 °C for 10 min. The supernatant was transferred to sample vials for LC-MS/MS analysis.
As a part of the system conditioning and quality control process, a pooled quality control sample (QC) was prepared by mixing equal volumes of all samples. The LC-MS analysis was performed on the Thermo UHPLC-Q system of Majorbio bio-pharma Technology Co. (Shanghai, China). A Thermo UHPLC-Q Exactive HF-X mass spectrometer was used to collect mass spectrum data, which was divided into two working modes: positive mode and negative mode. The data-related acquisition (DDA) mode was used for data acquisition. It was tested in the quality range of 70–1050 m/z. The LC/MS raw data were pre-processed using Progenesis QI (Waters Corporation, Milford, CT, USA) software (version 2.0). At the same time, we used the metabolite identification of a searchable database, which was the main database for the KEGG database (http://www.genome.jp/kegg/, accessed: 20 February 2023).
We uploaded a searchable database of the data matrix to the Majorbio cloud platform (https://cloud.majorbio.com, accessed: 15 March 2020) for data analysis. First, the data matrix was preprocessed: at least 80% of the metabolic signatures detected in each set of samples were retained. After filtration, minimum metabolite values were estimated for specific samples whose metabolite levels were below the lower quantization limit, and various metabolic characteristics were normalized to sum. The response intensity of the sample quality spectrum peak was normalized by the sum normalization method. At the same time, the variables of QC samples with a relative standard deviation (RSD) of >30% were removed, and log10 was used for subsequent analysis. Then, the R software package “ropls” (version 1.6.2) was used for orthogonal least partial binary discriminant analysis (OPLS-DA) and interactive verification to evaluate the stability of the model. According to the p-values generated by the OPLS-DA model and Student’s t-test, metabolites with p < 0.05 were significantly different metabolites.
Differential metabolites among the two groups were mapped into their biochemical pathways through metabolic enrichment and pathway analysis based on the KEGG database. Python packages “scipy.stats” (https://docs.scipy.org/doc/scipy/, accessed: 27 March 2023) was used to perform enrichment analysis to obtain the most relevant biological pathways for experimental treatments. Correlation analysis between the rumen microbiota, rumen and plasma metabolome were performed using the Spearman (https://cloud.majorbio.com/page/tools.html, accessed: 5 April 2023), any p-value below 0.05 was regarded as indicating a significant correlation.
3. Results
3.1. Characterization of Phenotypes
In this study, 10 buffaloes with the highest milk fat content (HF) and 10 buffaloes with the lowest milk fat content (LF) were selected for 16S, ITS, rumen metabolome, and plasma metabolome analyses. Among the phenotypes, the milk fat and lactose content was significantly different between the HF and LF (p < 0.001) (Table 1). Concentrations of rumen volatile fatty acids were quantified and found to be significantly higher (p < 0.05) in HF buffaloes for total volatile fatty acids (TVFAs) and acetate (Table S3).

Table 1.
Physiological parameters of HF and LF buffaloes.
3.2. Rumen Bacteria and Taxonomic Differences Between the HF and LF Buffaloes
In 16S rDNA sequencing, a total of 960,813 sequences were generated, with 48,040 ±1523 sequences (mean ± standard error of the mean [SEM]) per sample, and the average sequences in group HF and LF were 48,050 and 48,031, respectively (Table S4). The Chao, Shannon and coverage index of HF and LF buffaloes were not significantly different (Figure 1A–C). The partial least squares discriminant analysis (PLS-DA) showed separations between the groups based on bacterial phyla (Figure 1D) and genus (Figure 1E).
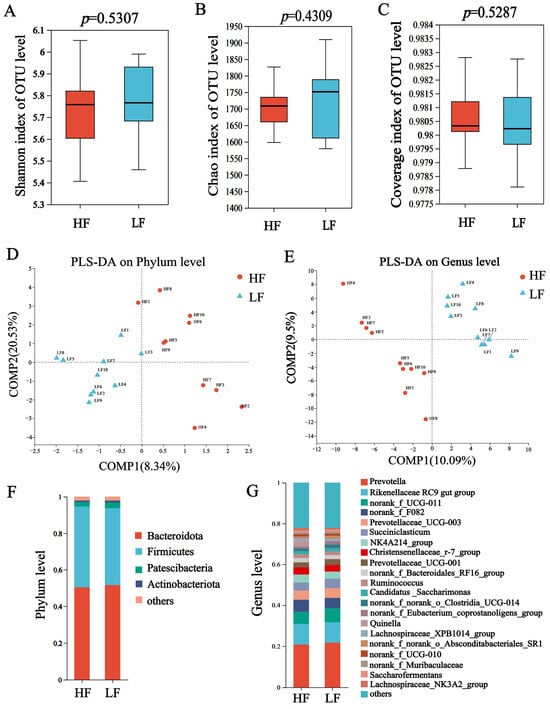
Figure 1.
Composition and differential bacteria in the rumen. (A) Shannon index. (B) Chao index. (C) Coverage index. (D) The partial least squares discriminant analysis at phylum level. (E) The partial least squares discriminant analysis at genus level. (F) Community structure at the phylum level. (G) Community structure at the genus level.
The dominant bacterial phyla included Bacteroidetes (55.98 ± 1.02%), Firmicutes (27.32 ± 1.14%), and Proteobacteria (7.32 ± 1.57%); the dominant bacterial genus was Prevotella (21.20 ± 0.45%), which was followed by Rikenellaceae_ RC9_ gut_ group (10.05 ± 0.05%), norank_f__UCG-011 (6.34 ±0.49%) and Succiniclasticum (4.20 ± 0.34%) (p < 0.05, Figure 1F,G). For differential abundance comparison analysis at the phylum level, the abundance of Synergistota was significantly higher in the rumen of HF buffaloes. At the genus level, Quinella, Selenomonas and Fretibacterium exhibited significantly higher abundances in the rumen of HF buffaloes (p < 0.05), while 12 genera showed significant enrichment in the rumen of LF buffaloes (p < 0.05; Figure 2).
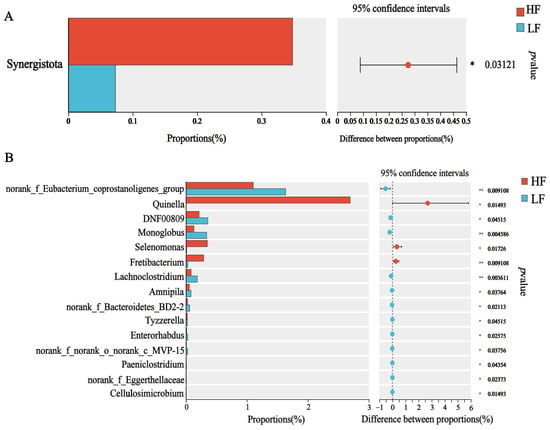
Figure 2.
Differential bacteria. * means p < 0.05, ** means p < 0.01. (A) At phylum level. (B) At genus level.
3.3. Rumen Fungi and Taxonomic Differences Between the HF and LF Buffaloes
In ITS sequencing, a total of 1,209,963 sequences were generated, with 60,498 ±2540 sequences (mean ± standard error of the mean [SEM]) per sample, and the average sequences in the HF and LF groups were 62,041 and 58,965, respectively (Table S5). The Chao, Shannon and coverage indexes of HF and LF buffaloes were not significantly different (Figure 3A–C). The partial least squares discriminant analysis (PLS-DA) showed separations between the groups based on fungi phyla (Figure 3D) and genus (Figure 3E).
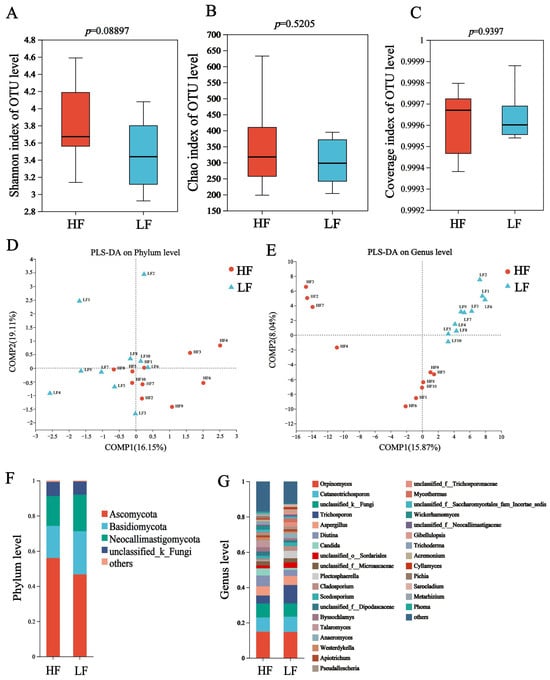
Figure 3.
Composition and differential fungi the rumen. (A) Shannon index. (B) Chao index. (C) Coverage index. (D) The partial least squares discriminant analysis at phylum level. (E) The partial least squares discriminant analysis at genus level. (F) Community structure at the phylum level. (G) Community structure at the genus level.
The dominant fungal phyla included Ascomycota (55.98 ± 1.02%), Basidiomycota (27.32 ± 1.14%), and Neocallimastigomycota (7.32 ± 1.57%); the dominant genus was Orpinomyces (14.83 ± 0.11%), which was followed by Cutaneotrichosporon (8.32 ± 0.41%), norank_f_UCG-011 (6.34 ± 0.49%) and Trichosporon (7.49 ± 2.90%) (Figure 3F,G). For differential abundance comparison analysis at the phylum level, there was no significant difference between HF and LF buffaloes (p > 0.05). At the genus level, the abundance of 14 rumen fungi, included Candida, Talaromyces, Cyrenella and Stilbella, was significantly higher in the rumen of HF buffaloes (p < 0.05), while Anaeromyces showed significant enrichment in the rumen of LF buffaloes (p < 0.05; Figure 4).
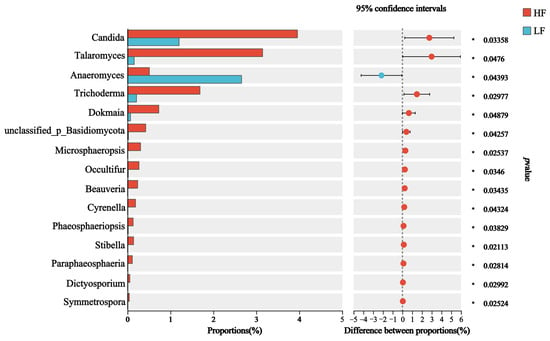
Figure 4.
Differential fungi at genus level. * means p < 0.05.
3.4. Rumen Metabolome
To discriminate the metabolic profiles across groups, we performed clustering analyses based on orthogonal partial least square discriminant analysis (OPLS-DA). The rumen samples from distinct groups were largely separated according to the OPLS-DA plots (Figure 5A). A total of 1451 compounds were identified in the rumen metabolome, and nine of these metabolites were identified in the HF group only, e.g., PE (15:0/16:0), LPC (18:1), and LPC (18:2) (Figure S2). After t-test filtering for the relative concentrations of rumen metabolites, 68 metabolites were significantly different between the two groups, 40 of which were significantly higher in the rumen of HF buffaloes (Figure 5B, Table S6). By clustering these differential metabolites, most of those clustered in the HF group were lipid and lipid-like molecules such as secoeremopetasitolide B and lucidenic acid J (Figure 5C,D). KEGG pathway enrichment analysis based on these 68 significantly different rumen metabolites revealed the enrichment of five pathways, which included “Bile secretion”, “Drug metabolism-cytochrome P450”, “Arginine and proline metabolism”, “Pentose and glucuronate interconversions”, and “ Phenylalanine metabolism”; among these, the “Pentose and glucuronate interconversions” pathway was significantly upregulated in the HF group (p < 0.05, DA score > 0.1) (Figure 5E).
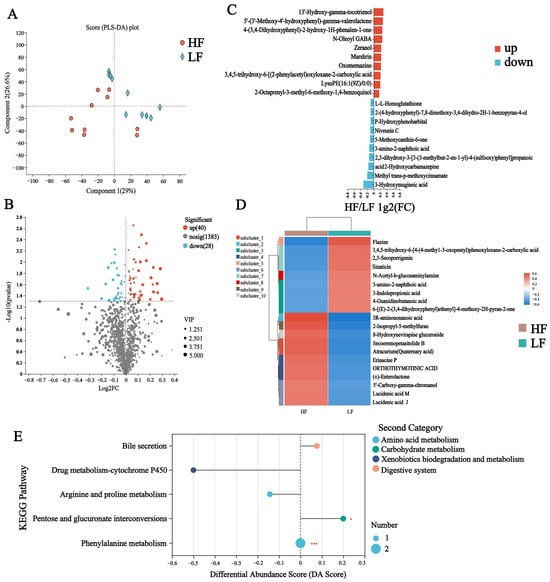
Figure 5.
Rumen metabolome of HF and LF buffaloes. (A) PLS-DA analysis of rumen metabolome. (B) Volcanogram of rumen differential metabolome. (C) Comparison of rumen metabolome between HF and LF buffaloes visualized. (D) Cluster analysis of differential rumen metabolites, the left is a dendrogram of metabolite clustering, on the right is the name of the metabolite, and the closer two metabolite branches are to each other, the closer they are in terms of expression. (E) Differential abundance score map of KEGG pathway of rumen metabolome. A positive DA score indicates that the expression trend of all annotated differential metabolites in the pathway was upregulated in the HF group, the dots are distributed to the right of the center axis and the longer the line segment, the more the overall expression of the pathway tends to be upregulated. * means p < 0.05, *** means p < 0.001.
3.5. Plasma Metabolome
Similar to the rumen, to discriminate the metabolic profiles across groups, we performed clustering analyses based on orthogonal partial least square discriminant analysis (OPLS-DA). The rumen samples from distinct groups were largely separated according to the OPLS-DA plots (Figure 6A). A total of 770 compounds were identified in the plasma metabolome; among these, three of the metabolites were identified in the HF group only, namely alpha-crocetin glucosyl ester, 3,4,5-trihydroxy-6-[(3-oxo-1,7-diphenylheptan-2-yl)oxy]oxane-2-carboxylic acid, and isovalerylglutamic acid (Figure S3). After t-test filtering for the relative concentrations of plasma metabolites, 42 metabolites were significantly different between the two groups, 36 of which were significantly higher in the rumen of HF buffaloes (Figure 6B, Table S7). By clustering these differential metabolites, most of those clustered in the HF group were lipid and lipid-like molecules such as LysoPE (0:0/18:2 (9Z, 12Z)), 5-tetradecenoic acid and hexadecanedioic acid (Figure 6C,D). KEGG pathway enrichment analysis based on these 42 significantly different plasma metabolites revealed the enrichment of six pathways, which included “Pyrimidine metabolism”, “Lysine degradation”, “Pantothenate and CoA biosynthesis”, “Choline metabolism in cancer” and “Glycerophospholipid metabolism”; among these, the “Pantothenate and CoA biosynthesis” pathway was significantly upregulated in the HF buffaloes (p < 0.05, DA score > 0.1) (Figure 6E).
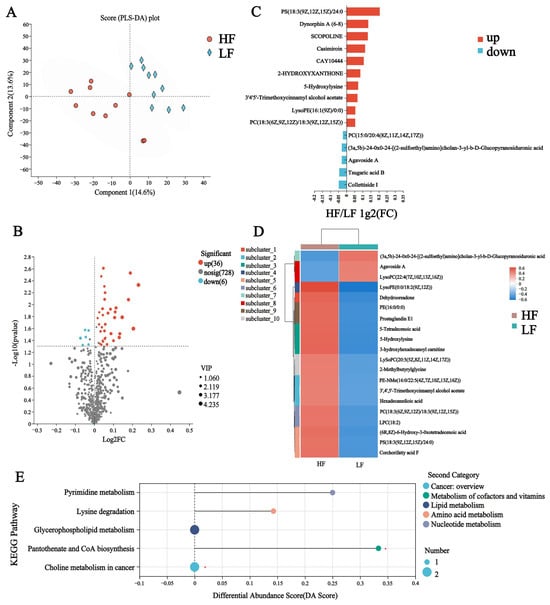
Figure 6.
Plasma metabolome of HF and LF buffaloes. (A) PLS-DA analysis of plasma metabolome. (B) Volcanogram of plasma differential metabolome. (C) Comparison of plasma metabolome between HF and LF buffaloes visualized. (D) Cluster analysis of differential plasma metabolites. On the left is a dendrogram of metabolite clustering, on the right is the name of the metabolite, and the closer two metabolite branches are to each other, the closer they are in terms of expression. (E) Differential abundance score map of KEGG pathway of plasma metabolome. A positive DA score indicates that the expression trend of all annotated differential metabolites in the pathway was upregulated in the HF group; the dots are distributed to the right of the center axis, and the longer the line segment, the more the overall expression of the pathway tends to be upregulated. * means p < 0.05.
3.6. Relationships Between the Rumen Microbiome, Rumen Metabolome and Plasma Metabolome
Spearman’s rank correlations between the rumen microbiota and rumen metabolites were assessed. The results showed that Quinella, Fretibacterium, Selenomonas, Cyrenella, Stilbella and Candida were significantly enriched in HF buffaloes and showed a significant positive correlation with rumen metabolites such as lucidenic acid J, secoeremopetasitolide B and erinacine P (p < 0.05). Meanwhile, they were negatively correlated with organic compounds such as 3-amino-2-naphthoic acid, N-acetyl-b-glucosaminylamine, and flazine (p < 0.05) (Figure 7A,B). Similarly, Spearman’s rank correlations between the rumen microbiota and plasma metabolites showed that Quinella, Fretibacterium and Selenomonas were significantly enriched in HF buffaloes and showed significant positive correlation with plasma metabolites such as corchorifatty acid F, PC (18:3 (6Z, 9Z, 12Z)/18:3(9Z, 12Z, 15Z)), hexadecanedioic acid, LysoPE (0:0/18:2 (9Z, 12Z)) and 5-tetradecenoic acid (p < 0.05). The rumen fungi such as Cyrenella, Stilbella and Beauveria showed significant positive correlations with dehydroadoreone, 3′,4′,5′-trimethoxycinnamyl alcohol acetate and prostaglandin E1. Meanwhile, Candida, Phaeosphaeriopsis and Microsphaeropsis showed significant negative correlations with LysoPC (22:4 (7Z, 10Z, 13Z, 16Z)) (Figure 7C,D). Spearman’s rank correlations between the differential rumen microbiota and rumen-specific metabolites were performed. The results showed that Quinella, Fretibacterium and Selenomonas were significantly enriched in the HF group and showed significant positive correlations with metabolites such as Bisnorbiotin, PE (15:0/16:0), LPC (18:1), and isovalerylglutamic acid (p < 0.05). Then, Paeniclostridium, Lachnoclostridium, and Monogobus were significantly and negatively correlated with isovalerylglutamic acid (p < 0.05) (Figures S4 and S5).
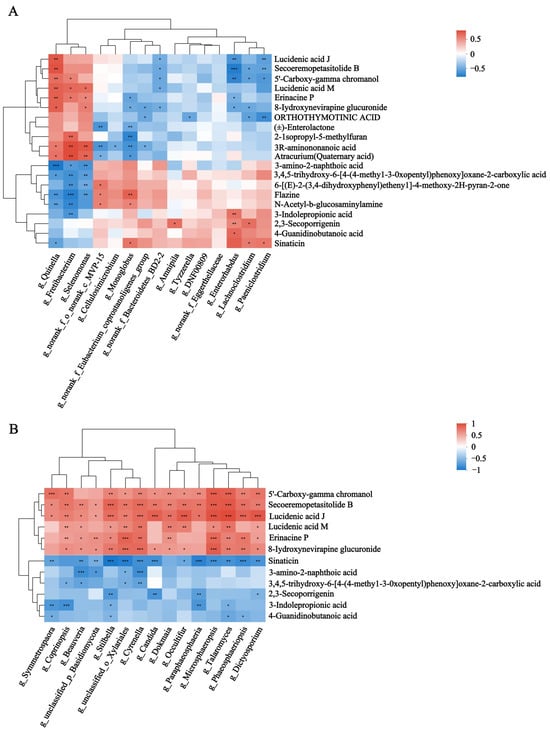
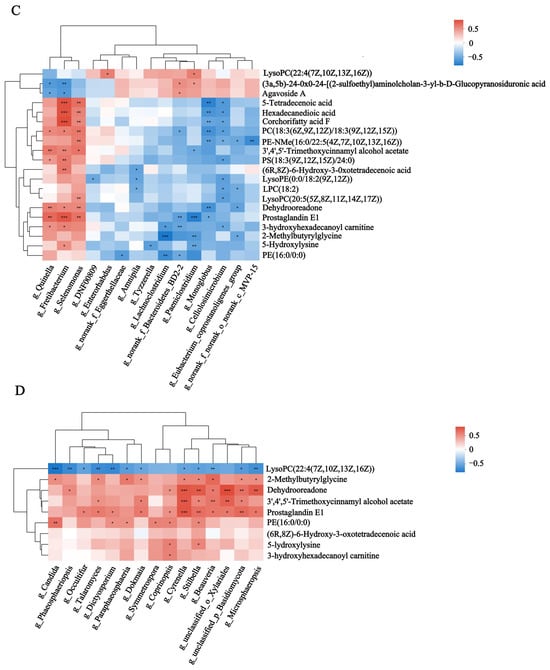
Figure 7.
Spearman’s rank correlations between the rumen microbiota and metabolites. (A) Differential bacteria and rumen differential metabolites. (B) Differential fungi and rumen differential metabolites. (C) Differential bacteria and plasma differential metabolites. (D) Differential fungi and plasma differential metabolites. * means p < 0.05, ** means p ≤ 0.01, *** means p ≤ 0.001.
To identify the rumen microbiota–metabolic interactions, Spearman’s rank correlations between the rumen microbiota, rumen and plasma metabolites were performed (R > 0.7/R < –0.7, p < 0.05). Among these correlations, Quienlla, Stilbella, Cyrenella, Microsphaeropsis, and Paraphaeosphaeria showed correlations with rumen and plasma metabolites, which included lucidenic acid J, N-acetyl-b-glucosaminylamine, flazine, corchorifatty acid F, LysoPE (16:1 (9Z)/0:0), hexadecanedioic acid, isovalerylglutamic acid, PC (18:3 (6Z, 9Z, 12Z)/18:3 (9Z, 12Z, 15Z)), and other metabolites (Figure 8).
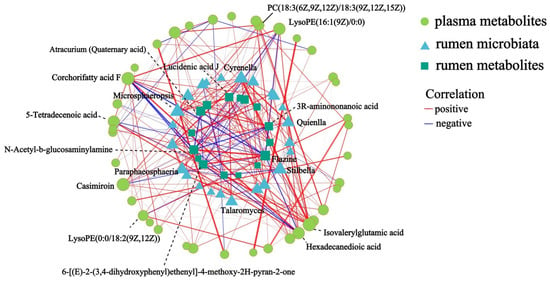
Figure 8.
Network analysis of rumen microbiota, rumen metabolites and plasma metabolites (R > 0.7/R < −0.7, p < 0.05). The size of the node in the graph indicates the degree size of the node, different colors indicate different species; the color of the connecting line indicates positive and negative correlation, red indicates positive correlation, blue indicates negative correlation; the thickness of the line indicates the size of the correlation coefficient, the thicker the line, the higher the correlation between the species; the shorter the line, the closer the connection between the node.
4. Discussion
The dominant rumen microbiota plays an important role in the lactation performance of the host. Current studies have shown that the dominant phyla of cows’ rumen microorganisms were Bacteroidetes, Firmicutes and Proteobacteria [], which is consistent with our study using 16S rDNA gene amplicon sequencing. Among these, Bacteroidetes mainly decompose non-fiber carbohydrates, while Firmicutes mainly decompose fibers, and there was a strong positive correlation between the milk fat yield and the proportion of Firmicute and Bacteroidetes []. The proportions of the Firmicutes and Bacteroidetes in the rumen of high yielding cows were significantly higher compared with low yielding cows []. Meanwhile, changes in diet or feeding preferences can also lead to changes in rumen microbiota, which in turn affect lactation performance. Exploring the mechanisms of rumen microbial metabolism of carbohydrates, proteins and sugars can be targeted to regulate rumen microbial fermentation and promote the production of milk component precursors through diets.
The relative abundance of some rumen microbiota has been found to have an effect on milk yield and milk fat content []. Furthermore, some studies have shown that rumen bacterial abundance was correlated with milk fat or protein in dairy cows [,]. In this study, the abundance of Quinella, Selenomonas and Fretibacterium was significantly higher in the HF buffaloes at the genus level. Quinella and Selenomonas all belong to the phylum Firmicutes [,]. A higher relative ruminal propionate concentration was also found by Kittelmann et al. (2014) in sheep with elevated populations of Quinella; it was hypothesized that Quinella would be conducive to lower methane production, and earlier studies have also supported that [,]. Further research found that Quinella genomic bins contained genes that code for pyruvate: ferredoxin oxidoreductase, which converts pyruvate to acetyl-CoA, and Quinella may be able to utilize cellulose and cellulodextrins released by other rumen microbiota using β-glucosidase []. In addition, β-xylosidase from Selenomonas has high β-xylosidase activity []. There are also studies that have found Schwartzia is a genus in the Firmicutes, which was more abundant in cows with higher milk production []. Li et al. (2020) found that Lachnoclostridium and Lachnospiraceae UCG-006 could inhibit short-chain fatty acid producing bacteria and thus inhibit the synthesis of milk fat, which was also confirmed by the significant enrichment of Lachnoclostridium in LF buffaloes in this study []. Milk fat contents have also been correlated with the abundance of Dialister, Megasphaera, Lachnospira, and Sharpea in the rumen []. Then, another study has shown that the proportion of Prevotella in the Bacteroidetes and the milk fat yield were significantly negatively correlated [,]. The relative abundance of rumen Prevotella increased significantly when cows developed low milk syndrome, while the relative abundance of unclassified_Lachnospiraceae, unclassified_Veillonellaceae, and Pseudobutyri-vibrio decreased significantly []. Additionally, plant cell wall polysaccharide-degrading enzymes expressed by rumen microbiota were key in regulating the production of milk fat synthesis precursors, suggesting that HF buffaloes may be producing more milk fat synthesis precursors such as volatile fatty acids. Notably, fewer studies have been conducted to correlate the effects of rumen fungi on milk fat synthesis, but that does not mean it is not important. In this study, the abundance of 14 rumen fungi, including Candida, Talaromyces, Cyrenella and Stilbella, was significantly higher in the rumen of HF buffaloes. This suggested that rumen fungi similarly play an important role in milk fat anabolism, which may require further investigation in the future. With the continuous development of sequencing technology, researchers have studied the diversity of rumen microorganisms and their enzyme genes using techniques such as macrogenomics, macrotranscriptomics, and macroproteomics. It opens the way to the discovery of more functional microorganisms and enzyme genes in the future, but there are still a large number of unexploited microorganisms in the rumen, and their isolation and expression in engineered bacteria will be promising for improving milk fat synthesis.
Alterations in the gastrointestinal microbiota affect metabolites [,] as well as metabolic pathways in dairy cows []. Ruminal microorganisms produced a large number of small-molecule metabolites during digestion in dairy cows, and these metabolites not only play a key role in colony messaging but also alter the metabolic state of their hosts [,]. Rumen metabolomics studies have shown that phospholipids, amino acids, fatty acids, carbohydrates, cholesterol esters and glycerides are the major metabolites in the rumen []. In the present study, it was found that the major differential metabolites in the rumen of high milk fat cows were lipid and lipid-like molecules such as triglycerides and phosphatidylethanolamine, which is consistent with the results of previous studies []. The synergistic effect of rumen microbes and their metabolites in dairy cows can increase the gene expression of hemicellulases, lipid synthases and transferase enzymes, which in turn increases the rumen’s ability to break down nutrients from the ration and ultimately promotes an increase in milk fat content []. Lactobacilli, Ruminalococcus and Clostridia in the phylum Firmicutes have bile salt hydrolase activity, which produces short fatty acids, lactate and antimicrobial substances involved in the regulation of traits such as lactation organisms and immune responses [,]. Another study found that bacteria of the Prevotella and Succinomonas in the rumen of dairy cows could affect the concentration of glutathione and phenylalanine in the rumen, which in turn affected the concentration of amino acids, such as glycine, serine, and threonine, in the serum of dairy cows, and regulated the production of milk lipids in cows []. It has been found that bacterial metabolites of Bacteroidetes could regulate the gluconeogenesis pathway by modulating bile acids, i.e., promoting cholesterol efflux through the dissociation of bile salts and thus regulating the concentration of triacylglycerol in the blood [,]. In this study, it was found that Synergistota was significantly enriched in the HF group. Bacteroidetes mainly decomposes non-fiber carbohydrates, which in turn increases the concentration of acetic acid in the rumen and promotes the synthesis of milk fat, which may be one of the reasons for the high milk fat content in the HF group, and the specific mechanism needs to be further explored. Eubacterium_xylanophium_group and Clostridium and Ruminalococcus play an important role in the fermentation of cellulose-rich feeds and resistant starch and can produce acetic and butyric acids to increase the production of volatile fatty acids, which in turn promotes the synthesis of milk fat [,]. Furthermore, some of the lipopolysaccharides produced by rumen microorganisms can damage the blood–milk barrier and then enter the mammary epithelial cells of dairy cows through the blood circulation to cause mastitis, leading to a decrease in milk fat []. Therefore, rumen metabolites may affect milk lipogenesis through immune responses and other metabolic pathways. However, milk fat synthesis is a complex process, and the molecular mechanisms of regulation need to be further investigated.
Changes in gastrointestinal microorganisms largely affect the metabolites in the blood []. Blood metabolome analysis showed that the high-fat content group enriched in carbohydrates had much higher levels of citric and succinic acids in their serum than the low-fat group, confirming that the tricarboxylic acid cycle is more active in the high-fat group, and pyruvic acid can be converted to acetyl coenzyme A by oxidative decarboxylation []. Acetyl coenzyme A can participate in the tricarboxylic acid cycle to produce adenosine triphosphate (ATP) to meet the higher energy requirements of cows with high milk fat content []. Plasma concentrations of phosphatidylcholine were found to be higher in cows with a high milk fat content than in cows with a low milk fat content, which were absorbed by the mammary gland for triglyceride synthesis []. Lipid-related metabolites were increased in the group of cows with high milk fat content, whereas amino acid metabolism was enhanced and fatty acid metabolism was blocked in cows under conditions of reduced milk fat []. In the present study, lipid and lipid-like molecules such as LysoPE (0:0/18:2 (9Z, 12Z)) were found to be significantly upregulated in the HF group. Yamamoto et al. found that lysoPE 18:2 could be involved in the formation of lipid droplets and increased the milk fat content []. It has recently been shown that cows at peak lactation have higher LysoPE (16:0) in the rumen, which increases phospholipid levels in milk []. Metabolites that differed between groups in plasma samples from cows with different milk fat content were mainly enriched in the choline metabolism, glycerophospholipid metabolism, and amino acid metabolism [], which is consistent with the results of this study. On the other hand, milk fat content was positively correlated with the lipopolysaccharide biosynthesis pathway, amino acid biosynthesis pathway, valine biosynthesis pathway, leucine biosynthesis pathway, and isoleucine biosynthesis pathway []. Meanwhile, the relationship between milk fat content and microorganisms and metabolites is complex and diverse. Our current study demonstrates a positive correlation between specific microbiota and both rumen metabolites and plasma metabolites. The rumen microbiota such as Quienlla, Stilbella, Cyrenella, Microsphaeropsis, and Paraphaeosphaeria showed correlations with rumen and plasma metabolites, including lucidenic acid J, N-acetyl-b-glucosaminylamine, flazine, corchorifatty acid F, and LysoPE (16:1 (9Z)/0:0). Spearman’s correlation results showed that the metabolites that positively correlated with milk fat content were mainly lipids and organic acids, suggesting that these metabolites provide more milk fat precursors and metabolic energy to the mammary glands of the HF buffaloes via the bloodstream. Additionally, the phenotype-associated metabolites in both the rumen and serum were also significantly correlated with the rumen microbiota, suggesting a responsive relationship between these metabolites and the rumen microbiota []. In summary, the composition of rumen microbiota affected both microbial and host metabolism, which, in turn, impacted the host milk fat production traits. However, milk fat synthesis is the result of a concerted effort between gastrointestinal microbes and the organism. Not only do rumen microbes and host metabolites have an impact on milk lipid synthesis, but mammary lactation genes and signaling pathways also play a key role in the regulation of lipid synthesis. Therefore, it is necessary to further investigate the regulatory mechanism of milk fat synthesis with the help of genomics and the transcriptome, which is of great scientific significance, as it can help make full use of the lactation potential of buffaloes and produce high-quality milk.
5. Conclusions
There are differences in the rumen microbial composition and host metabolites of Bingangjiang buffaloes with high and low milk fat content. These changes of microbiota may lead to alterations in fatty acids biosynthesis precursors in the rumen and plasma, and it ultimately affected milk fat content. Our findings associated Quienlla, Stilbella, Cyrenella, Microsphaeropsis and Paraphaeosphaeria with the rumen metabolites lucidenic acid J, secoeremopetasitolide B and erinacine P and the plasma metabolites corchorifatty acid F and PC (18:3 (6Z, 9Z, 12Z)/18:3 (9Z, 12Z, 15Z)), which may be key contributors to high milk fat content in HF buffaloes. The role of these microorganisms can be further validated in the future. Moreover, the mechanism of formation of buffalo milk fat content at the organizational and cellular levels requires further investigation.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ani15020248/s1, Table S1: Feed ingredients of the buffaloes; Table S2: Concentrate mixture of the buffaloes; Table S3: The concentration of VFAs in rumen fluid of HF and LF buffaloes; Table S4: Summary of bacterial sequence data generated from rumen samples of HF and LF buffaloes; Table S5: Summary of fungal sequence data generated from rumen samples of HF and LF buffaloes; Table S6: Rumen differential metabolite information; Table S7: Plasma differential metabolite information; Figure S1: Histogram of milk fat content of 75 buffaloes; Figure S2: Rumen metabolites Venn chart; Figure S3: Plasma metabolites Venn chart; Figure S4: Correlation between rumen microbiota and rumen metabolites. Correlation between rumen bacteria and rumen metabolites. Correlation between rumen fungi and rumen metabolites; Figure S5: Correlation between rumen microbiota and plasma metabolites. (A) Correlation between rumen bacteria and plasma metabolites. (B) Correlation between rumen fungi and plasma metabolites. * means p < 0.05, ** means p ≤ 0.01, *** means p ≤ 0.001.
Author Contributions
Y.Y.: conceptualization, methodology, data curation, formal analysis, investigation, and writing—original draft. R.F.: methodology, formal analysis and writing—original draft. C.J.: methodology and writing—reviewing and editing. L.H.: formal analysis, methodology, and writing—reviewing. H.G.: data curation, formal analysis, investigation. B.F.: methodology and writing—reviewing and editing. M.Q.: formal analysis and methodology. Q.L.: data curation. J.L.: conceptualization, methodology, investigation, supervision, project administration, funding acquisition, validation, and writing—reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the projects of the “Yunnan Revitalization Talent Support Program”, China; the National Natural Science Foundation of China (32260841, U2202203); Key Project of Basic Research Program of Science and Technology Department of Yunnan Province (202201AS070063) and Yunnan Provincial Department of Education project (2024Y308, FWCY-BSPY2024087).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care Committee at the Faculty of Animal Science and Technology, Yunnan Agricultural University (Kunming, P. R. China, approval No.: YNAU202205020).
Informed Consent Statement
The study did not involve human trials.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: NCBI–PRJNA1205516.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gernot, O.; Arno, H.; Maryna de, W.; Mai Nguyen, T.P. The chemical composition of milk from free-ranging African buffalo (Syncerus caffer). S. Afr. J. Wildl. Res. 2009, 9, 97–102. [Google Scholar]
- Spanghero, M.; Susmel, P. Chemical composition and energy content of buffalo milk. J. Dairy Res. 1996, 63, 629–633. [Google Scholar] [CrossRef]
- Vargas-Bello-Pérez, E.; Loor, J.J.; Garnsworthy, P.C. Effect of different exogenous fatty acids on the cytosolic triacyl-glycerol content in bovine mammary cells. Anim. Nutr. 2019, 5, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Ramella, M.; Pateiro, M.; Maggiolino, A.; Faccia, M.; Franco, D.; De Palo, P.; Lorenzo, J.M. Buffalo Milk as a Source of Probiotic Functional Products. Microorganisms 2021, 9, 2303. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Pan, B.; Ahmad, M.J.; Zhang, X.; Chen, C.; Yao, Z.; Lv, H.; Wei, K.; Yang, L. Summer Buffalo Milk Produced in China: A Desirable Diet Enriched in Polyunsaturated Fatty Acids and Amino Acids. Foods 2022, 11, 3475. [Google Scholar] [CrossRef] [PubMed]
- Tishkoff, S.A.; Reed, F.A.; Ranciaro, A.; Voight, B.F.; Babbitt, C.C.; Silverman, J.S.; Powell, K.; Mortensen, H.M.; Hirbo, J.B.; Osman, M.; et al. Convergent adaptation of human lactase persistence in Africa and Europe. Nat. Genet. 2007, 39, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Xie, X.; Xue, M.; Valencak, T.G.; Liu, J.; Sun, H. The Effects of Non-Fiber Carbohydrate Content and Forage Type on Rumen Microbiome of Dairy Cows. Animals 2021, 11, 3519. [Google Scholar] [CrossRef]
- Zhao, W.; Abdelsattar, M.M.; Wang, X.; Zhang, N.; Chai, J. In Vitro Modulation of Rumen Fermentation by Microbiota from the Recombination of Rumen Fluid and Solid Phases. Microbiol. Spectr. 2023, 11, e0338722. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Y.; Huang, G.; Zheng, N.; Zhao, S.; Wang, J. Ruminal bacterial community is associated with the vari-ations of total milk solid content in Holstein lactating cows. Anim. Nutr. 2022, 9, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Jakaitis, B.M.; Denning, P.W. Human breast milk and the gastrointestinal innate immune system. Clin. Perinat. 2014, 41, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, C.; Guo, G.; Huo, W.J.; Zhang, S.L.; Pei, C.X.; Zhang, Y.L.; Wang, H. Effects of branched-chain volatile fatty acids on lactation performance and mRNA expression of genes related to fatty acid synthesis in mammary gland of dairy cows. Animal 2018, 12, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, P.; Chen, F.; Zhou, J.; Guo, A.; Shi, K.; Zhang, Q. Multi-omics analyses reveal that the gut microbiome and its me-tabolites promote milk fat synthesis in Zhongdian yak cows. PeerJ 2022, 10, e14444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Y.; Liu, Y.J.; Yin, Y.Y.; Jin, W.; Mao, S.Y.; Liu, J.H. Response of rumen microbiota, and metabolic profiles of rumen fluid, liver and serum of goats to high-grain diets. Animal 2019, 13, 1855–1864. [Google Scholar] [CrossRef]
- Krempaský, M.; Maskaľová, I.; Bujňák, L.; Vladimír, V. Ketone bodies in blood of dairy cows: Prevalence and monitoring of sub-clinical ketosis. Acta Vet. Brno 2014, 83, 411–416. [Google Scholar] [CrossRef]
- Soga, T.; Baran, R.; Suematsu, M.; Ueno, Y.; Ikeda, S.; Sakurakawa, T.; Kakazu, Y.; Ishikawa, T.; Robert, M.; Nishioka, T.; et al. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J. Biol. Chem. 2006, 281, 16768–16776. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Z.; Wang, D.M.; Wang, B.; Wang, J.K.; Liu, H.Y.; Guan, L.L.; Liu, J.X. Metabolomics of four biofluids from dairy cows: Potential biomarkers for milk production and quality. J. Proteome Res. 2015, 14, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ogunade, I.M.; Arriola, K.G.; Qi, M.; Vyas, D.; Staples, C.R.; Adesogan, A.T. Effects of the dose and viability of Sac-charomyces cerevisiae. 2. Ruminal fermentation, performance of lactating dairy cows, and correlations between ruminal bac-teria abundance and performance measures. J. Dairy Sci. 2017, 100, 8102–8118. [Google Scholar] [CrossRef] [PubMed]
- Pineda, P.S.; Flores, E.B.; Herrera, J.R.V.; Low, W.Y. Opportunities and Challenges for Improving the Productivity of Swamp Buffaloes in Southeastern Asia. Front. Genet. 2021, 12, 629861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zhang, Y.; Wang, S.Q.; Miao, Y.W.; Liu, X.H.; Yin, K.L.; Duan, D.R.; Liu, D.J.; Li, W.Z. Comparative Analysis of Milk Compositions of Binglangjiang Buffalo, Murrah Buffalo and Holstein Cow. China Dairy Cattle 2012, 7, 24–26. (In Chinese) [Google Scholar]
- Hu, W.L.; Liu, J.X.; Ye, J.A.; Wu, Y.M.; Guo, Y.Q. Effect of tea saponin on rumen fermentation in vitro. Anim. Feed. Sci. Technol. 2005, 120, 333–339. [Google Scholar] [CrossRef]
- Yu, Y.; Zi, Y.J.; Fu, R.Q.; Fu, B.L.; Li, C.H.; Lv, Y.Q.; Li, Z.; Wang, H.Y.; Leng, J. Effects of dietary energy levels on microorganisms and short-chain fatty acids of rumen and tight junction proteins in Honghe Yellow cattle. Front. Microbiol. 2024, 15, 1335818. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Env. Microb. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Xue, Y.; Seddik, H.E.; Yin, Y.; Hu, F.; Mao, S. Dynamic changes of plasma metabolome in response to severe feed restriction in pregnant ewes. Metabolites 2019, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Indugu, N.; Vecchiarelli, B.; Baker, L.D.; Ferguson, J.D.; Vanamala, J.K.P.; Pitta, D.W. Comparison of rumen bacterial com-munities in dairy herds of different production. BMC Microbiol. 2017, 17, 190. [Google Scholar] [CrossRef]
- Jami, E.; White, B.A.; Mizrahi, I. Potential role of the bovine rumen microbiome in modulating milk composition and feed effi-ciency. PLoS ONE 2014, 9, e85423. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.Y.; Sun, H.Z.; Wu, X.H.; Liu, J.X.; Guan, L.L. Multi-omics reveals that the rumen microbiome and its metab-olome to-gether with the host metabolome contribute to individualized dairy cow performance. Microbiome 2020, 8, 64. [Google Scholar] [CrossRef]
- Sun, P.; Wang, J.Q.; Deng, L.F. Effects of Bacillus subtilis natto on milk production, rumen fermentation and ruminal microbi-ome of dairy cows. Animal 2013, 7, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.X.; Qin, C.B.; Xu, Y.X.; Song, X.H.; Fu, Y.H.; Li, R.J.; Liu, Q.Y.; Shi, D.S. Multi-omics reveals the mechanism of rumen microbiome and its metabolome together with host metabolome participating in the regulation of milk production traits in dairy buffaloes. Front. Microbiol. 2024, 15, 1301292. [Google Scholar] [CrossRef] [PubMed]
- Marchandin, H.; Teyssier, C.; Campos, J.; Jean-Pierre, H.; Roger, F.; Gay, B.; Carlier, J.P.; Jumas-Bilak, E. Negativicoccus suc-cinicivorans gen. nov.; sp. nov.; isolated from human clinical samples, emended description of the family Veillonel-laceae and description of Negativicutes classis nov.; Selenomonadales ord. nov. and Acidaminococcaceae fam. nov. in the bacterial phy-lum Firmicutes. Int. J. Syst. Evol. Microbiol. 2010, 60, 1271–1279. [Google Scholar]
- Kojima, S.; Kamio, Y. Molecular basis for the maintenance of envelope integrity in Selenomonas ruminantium: Cadaverine biosyn-thesis and covalent modification into the peptidoglycan play a major role. J. Nutr. Sci. Vitaminol. 2012, 58, 153–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kittelmann, S.; Pinares-Patiño, C.S.; Seedorf, H.; Kirk, M.R.; Ganesh, S.; McEwan, J.C.; Janssen, P.H. Two different bacterial community types are linked with the low-methane emission trait in sheep. PLoS ONE 2014, 9, e103171. [Google Scholar] [CrossRef] [PubMed]
- Vicini, J.L.; Brulla, W.J.; Davis, C.L.; Bryant, M.P. Quin’s oval and other microbiota in the rumens of molasses-fed sheep. Appl. Env. Microbiol. 1987, 53, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Altermann, E.; Leahy, S.C.; Jauregui, R.; Jonker, A.; Henderson, G.; Kittelmann, S.; Attwood, G.T.; Kamke, J.; Waters, S.M.; et al. Genomic insights into the physiology of Quinella, an iconic uncultured rumen bacte-rium. Nat. Commun. 2022, 13, 6240. [Google Scholar] [CrossRef]
- Jordan, D.B. Beta-D-xylosidase from Selenomonas ruminantium: Catalyzed reactions with natural and artificial substrates. Appl. Biochem. Biotechnol. 2008, 146, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, F.; Lu, J.J.; Shi, J.L.; Guan, J.Q.; Yan, F.F.; Li, B.L.; Huo, G.C. Probiotic Mixture of Lactobacillus plantarum Strains Improves Lipid Metabolism and Gut Microbiota Structure in High Fat Diet-Fed Mice. Front. Microbiol. 2020, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Pitta, D.W.; Indugu, N.; Vecchiarelli, B.; Hennessy, M.; Baldin, M.; Harvatine, K.J. Effect of 2-hydroxy-4-(methylthio) butanoate (HMTBa) supplementation on rumen bacterial populations in dairy cows when exposed to diets with risk for milk fat depres-sion. J. Dairy Sci. 2020, 103, 2718–2730. [Google Scholar] [CrossRef]
- Hassan, F.U.; Ebeid, H.M.; Tang, Z.H.; Li, M.W.; Peng, L.J.; Peng, K.P.; Liang, X.; Yang, C.J. A mixed mhytogenic modulates the rumen bacteria composition and milk fatty acid profile of water buffaloes. Front. Vet. Sci. 2020, 7, 569. [Google Scholar] [CrossRef]
- Zeng, H.; Guo, C.; Sun, D.; Seddik, H.E.; Mao, S.Y. The Ruminal Microbiome and Metabolome Alterations Associated with Di-et-Induced Milk Fat Depression in Dairy Cows. Metabolites 2019, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, F.J.; Doherty, M.L. Production diseases of the transition cow. Vet. J. 2008, 176, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, H.; Lu, C.; Kang, Z.; Luo, Y.; Feng, L.; Lu, X. Fermented milk supplemented with probiotics and prebiotics can effectively alter the intestinal microbiota and immunity of host animals. J. Dairy Sci. 2012, 95, 4813–4822. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.S.; Oikonomou, G.; Lima, S.F.; Bicalho, M.L.; Ganda, E.K.; Filho, J.C.; Lorenzo, G.; Trojacanec, P.; Bicalhoa, R.C. Prepartum and postpartum rumen fluid microbiomes: Characterization and correlation with production traits in dairy cows. Appl. Environ. Microb. 2015, 81, 1327–1337. [Google Scholar] [CrossRef]
- Luu, M.; Visekruna, A. Microbial metabolites: Novel therapeutic tools for boosting cancer therapies. Trends Cell Biol. 2021, 31, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Tardón, D.C.; Hoffmann, C.; Santos, F.C.R.; Decaris, N.; Pinheiro, F.A.; Queiroz, L.L.; Hurley, D.J.; Gomes, V. Relationships among Indicators of Metabolism, Mammary Health and the Microbiomes of Periparturient Holstein Cows. Animals 2021, 12, 3. [Google Scholar] [CrossRef]
- Xue, M.Y.; Sun, H.Z.; Wu, X.H.; Guan, L.L.; Liu, J.X. Assessment of rumen bacteria in dairy cows with varied milk protein yield. J. Dairy Sci. 2019, 102, 5031–5041. [Google Scholar] [CrossRef]
- Hu, H.; Shao, W.; Liu, Q.; Liu, N.; Wang, Q.; Xu, J.; Zhang, X.; Weng, Z.; Lu, Q.; Jiao, L.; et al. Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nat. Commun. 2022, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Cholewińska, P.; Wołoszyńska, M.; Michalak, M.; Czyż, K.; Rant, W.; Smoliński, J.; Wyrostek, A.; Wojnarowski, K. Influence of selected factors on the Firmicutes, Bacteroidetes phyla and the Lactobacillaceae family in the digestive tract of sheep. Sci. Rep. 2021, 11, 23801. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.; Barbosa-da-Silva, S.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Differences and similarities in hepatic lipogenesis, gluconeogenesis and oxidative imbalance in mice fed diets rich in fructose or sucrose. Food Funct. 2015, 6, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.V.; Abdel-Hamid, A.M.; Dutta, S.; D’Alessandro-Gabazza, C.N.; Wefers, D.; Farris, J.A.; Bajaj, S.; Wawrzak, Z.; Atomi, H.; Mackie, R.I.; et al. Degradation of complex arabinoxylans by human colonic Bacteroidetes. Nat. Commun. 2021, 12, 459. [Google Scholar] [CrossRef]
- Gaffney, J.; Embree, J.; Gilmore, S.; Embree, M. Ruminococcus bovis sp. nov.; a novel species of amylolytic Ruminococcus isolated from the rumen of a dairy cow. Int. J. Syst. Evol. Microbiol. 2021, 71, 004924. [Google Scholar] [CrossRef] [PubMed]
- Maifeld, A.; Bartolomaeus, H.; Löber, U.; Avery, E.G.; Steckhan, N.; Markó, L.; Wilck, N.; Hamad, I.; Šušnjar, U.; Mähler, A.; et al. Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat. Commun. 2021, 12, 1970. [Google Scholar] [CrossRef]
- Hu, X.Y.; Li, S.; Mu, R.Y.; Guo, J.; Zhao, C.J.; Cao, Y.G.; Zhang, N.S.; Fu, Y.H. The Rumen Microbiota Contributes to the De-velopment of Mastitis in Dairy Cows. Microbiol. Spectr. 2022, 10, e0251221. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.J.; Stevenson, D.M.; Mertens, D.R. Shifts in bacterial community composition in the rumen of lactating dairy cows under milk fat-depressing conditions. J. Dairy Sci. 2010, 93, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Ilves, A.; Harzia, H.; Ling, K.; Ots, M.; Soomets, U.; Kilk, K. Alterations in milk and blood metabolomes during the first months of lactation in dairy cows. J. Dairy Sci. 2012, 95, 5788–5797. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Sakurai, T.; Chen, Z.; Inoue, N.; Chiba, H.; Hui, S.P. Lysophosphatidylethanolamine affects lipid ac-cumulation and metabolism in a human liver-derived cell line. Nutrients 2022, 14, 579. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Jiang, X.; Song, Y.; Wang, D.; Liu, H.; Wu, S.; Yao, J. Multiomics analysis revealed that the metabolite profile of raw milk is associated with the lactation stage of dairy cows and could be affected by variations in the ruminal microbiota. J. Dairy Sci. 2024, 107, 8709–8721. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Xu, M.; Gao, K.; Wang, L.; Yang, X.; Wen, X.; Xiao, H.; Li, M.; Jiang, Z. Mammary tissue proteomics in a pig model indicates that dietary valine supplementation increases milk fat content via increased de novo synthesis of fatty acid. Food Sci. Nutr. 2021, 9, 6213–6223. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).