The Grouping Patterns of Cervus canadensis songaricus in the Tianchi Bogda Peak Nature Reserve of Tianshan Mountain, Northwestern China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
- (1)
- Adult males: large-sized normally with branched antlers; when antlers cast in late winter-early spring, there are visible signs of shedding on the head.
- (2)
- Adult females: medium-sized, antlerless and often accompanied by calves.
- (3)
- Yearlings: smaller than adults and, if males, provided with non-branched and slender antlers.
- (1)
- Single female groups: only one adult female.
- (2)
- Single male groups: only one adult male.
- (3)
- Female groups: groups consisting of two or more adult females.
- (4)
- Male groups: groups consisting of two or more adult males.
- (5)
- Mixed groups: groups consisting of females and males.
- (6)
- Mother-kid groups: groups consisting of females and kids, excluding males.
- (7)
- Young groups: containing subadults or calves.
2.3. Data Analysis
3. Results
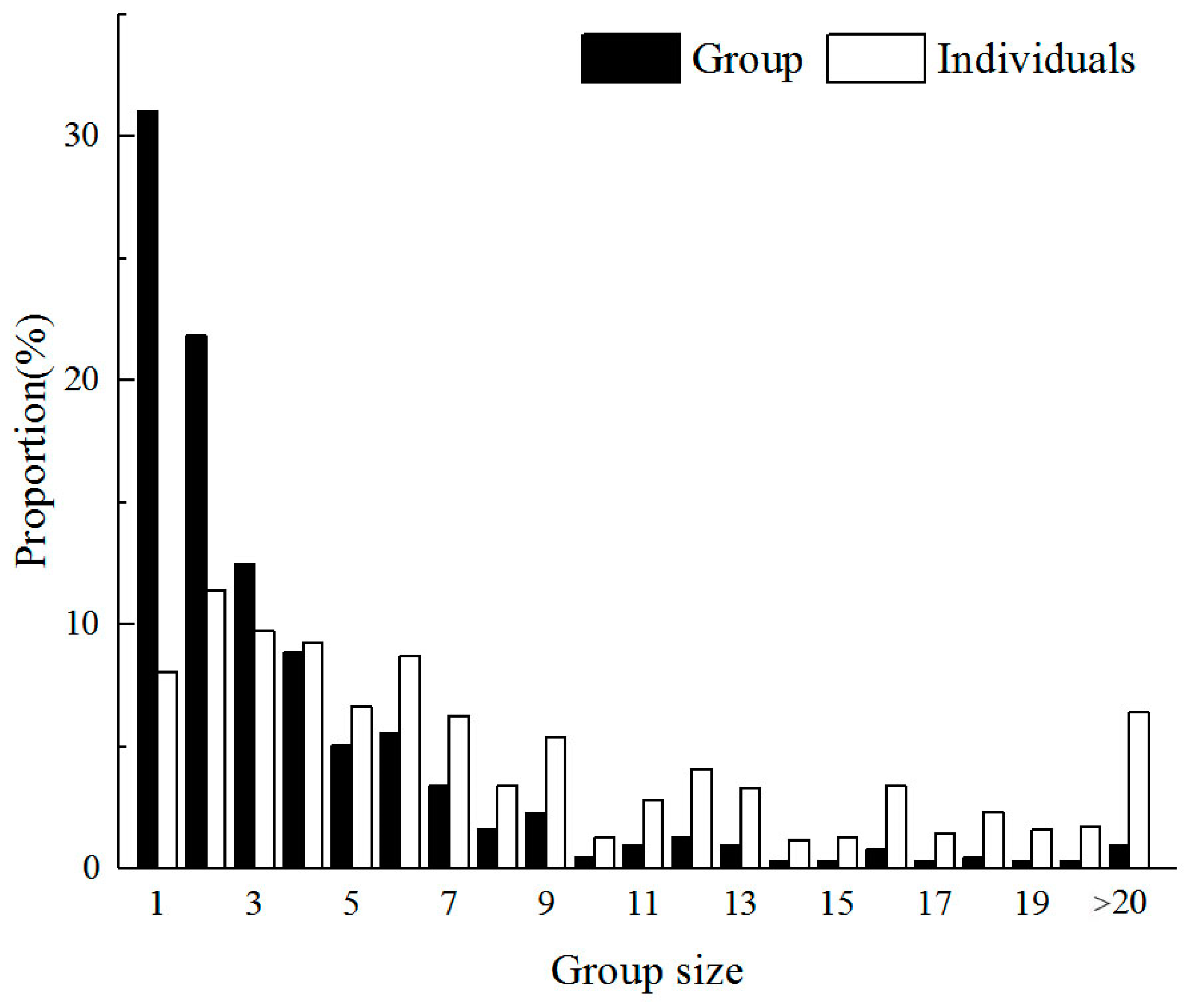
3.1. Overall Group Size Distribution
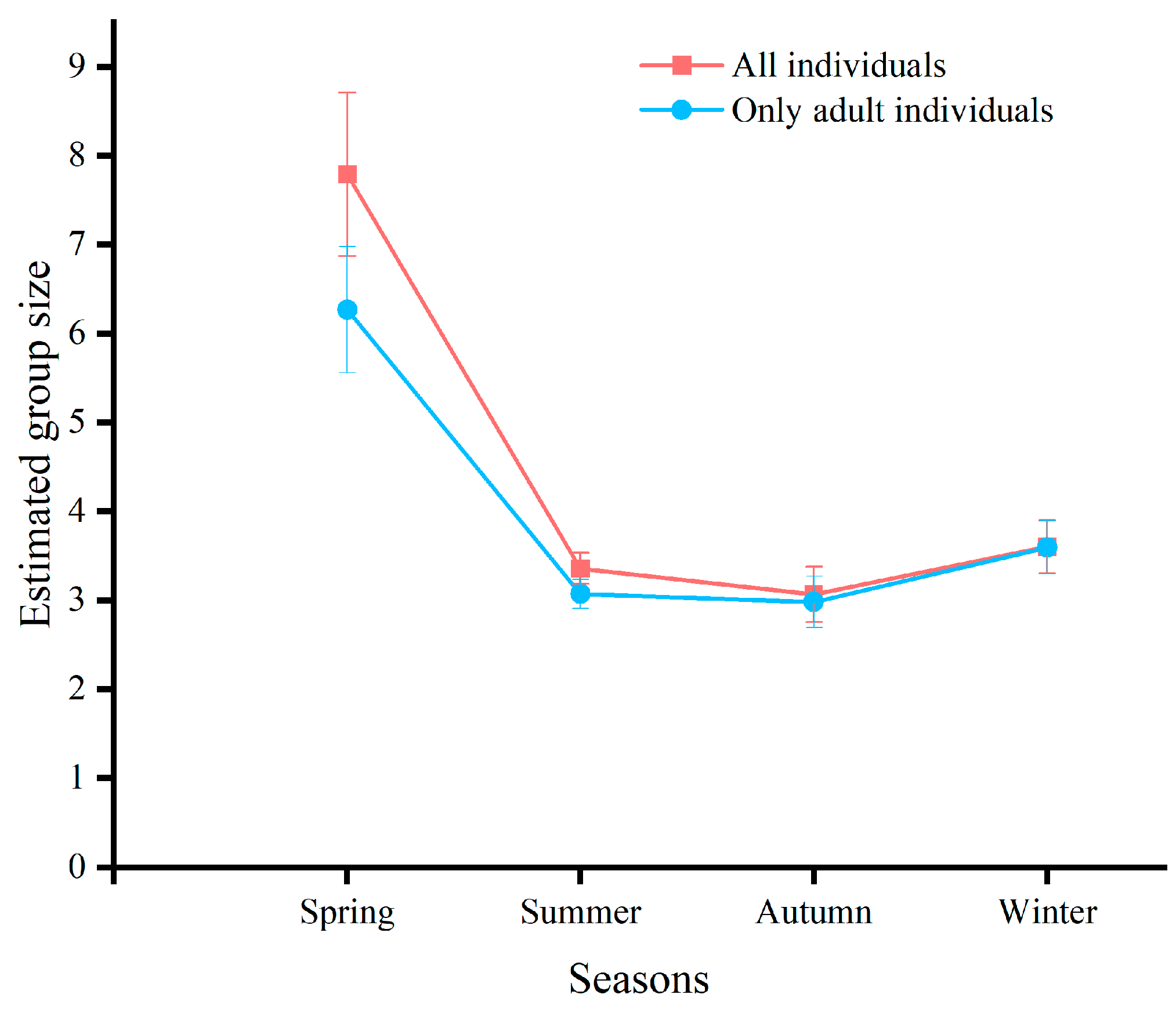
3.2. Seasonal Variability in Group Size
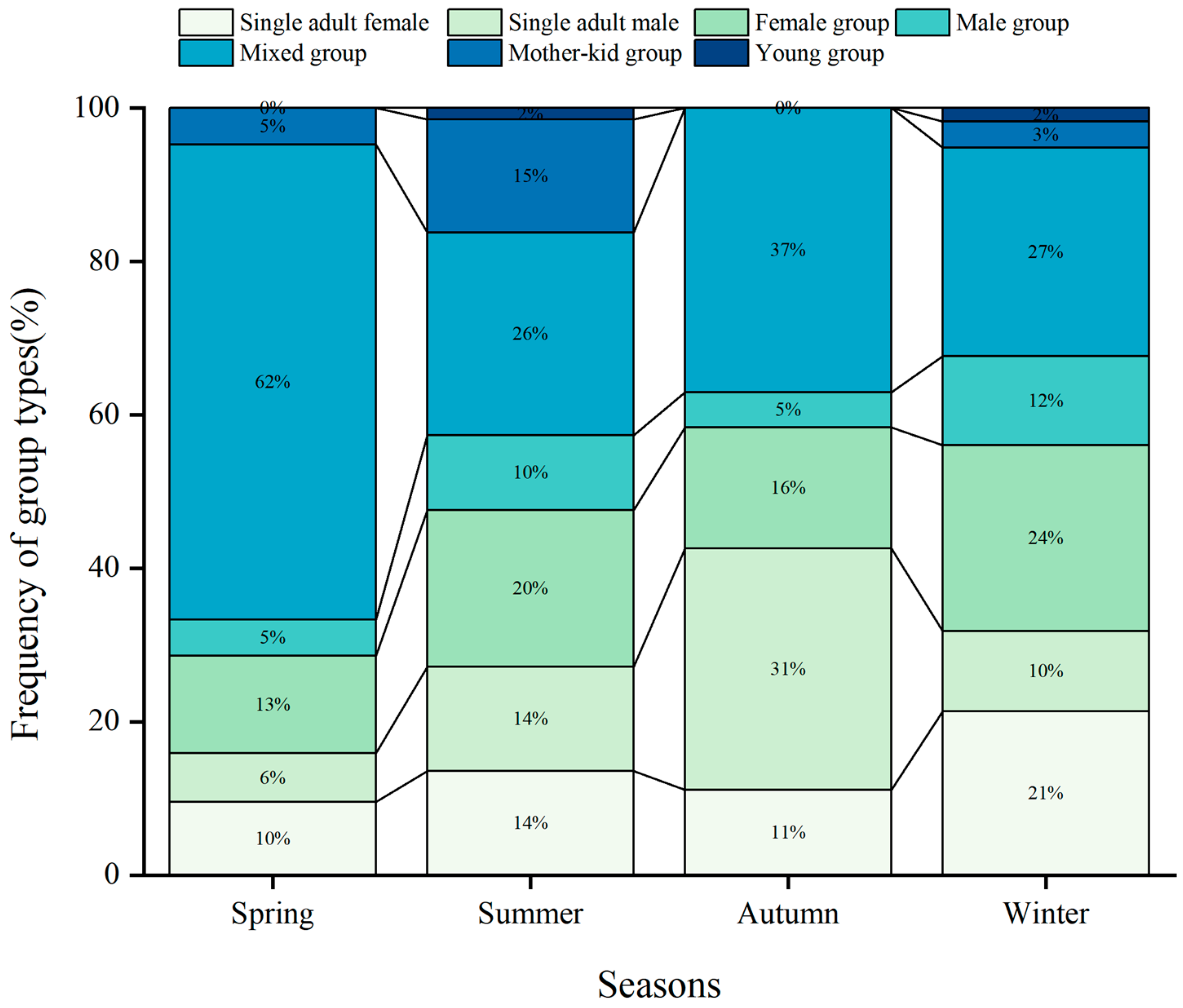
3.3. Seasonal Variability in Frequency of Different Group Types
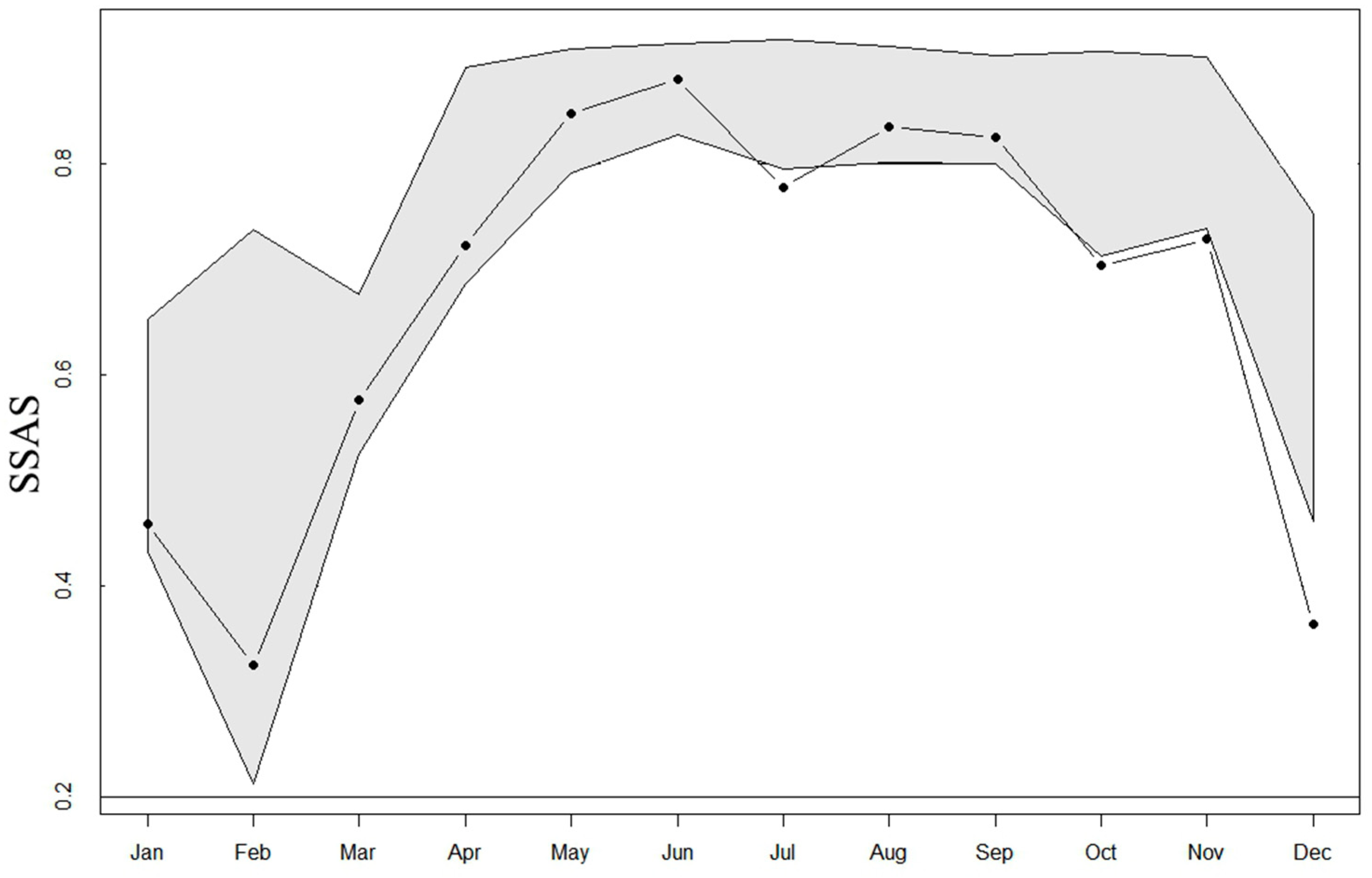
3.4. Sex Segregation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raman, T.R.S. Factors influencing seasonal and monthly changes in the group size of chital or axis deer in southern India. J. Biosci. 1997, 22, 203–218. [Google Scholar] [CrossRef]
- Shi, J.B.; Dunbar, R.I.M.; Buckland, D.; Miller, D. Dynamics of grouping patterns and social segregation in feral goats (Capra hircus) on the Isle of Rum, NW Scotland. Mammalia 2005, 69, 185–199. [Google Scholar] [CrossRef]
- Wang, M.Y.; Alves, J.; da Silva, A.A.; Yang, W.K.; Ruckstuhl, K.E. The effect of male age on patterns of sexual segregation in Siberian ibex. Sci. Rep. 2018, 8, 13095. [Google Scholar] [CrossRef]
- Lagory, K.E. Habitat, group-size, and the behavior of white-tailed deer. Behaviour 1986, 98, 168–179. [Google Scholar] [CrossRef]
- Lima, S.L. Back to the basics of antipredator vigilance—The group-size effect. Anim. Behav. 1995, 49, 11–20. [Google Scholar] [CrossRef]
- Han, L.; Blanks, D.; Wang, M.Y.; Yang, W.K.; Alves Da Silva, A.; Alves, J. Grouping patterns and social organization in Siberian ibex (Capra sibirica): Feeding strategy matters. Folia Zool. 2019, 68, 35–42. [Google Scholar]
- Jarman, P.J. Social-organization of antelope in relation to their ecology. Behaviour 1974, 48, 215–267. [Google Scholar] [CrossRef]
- Gerard, J.F.; Bideau, E.; Maublanc, M.L.; Loisel, P.; Marchal, C. Herd size in large herbivores: Encoded in the individual or emergent? Biol. Bull. 2002, 202, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Pulliam, H.R. Advantages of flocking. Theor. Biol. 1973, 38, 419–422. [Google Scholar] [CrossRef]
- Fryxell, J.M. Forage quality and aggregation by large herbivores. Am. Nat. 1991, 138, 478–498. [Google Scholar] [CrossRef]
- Ruckstuhl, K.; Neuhaus, P. Sexual segregation in ungulates: A new approach. Behaviour 2000, 137, 361–377. [Google Scholar] [CrossRef]
- Yearsley, J.M.; Javier Pérez-Barbería, F. Does the activity budget hypothesis explain sexual segregation in ungulates? Anim. Behav. 2005, 69, 257–267. [Google Scholar] [CrossRef]
- Bowyer, R.T. Sexual Segregation in Ungulates; Johns Hopkins University Press: Baltimore, MD, USA, 2022. [Google Scholar]
- Polziehn, R.O.; Strobeck, C. Phylogeny of Wapiti, Red Deer, Sika Deer, and Other North American Cervids as Determined from Mitochondrial DNA. Mol. Phylogenet. Evol. 1998, 10, 249–258. [Google Scholar] [CrossRef]
- Kuwayama, R.; Ozawa, T. Phylogenetic Relationships among European Red Deer, Wapiti, and Sika Deer Inferred from Mitochondrial DNA Sequences. Mol. Phylogenet. Evol. 2000, 15, 115–123. [Google Scholar] [CrossRef]
- Randi, E.; Pierpaoli, M.; Beaumont, M.; Ragni, B.; Sforzi, A. Genetic Identification of Wild and Domestic Cats (Felis silvestris) and Their Hybrids Using Bayesian Clustering Methods. Mol. Biol. Evol. 2001, 18, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Ludt, C.J.; Schroeder, W.; Rottmann, O.; Kuehn, R. Mitochondrial DNA phylogeography of red deer (Cervus elaphus). Mol. Phylogenet. Evol. 2004, 31, 1064–1083. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, G.; Grassi, C.; Nali, C.; Petiti, A.; Loppi, S.; Tognotti, L. Leaves of Pittosporum tobira as indicators of airborne trace element and PM10 distribution in central Italy. Atmos. Environ. 2006, 40, 4025–4036. [Google Scholar] [CrossRef]
- Heckeberg, N.S. The systematics of the Cervidae: A total evidence approach. PeerJ 2020, 8, e8114. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz, P.; Matosiuk, M.; Świsłocka, M.; Zachos, F.E.; Hajji, G.M.; Saveljev, A.P.; Seryodkin, I.V.; Farahvash, T.; Rezaei, H.R.; Torshizi, R.V.; et al. Phylogeny and evolution of the genus Cervus (Cervidae, Mammalia) as revealed by complete mitochondrial genomes. Sci. Rep. 2022, 12, 16381. [Google Scholar] [CrossRef] [PubMed]
- Lovari, S.; Franceschi, S.; Chiatante, G.; Fattorini, L.; Fattorini, N.; Ferretti, F. Climatic changes and the fate of mountain herbivores. Clim. Change 2020, 162, 2319–2337. [Google Scholar] [CrossRef]
- Brook, S.M.; Thakur, M.; Ranjitsinh, M.K.; Donnithorne-Tait, D.; Ahmad, K. Cervus hanglu ssp. hanglu. The IUCN Red List of Threatened Species. 2017. Available online: https://www.iucnredlist.org/species/113259123/113281791 (accessed on 13 January 2025).
- Clutton-Brock, T.H.; Guinness, F.E.; Albon, S.D. Red Deer: Behavior and Ecology of Two Sexes; University of Chicago Press: Chicago, IL, USA, 1982. [Google Scholar]
- Mahmut, H.; Masuda, R.; Onuma, M.; Takahashi, M.; Nagata, J.; Suzuki, M.; Ohtaishi, N. Molecular phylogeography of the red deer (Cervus elaphus) populations in Xinjiang of China: Comparison with other Asian, European, and North American populations. Zool. Sci. 2002, 19, 485–495. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X. Xinjiang Tianshan World Natural Heritage; Chine Science Press: Beijing, China, 2017. [Google Scholar]
- Bai, H.; Yin, L.; Su, H.; Gao, F.; Mu, Y.; Zhang, Y.; Tong, Y.; Xu, W.; Cao, Q.; Yang, W.; et al. Camera trapping survey of birds and mammals in the Tianchi Bogda Peak Nature Reserve of Xinjiang. China. Arid Land Geogr. 2022, 45, 847–859. [Google Scholar]
- Zhu, X.S.; Wang, M.Y.; Yang, W.K.; David, B. Social structure of Asiatic ibex (Capra sibirica) in Middle Tien Shan, Xinjiang. Acta Theriol. Sin. 2016, 36, 56–63. [Google Scholar]
- Bonenfant, C.; Gaillard, J.-M.; Dray, S.; Loison, A.; Royer, M.; Chessel, D. Testing sexual segregation and aggregation: Old ways are best. Ecology 2007, 88, 3202–3208. [Google Scholar] [CrossRef] [PubMed]
- Georgii, B. Activity patterns of female red deer (Cervus elaphus L.) in the Alps. Oecologia 1981, 49, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Ma, J.Z.; Bao, J. Seasonal change pattern of breeding behavior of Tianshan wapiti in captive population. Heilongjiang Anim. Sci. Vet. Med. 2008, 7, 100–101. [Google Scholar]
- Moen, A.N. Wildlife Ecology; WH Freeman and Co.: San Francisco, CA, USA, 1973; p. 457. [Google Scholar]
- Ozoga, J.J.; Verme, L.J. Activity patterns of white-tailed deer during estrus. J. Wildl. Manag. 1975, 39, 679–683. [Google Scholar] [CrossRef]
- Berger, A.; Scheibe, K.M.; Brelurut, A.; Schober, E.; Streich, W. Seasonal variation of diurnal and ultradian rhythms in red deer. Biol. Rhythm Res. 2002, 33, 237–253. [Google Scholar] [CrossRef]
- Westoby, M. An analysis of diet selection by large generalist herbivores. Am. Nat. 1974, 108, 290–304. [Google Scholar] [CrossRef]
- Moen, A.N. Seasonal changes in heart rates, activity, metabolism, and forage intake of white-tailed deer. J. Wildl. Manag. 1978, 42, 715–738. [Google Scholar] [CrossRef]
- Pépin, D.; Renaud, P.C.; Dumont, B.; Decuq, F. Time budget and 24-h temporal rest–activity patterns of captive red deer hinds. Appl. Anim. Behav. Sci. 2006, 101, 339–354. [Google Scholar] [CrossRef]
- Gulijiang, H.; Liang, Y. Forest fire prevention and control countermeasures in Xinjiang Tianchi Bogda Peak Nature Reserve. For. Fire Prev. 2019, 2, 8–11. (In Chinese) [Google Scholar]
- Rizihan, A.; Aysajan, T.; Amila, A.; Zhou, C.L.; Mahmut, H. Winter food habits of Tianshan red deer (Cervus elaphus songaricus) in Nanshan mountains of Urumqi, China. Acta Theriol. Sin. 2013, 33, 326–332. [Google Scholar]
- Moen, A.N. Energy conservation by white-tailed deer in the winter. Ecology 1976, 57, 192–198. [Google Scholar] [CrossRef]

| Group Type | Season | |||
|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | |
| Single adult female | 1 | 1 | 1 | 1 |
| Single adult male | 1 | 1 | 1 | 1 |
| Female group | 3.88 ± 0.72 | 3.28 ± 0.25 | 3.47 ± 0.44 | 3.31 ± 0.32 |
| Male group | 2.67 ± 0.33 | 3 ± 0.25 | 2.6 ± 0.40 | 2.95 ± 0.31 |
| Mixed group | 10.03 ± 1.21 | 5.49 ± 0.41 | 5.33 ± 0.65 | 6.81 ± 0.72 |
| Mother-kid group | 17 ± 1 | 4.46 ± 0.45 | — | 7.83 ± 3.4 |
| Young group | — | 1.25 ± 0.25 | — | 1 |
| All individuals | 7.79 ± 0.92 | 3.36 ± 0.17 | 3.06 ± 0.31 | 3.60 ± 0.30 |
| Only adult individuals | 6.27 ± 0.71 | 3.07 ± 0.16 | 2.98 ± 0.29 | 3.49 ± 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Blank, D.; Xu, F. The Grouping Patterns of Cervus canadensis songaricus in the Tianchi Bogda Peak Nature Reserve of Tianshan Mountain, Northwestern China. Animals 2025, 15, 247. https://doi.org/10.3390/ani15020247
Ma X, Blank D, Xu F. The Grouping Patterns of Cervus canadensis songaricus in the Tianchi Bogda Peak Nature Reserve of Tianshan Mountain, Northwestern China. Animals. 2025; 15(2):247. https://doi.org/10.3390/ani15020247
Chicago/Turabian StyleMa, Xuejun, David Blank, and Feng Xu. 2025. "The Grouping Patterns of Cervus canadensis songaricus in the Tianchi Bogda Peak Nature Reserve of Tianshan Mountain, Northwestern China" Animals 15, no. 2: 247. https://doi.org/10.3390/ani15020247
APA StyleMa, X., Blank, D., & Xu, F. (2025). The Grouping Patterns of Cervus canadensis songaricus in the Tianchi Bogda Peak Nature Reserve of Tianshan Mountain, Northwestern China. Animals, 15(2), 247. https://doi.org/10.3390/ani15020247





