Metabolic Performance of Mealworms and Black Soldier Fly Larvae Reared on Food and Agricultural Waste and By-Products
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mealworms and BSF Larvae
2.2. Feed Substrates
2.3. Rearing Experiments
2.4. Larval CO2 Production Rates
2.5. Analytical Procedures
2.6. Substrate Conversion Efficiency
2.7. Growth and Metabolic Performance of Mealworms and BSF Larvae
3. Results
3.1. Feed Substrates
3.2. Growth and Metabolic Performance of Mealworms
3.3. Growth and Metabolic Performance of BSF Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caparros Megido, R.; Francis, F.; Haubruge, E.; le Gall, P.; Tomberlin, J.K.; Miranda, C.D.; Jordan, H.R.; Picard, C.J.; Pino, M.J.M.; Ramos-Elordy, J.; et al. A worldwide overview of the status and prospects of edible insect production. Entomol. Gen. 2024, 44, 3–27. [Google Scholar] [CrossRef]
- El Deen, S.N.; Spranghers, T.; Baldacchino, F.; Deruytter, D. The effects of the particle size of four different feeds on the larval growth of Tenebrio molitor (Coleoptera: Tenebrionidae). Eur. J. Entomol. 2022, 119, 242–249. [Google Scholar] [CrossRef]
- Peguero, D.A.; Gold, M.; Velasquez, L.; Niu, M.; Zurbrügg, C.; Mathys, A. Physical pretreatment of three biowastes to improve black soldier fly larvae bioconversion efficiency. Waste Manag. 2024, 178, 280–291. [Google Scholar] [CrossRef]
- Urs, K.C.D.; Hopkins, T.L. Effect of Moisture on Growth Rate and Development of Two Strains of Tenebrio molitor L. (Coleoptera, Tenebrionidae). J. Stored Prod. Res. 1973, 8, 291–297. [Google Scholar] [CrossRef]
- Bekker, N.S.; Heidelbach, S.; Vestergaard, S.Z.; Nielsen, M.E.; Riisgaard-Jensen, M.; Zeuner, E.J.; Bahrndorff, S.; Eriksen, N.T. Impact of substrate moisture content on growth and metabolic performance of black soldier fly larvae. Waste Manag. 2021, 127, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Morales-Ramos, J.; Rojas, M. Effect of larval density on food utilization efficiency of Tenebrio molitor (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2015, 108, 2259–2267. [Google Scholar] [CrossRef]
- Parra Paz, A.; Carrejo, N.; Gómez Rodríguez, C. Effects of larval density and feeding rates on the bioconversion of vegetable waste using black soldier fly larvae Hermetia illucens (L.), (Diptera: Stratiomyidae). Waste Biomass Valorization 2015, 6, 1059–1065. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.; Dicke, M.; van Loon, J. Influence of larval density and dietary nutrient concentration on performance, body protein, and fat contents of black soldier fly larvae (Hermetia illucens). Entomol. Exp. Appl. 2018, 166, 761–770. [Google Scholar] [CrossRef]
- Deruytter, D.; Coudro, C.L.; Claeys, J. The effects of density on the growth and temperature production of Tenebrio molitor larvae. Sustainability 2022, 14, 6234. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, Z.; Li, N.; Yu, Y.; Cai, M.; Zheng, L.; Zhu, F.; Huang, F.; Tomberlin, J.K.; ur Rehman, K.; et al. Cellulose-degrading bacteria improve conversion efficiency in the co-digestion of dairy and chicken manure by black soldier fly larvae. J. Environ. Manag. 2023, 348, 119156. [Google Scholar] [CrossRef]
- Khanal, P.; Pandey, D.; Næss, G.; Cabrita, A.R.J.; Fonseca, A.J.M.; Maia, M.R.G.; Timilsina, B.; Veldkamp, T.; Sapkota, R.; Overrein, H. Yellow mealworms (Tenebrio molitor) as an alternative animal feed source: A comprehensive characterization of nutritional values and the larval gut microbiome. J. Clean. Prod. 2023, 389, 136104. [Google Scholar] [CrossRef]
- Muurmann, A.T.; Banovic, M.; Gilbert, M.T.P.; Sogari, G.; Limborg, M.T.; Sicheritz-Pontén, T.; Bahrndorff, S. Framework for valorizing waste- and by-products through insects and their microbiomes for food and feed. Food Res. Int. 2024, 187, 114358. [Google Scholar] [CrossRef]
- van Broekhoven, S.; Oonincx, D.G.A.B.; van Huis, A.; van Loon, J.J.A. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, H.; Chen, G.; Qiao, L.; Li, J.; Liu, B.; Liu, Z.; Li, M.; Liu, X. Growth performance and nutritional profile of mealworms reared on corn stover, soybean meal, and distillers’ grains. Eur. Food Res. Technol. 2019, 245, 2631–2640. [Google Scholar] [CrossRef]
- Montalbán, A.; Sánchez, C.J.; Hernández, F.; Schiavone, A.; Madrid, J.; Martínez-Miró, S. Effects of agro-industrial byproduct-based diets on the growth performance, digestibility, nutritional and microbiota composition of mealworm (Tenebrio molitor L.). Insects 2022, 13, 323. [Google Scholar] [CrossRef]
- Vrontaki, M.; Adamaki-Sotiraki, C.; Rumbos, C.I.; Anastasiadis, A.; Athanassiou, C.G. Bridging the gap: Scaling up the sustainable production of the yellow mealworm with agricultural by-products—Insights into larval growth and body composition. Agriculture 2024, 14, 520. [Google Scholar] [CrossRef]
- Vrontaki, M.; Adamaki-Sotiraki, C.; Rumbos, C.I.; Anastasiadis, A.; Athanassiou, C.G. Valorization of local agricultural by-products as nutritional substrates for Tenebrio molitor larvae: A sustainable approach to alternative protein production. Environ. Sci. Pollut. Res. 2024, 31, 35760–35768. [Google Scholar] [CrossRef]
- Chia, S.Y.; Tanga, C.M.; Khamis, F.M.; Mohamed, S.A.; Salifu, D.; Sevgan, S.; Fiaboe, K.K.M.; Niassy, S.; van Loon, J.J.A.; Dicke, M.; et al. Threshold temperatures and thermal requirements of black soldier fly Hermetia illucens: Implications for mass production. PLoS ONE 2018, 13, e0206097. [Google Scholar] [CrossRef] [PubMed]
- Surendra, K.C.; Tomberlin, J.K.; van Huis, A.; Cammack, J.A.; Heckmann, L.-H.L.; Khanal, S.K. Rethinking organic wastes bioconversion: Evaluating the potential of the black soldier fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae) (BSF). Waste Manag. 2020, 117, 58–80. [Google Scholar] [CrossRef]
- Hosseindoust, A.; Ha, S.H.; Mun, J.Y.; Kim, S. A metanalysis to evaluate the effects of substrate sources on the nutritional performance of black soldier fly larvae: Implications for sustainable poultry feed. Poult. Sci. 2024, 103, 103299. [Google Scholar] [CrossRef]
- Eriksen, N.T. Metabolic performance and feed efficiency of black soldier fly larvae. Front. Bioeng. Biotechnol. 2024, 12, 1397108. [Google Scholar] [CrossRef] [PubMed]
- Maino, J.L.; Kearney, M.R. Ontogenetic and interspecific metabolic scaling in insects. Am. Nat. 2014, 184, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Laganaro, M.; Bahrndorff, S.; Eriksen, N.T. Growth and metabolic performance of black soldier fly larvae grown on low and high-quality substrates. Waste Manag. 2021, 121, 198–205. [Google Scholar] [CrossRef]
- Hansen, R.J.; Nielsen, S.H.M.; Johansen, M.; Nielsen, F.K.; Dragsbæk, F.B.; Sørensen, O.S.B.; Eriksen, N.T. Metabolic performance of black soldier fly larvae during entomoremediation of brewery waste. J. Appl. Entomol. 2023, 147, 423–431. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.-P.; Van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Eberle, S.; Schaden, L.M.; Tintner, J.; Stauffer, C.; Schebeck, M. Effect of temperature and photoperiod on development, survival, and growth rate of mealworms, Tenebrio molitor. Insects 2022, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Özsoy, A.N. Modeling of development and water consumption of mealworm (Tenebrio molitor L., 1758) (Coleoptera: Tenebrionidae) larvae using nonlinear growth curves and polynomial functions. Turk. J. Entomol. 2019, 43, 253–262. [Google Scholar] [CrossRef]
- Riisgård, H.U. No foundation of a ’3/4 power scaling law’ for respiration in biology. Ecol. Lett. 1998, 1, 71–73. [Google Scholar] [CrossRef]
- Roels, J.A. Application of macroscopic principles to microbial metabolism. Biotechnol. Bioeng. 1980, 22, 2457–2514. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Deruytter, D.; Rumbos, C.I.; Adamaki-Sotiraki, C.; Tournier, L.; Ageorges, V.; Coudron, C.L.; Yakti, W.; Ulrichs, C.; Spranghers, T.; Berrens, S.; et al. Make it a standard? The creation and variability assessment of a consensus standard protocol for Tenebrio molitor larvae feeding trials. J. Insects Food Feed. 2024, 1–11. [Google Scholar] [CrossRef]
- Deruytter, D.; Gasco, L.; Yakti, W.; Katz, H.; Coudron, C.L.; Gligorescu, A.; Frooninckx, L.; Noyens, I.; Meneguz, M.; Grosso, F.; et al. Standardising black soldier fly larvae feeding experiments: An initial protocol and variability estimates. J. Insects Food Feed 2024, 10, 1685–1995. [Google Scholar] [CrossRef]
- Frooninckx, L.; Broeckx, L.; Goossens, S.; Wuyts, A.; Van Miert, S. Optimizing substrate moisture content for enhanced larval survival and growth performance in Hermetia illucens: Exploring novel approaches. Discov. Anim. 2024, 1, 7. [Google Scholar] [CrossRef]
- Li, L.; Stasiak, M.; Li, L.; Xie, B.; Fu, Y.; Gidzinski, D.; Dixon, M.; Liu, H. Rearing Tenebrio molitor in BLSS: Dietary fiber affects larval growth, development, and respiration characteristics. Acta Astronaut. 2016, 118, 130–136. [Google Scholar] [CrossRef]
- Bjørge, J.D.; Overgaard, J.; Malte, H.; Gianotten, N.; Heckmann, L.-H. Role of temperature on growth and metabolic rate in the tenebrionid beetles Alphitobius diaperinus and Tenebrio molitor. J. Insect Physiol. 2018, 107, 89–96. [Google Scholar] [CrossRef]
- Shojaaddini, M. Applicability of black soldier fly and yellow mealworm in municipal food waste bioconversion: Assessment of efficiency, nutritional proficiency, and safety. J. Asia-Pac. Entomol. 2024, 27, 102306. [Google Scholar] [CrossRef]
- Gold, M.; Cassar, C.M.; Zurbrügg, C.; Kreuzer, M.; Boulos, S.; Diener, S.; Mathys, A. Biowaste treatment with black soldier fly larvae: Increasing performance through the formulation of biowastes based on protein and carbohydrates. Waste Manag. 2020, 102, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Minkevich, I.G.; Dedyukhina, E.G.; Chistyakova, T.I. The effect of lipid content on the elemental composition and energy capacity of yeast biomass. Appl. Microbiol. Biotechnol. 2010, 88, 799–806. [Google Scholar] [CrossRef]
- Ferral, N.; Gomez, N.; Holloway, K.; Neeter, H.; Fairfield, M.; Pollman, K.; Huang, Y.-W.; Hou, C. The extremely low energy cost of biosynthesis in holometabolous insect larvae. J. Insect Physiol. 2020, 120, 103988. [Google Scholar] [CrossRef]
- Brown, J.H.P.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Coutchié, P.A.; Machin, J. Allometry of water vapor absorption in two species of tenebrionid beetle larvae. Am. J. Physiol. 1984, 247, R230–R236. [Google Scholar] [CrossRef]
- Danieli, P.P.; Lussiana, C.; Gasco, L.; Amici, A.; Ronchi, B. The effects of diet formulation on the yield, proximate composition, and fatty acid profile of the black soldier fly (Hermetia illucens L.) prepupae intended for animal feed. Animals 2019, 9, 178. [Google Scholar] [CrossRef]
- Rho, M.S.; Lee, K.P. Behavioural and physiological regulation of protein and carbohydrates in mealworm larvae: A geometric analysis. J. Insect Physiol. 2022, 136, 104329. [Google Scholar] [CrossRef] [PubMed]
- Syahrulawal, L.; Torske, M.O.; Sapkota, R.; Næss, G.; Khanal, P. Improving the nutritional values of yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae) larvae as an animal feed ingredient: A review. J. Anim. Sci. Biotechnol. 2023, 14, 146. [Google Scholar] [CrossRef]
- Eriksen, N.T. Dynamic modelling of feed assimilation, growth, lipid accumulation, and CO2 production in black soldier fly larvae. PLoS ONE 2022, 17, e0276605. [Google Scholar] [CrossRef] [PubMed]
- Boulos, S.; Tännler, A.; Nyström, L. Nitrogen-to-protein conversion factors for edible insects on the Swiss market: T. molitor, A. domesticus, and L. migratoria. Front. Nutr. 2020, 7, 89. [Google Scholar] [CrossRef]
- Eggink, K.; Dalsgaard, J. Chitin contents in different black soldier fly (Hermetia illucens) life stages. J. Insects Food Feed 2023, 9, 855–864. [Google Scholar] [CrossRef]
- Eggink, K.M.; Lund, I.; Pedersen, P.B.; Hansen, B.W.; Dalsgaard, J. Biowaste and by-products as rearing substrates for black soldier fly (Hermetia illucens) larvae: Effects on larval body composition and performance. PLoS ONE 2022, 17, e0275213. [Google Scholar] [CrossRef] [PubMed]
- Bordiean, A.; Krzyzaniak, M.; Stolarski, M.J.; Peni, D. Growth potential of yellow mealworm reared on industrial residues. Agriculture 2020, 10, 599. [Google Scholar] [CrossRef]
- Bordiean, A.; Krzyzaniak, M.; Aljewic, M.; Stolarski, M.J. Influence of different diets on growth and nutritional composition of yellow mealworm. Foods 2022, 11, 3075. [Google Scholar] [CrossRef] [PubMed]
- Bruno, D.; Bonelli, M.; Valoroso, M.C.; Roma, D.; Montali, A.; Pellegrino, M.G.; Marzari, M.; Caccia, S.; Tettamanti, G.; Casartelli, M. Black soldier fly larvae efficiently bioconvert the organic fraction of municipal solid waste thanks to their midgut functional plasticity. J. Insects Food Feed. 2024, 11, 157–172. [Google Scholar] [CrossRef]
- Liu, Z.; Minor, M.; Morel, P.C.H.; Najar-Rodriguez, A.J. Bioconversion of three organic wastes by black soldier fly (Diptera: Stratiomyidae) larvae. Environ. Entomol. 2018, 47, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef] [PubMed]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef] [PubMed]
- Galassi, G.; Jucker, C.; Parma, P.; Lupi, D.; Crovetto, G.M.; Savoldelli, S.; Colombini, S. Impact of agro-industrial byproducts on bioconversion, chemical composition, in vitro digestibility, and microbiota of the black soldier fly (Diptera: Stratiomyidae) larvae. J. Insect Sci. 2021, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.Y.; Tanga, C.M.; Osuga, I.M.; Mohamed, S.A.; Khamis, F.M.; Salifu, D.; Sevgan, S.; Fiaboe, K.K.M.; Niassy, S.; van Loon, J.J.A.; et al. Effects of waste stream combinations from brewing industry on performance of Black Soldier Fly, Hermetia illucens (Diptera: Stratiomyidae). PeerJ 2018, 6, e588. [Google Scholar] [CrossRef] [PubMed]
- Jucker, C.; Leonardi, M.G.; Rigamonti, I.; Lupi, D.; Savoldelli, S. Brewery’s waste streams as a valuable substrate for black soldier fly Hermetia illucens (Diptera: Stratiomyidae). J. Entomol. Acarol. Res. 2019, 51, 8876. [Google Scholar] [CrossRef]
 , dashed curves predicted by Equation (6)), and specific feed assimilation rate, a (dashed curves, grey scale predicted by Equation (8)). Right panels compare the specific CO2 production rate to specific growth rate (curve predicted by Equations (2)–(8)). Data points represent averages of 5 replicate cultures ± standard deviation. Extinction of individual larval cultures indicated by †.
, dashed curves predicted by Equation (6)), and specific feed assimilation rate, a (dashed curves, grey scale predicted by Equation (8)). Right panels compare the specific CO2 production rate to specific growth rate (curve predicted by Equations (2)–(8)). Data points represent averages of 5 replicate cultures ± standard deviation. Extinction of individual larval cultures indicated by †.
 , dashed curves predicted by Equation (6)), and specific feed assimilation rate, a (dashed curves, grey scale predicted by Equation (8)). Right panels compare the specific CO2 production rate to specific growth rate (curve predicted by Equations (2)–(8)). Data points represent averages of 5 replicate cultures ± standard deviation. Extinction of individual larval cultures indicated by †.
, dashed curves predicted by Equation (6)), and specific feed assimilation rate, a (dashed curves, grey scale predicted by Equation (8)). Right panels compare the specific CO2 production rate to specific growth rate (curve predicted by Equations (2)–(8)). Data points represent averages of 5 replicate cultures ± standard deviation. Extinction of individual larval cultures indicated by †.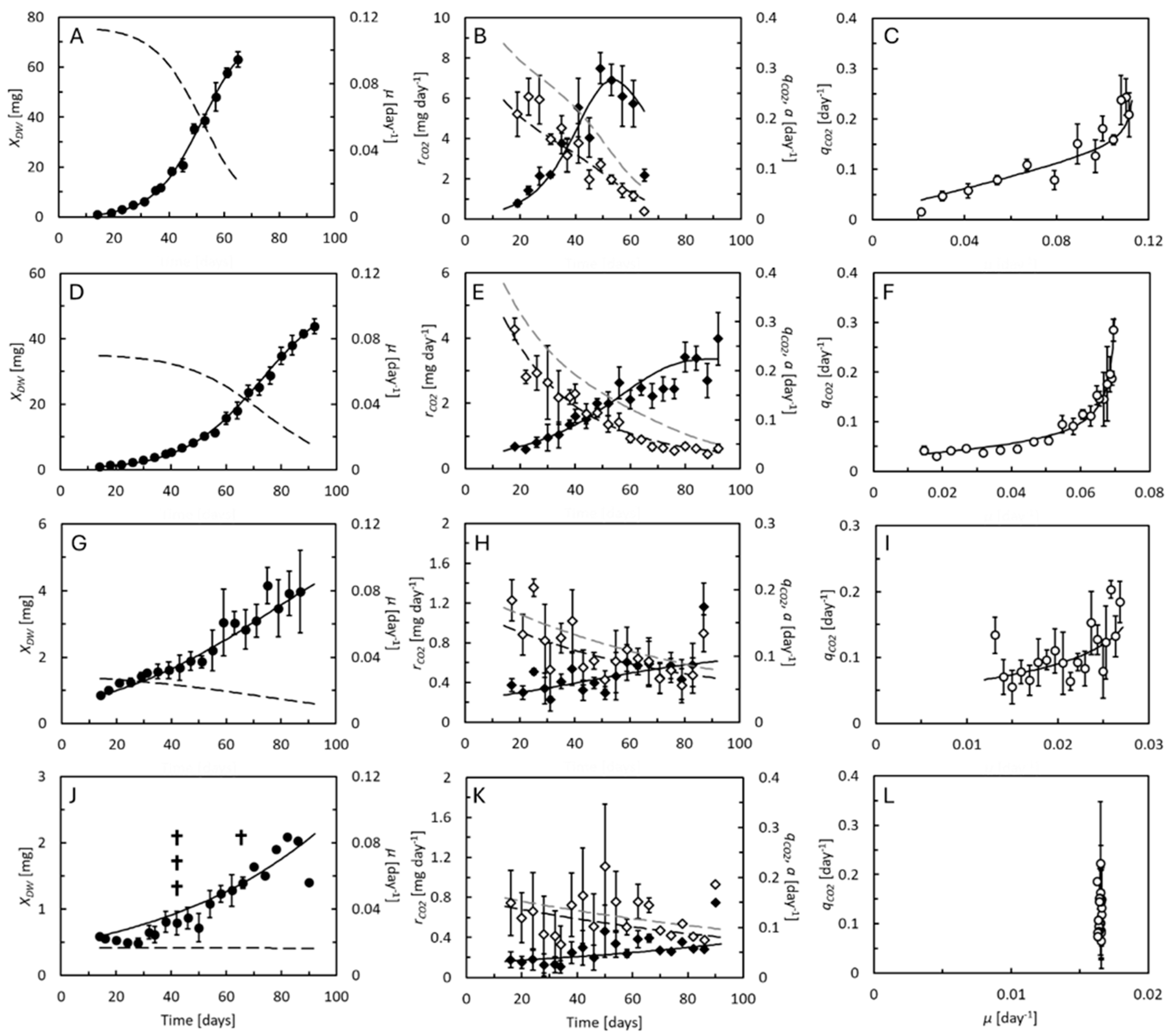
 ). Insets in panels (B,C) show the and NGE* on brewers’ spent grain on expanded scales. Data points represent average values of 5 replicate cultures (standard deviations indicated in Figure 1). Curves are model predictions from Figure 1.
). Insets in panels (B,C) show the and NGE* on brewers’ spent grain on expanded scales. Data points represent average values of 5 replicate cultures (standard deviations indicated in Figure 1). Curves are model predictions from Figure 1.
 ). Insets in panels (B,C) show the and NGE* on brewers’ spent grain on expanded scales. Data points represent average values of 5 replicate cultures (standard deviations indicated in Figure 1). Curves are model predictions from Figure 1.
). Insets in panels (B,C) show the and NGE* on brewers’ spent grain on expanded scales. Data points represent average values of 5 replicate cultures (standard deviations indicated in Figure 1). Curves are model predictions from Figure 1.
 , dashed curves predicted by Equation (6)), and specific feed assimilation rate, a (dashed curves, grey scale predicted by Equation (8)). Right panels compare the specific CO2 production rate to specific growth rate (curve predicted by Equations (2)–(8)). Data points represent averages of 5 replicate cultures ± standard deviation.
, dashed curves predicted by Equation (6)), and specific feed assimilation rate, a (dashed curves, grey scale predicted by Equation (8)). Right panels compare the specific CO2 production rate to specific growth rate (curve predicted by Equations (2)–(8)). Data points represent averages of 5 replicate cultures ± standard deviation.
 , dashed curves predicted by Equation (6)), and specific feed assimilation rate, a (dashed curves, grey scale predicted by Equation (8)). Right panels compare the specific CO2 production rate to specific growth rate (curve predicted by Equations (2)–(8)). Data points represent averages of 5 replicate cultures ± standard deviation.
, dashed curves predicted by Equation (6)), and specific feed assimilation rate, a (dashed curves, grey scale predicted by Equation (8)). Right panels compare the specific CO2 production rate to specific growth rate (curve predicted by Equations (2)–(8)). Data points represent averages of 5 replicate cultures ± standard deviation.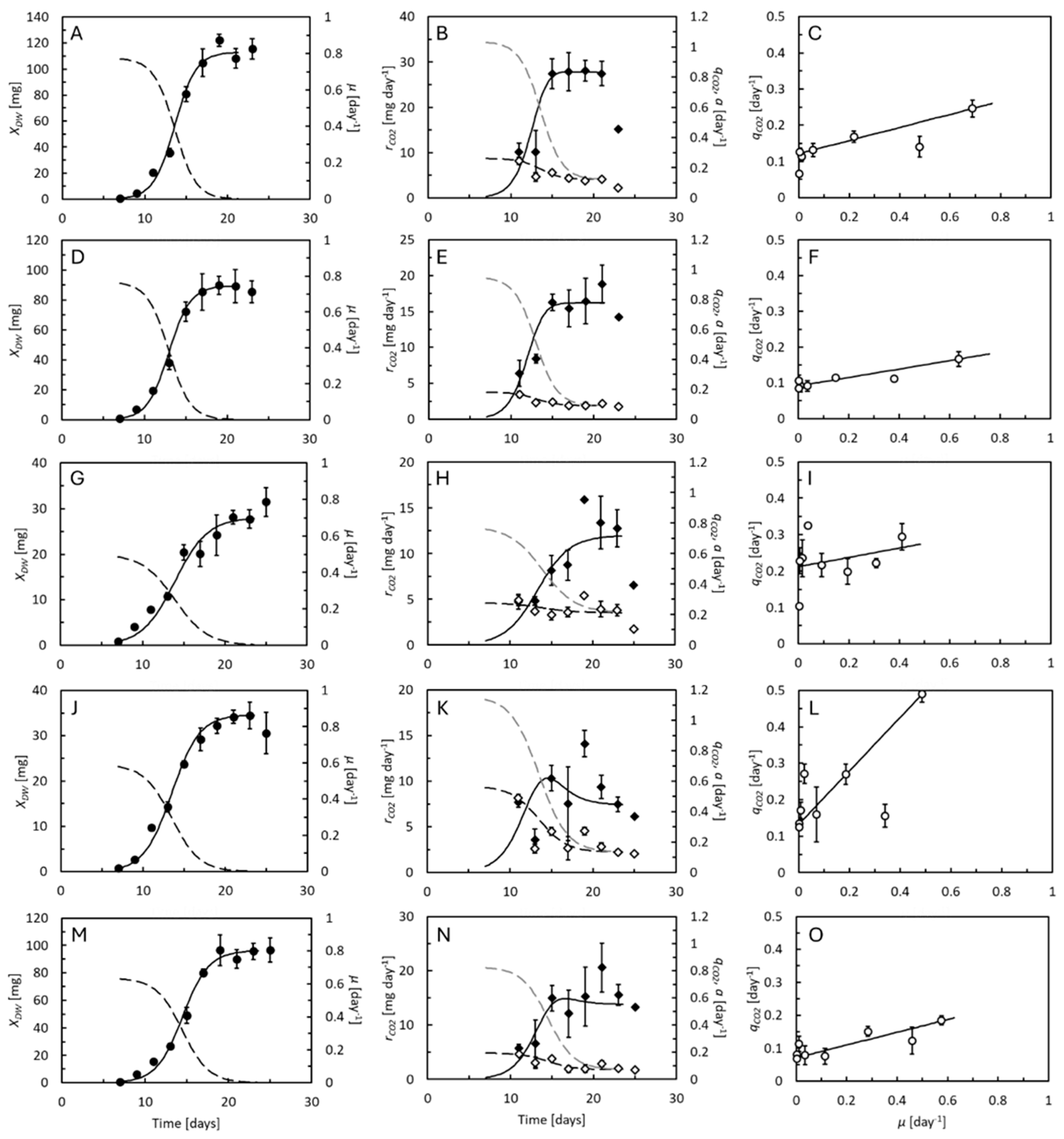
 , solid curve), deproteinized grass (♦, dashed curve), and biopulp (Δ, dotted curve). Data points represent average values of 5 replicate cultures (standard deviation indicated in Figure 3). Curves are model predictions from Figure 3.
, solid curve), deproteinized grass (♦, dashed curve), and biopulp (Δ, dotted curve). Data points represent average values of 5 replicate cultures (standard deviation indicated in Figure 3). Curves are model predictions from Figure 3.
 , solid curve), deproteinized grass (♦, dashed curve), and biopulp (Δ, dotted curve). Data points represent average values of 5 replicate cultures (standard deviation indicated in Figure 3). Curves are model predictions from Figure 3.
, solid curve), deproteinized grass (♦, dashed curve), and biopulp (Δ, dotted curve). Data points represent average values of 5 replicate cultures (standard deviation indicated in Figure 3). Curves are model predictions from Figure 3.

| Substrate | Wheat Bran 1 | Chicken Feed 2 | Rapeseed Cake 1,2 | Brewers’ Spent Grain 1 | Brewers’ Spent Grain 2 | Deproteinized Grass 1,2 | Biopulp 2 | |
|---|---|---|---|---|---|---|---|---|
| Component | Unit | |||||||
| Fat | % DW | 3.5 | 0.8 | 14.0 | 9.3 | 14.6 | 1.9 | 13.7 |
| Carbohydrate | % DW | 32.9 | 55.7 | 11.5 | 7.8 | 0.0 | 29.9 | 33.5 |
| Protein | % DW | 14.9 | 18.4 | 30.8 | 26.3 | 26.3 | 23.7 | 22.6 |
| Dietary fibers | % DW | 45.7 | 21.9 | 36.6 | 53.1 | 58.4 | 37.0 | 21.2 |
| Ash | % DW | 2.9 | 3.3 | 7.2 | 3.5 | 3.9 | 7.6 | 8.9 |
| Feed Substrate | Wheat Bran | Rapeseed Cake | Brewers’ Spent Grain | Deproteinized Grass | |
|---|---|---|---|---|---|
| Experimental variables | |||||
| XDW,14 | mg | 0.99 ± 0.07 | 0.83 ± 0.04 | 0.85 ± 0.05 | 0.60 ± 0.03 |
| tp | days | 65 | 93 | n.d. | n.d. |
| Survival rate | % | 98 ± 1 | 96 ± 2 | 91 ± 4 | 0–20 |
| μ | day−1 | 0.10 ± 0.00 | 0.06 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.01 |
| aDay19 | day−1 | 0.34 ± 0.00 | 0.37 ± 0.05 | 0.17 ± 0.01 | 0.16 ± 0.04 |
| SCE | - | 0.14 ± 0.01 | 0.12 ± 0.00 | 0.02 ± 0.00 | 0.00 |
| Model parameters | |||||
| XDW,max | mg | 78 ± 0 | 56 ± 3 | 7 ± 6 | n.d. |
| μmax | day−1 | 0.11 ± 0.00 | 0.07 ± 0.00 | 0.03 ± 0.01 | 0.02 |
| Y | - | 1.08 ± 0.08 | 0.36 ± 0.32 | 0.79 ± 0.14 | 0.39 |
| m | day−1 | 0.03 ± 0.00 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.02 |
| b | - | 0.53 ± 0.1 | 0.43 ± 0.1 | 0.51 ± 0.1 | 0.53 |
| NGE*avg | - | 0.40 ± 0.01 | 0.37 ± 0.03 | 0.17 ± 0.02 | 0.16 |
| NGE*DW,avg | - | 0.32 ± 0.01 | 0.28 ± 0.02 | 0.13 ± 0.02 | 0.12 |
| Larval composition | |||||
| XWW | mg | 185 ± 9 | 129 ± 7 | 12 ± 4 | 6 |
| δDW | % WW | 37 ± 0 | 42 ± 1 | 34 ± 1 | 23 |
| δC | % DW | 59 ± 3 | 59 ± 3 | 60 ± 15 | 53 |
| δN | %DW | 8.3 ± 0.3 | 8.6 ± 0.7 | 12.1 ± 2.4 | 10.3 |
| δprotein | % DW | 39 ± 2 | 40 ± 3 | 56 ± 11 | 48 |
| δlipid | % DW | 29 ± 1 | 26 ± 1 | n.d. | n.d. |
| δash | % DW | 2 ± 0 | 2 ± 0 | n.d. | n.d. |
| Feed Substrate | Chicken Feed | Rapeseed Cake | Brewers’ Spent Grain | Deproteinized Grass | Biopulp | |
|---|---|---|---|---|---|---|
| Experimental variables | ||||||
| XDW,7 | mg | 0.67 ± 0.10 | 0.92 ± 0.10 | 0.85 ± 0.07 | 0.73 ± 0.08 | 0.82 ± 0.03 |
| tp | days | 23 | 23 | 25 | 25 | 25 |
| Survival rate | % | 99 ± 1 | 97 ± 2 | 99 ± 1 | 98 ± 2 | 96 ± 2 |
| μ | day−1 | 0.85 ± 0.03 | 0.76 ± 0.04 | 0.56 ± 0.03 | 0.65 ± 0.02 | 0.73 ± 0.04 |
| SCE | - | 0.35 ± 0.01 | 0.29 ± 0.01 | 0.12 ± 0.01 | 0.14 ± 0.02 | 0.35 ± 0.02 |
| Model parameters | ||||||
| XDW.max | mg | 113 ± 11 | 90 ± 6 | 28 ± 1 | 35 ± 2 | 96 ± 1 |
| μmax | day−1 | 0.77 ± 0.03 | 0.77 ± 0.07 | 0.50 ± 0.05 | 0.59 ± 0.03 | 0.63 ± 0.01 |
| amax | day−1 | 1.04 ± 0.08 | 0.95 ± 0.07 | 0.96 ± 0.06 | 1.16 ± 0.03 | 0.81 ± 0.03 |
| Y | - | 0.18 ± 0.06 | 0.12 ± 0.03 | 0.13 ± 0.11 | 0.73 ± 0.05 | 0.19 ± 0.04 |
| m | day−1 | 0.12 ± 0.01 | 0.09 ± 0.01 | 0.21 ± 0.03 | 0.13 ± 0.01 | 0.07 ± 0.00 |
| 1 NGE*avg | - | 0.50 ± 0.04 | 0.56 ± 0.01 | 0.33 ± 0.02 | 0.34 ± 0.01 | 0.56 ± 0.01 |
| NGE*DW,avg | - | 0.43 ± 0.03 | 0.47 ± 0.01 | 0.28 ± 0.02 | 0.35 ± 0.01 | 0.46 ± 0.01 |
| Larval composition | ||||||
| XWW | mg | 354 ± 24 | 251 ± 21 | 115 ± 11 | 118 ± 17 | 283 ± 25 |
| δDW | % WW | 32 ± 0 | 33 ± 1 | 28 ± 1 | 26 ± 2 | 35 ± 2 |
| δC | % DW | 55 ± 2 | 54 ± 2 | 55 ± 3 | 44 ± 1 | 55 ± 2 |
| δN | %DW | 6.3 ± 0.4 | 7.2 ± 0.1 | 7.7 ± 0.3 | 7.2 ± 0.2 | 6.1 ± 0.3 |
| δprotein | % DW | 30 ± 2 | 34 ± 1 | 36 ± 1 | 34 ± 1 | 29 ± 1 |
| δlipid | % DW | 20 ± 2 | 28 ± 1 | 26 ± 1 | 13 ± 2 | 40 ± 2 |
| δash | % DW | 12 ± 0 | 9 ± 1 | 6 ± 1 | 20 ± 2 | 11 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, F.K.; Hansen, R.J.; Muurmann, A.T.; Bahrndorff, S.; Eriksen, N.T. Metabolic Performance of Mealworms and Black Soldier Fly Larvae Reared on Food and Agricultural Waste and By-Products. Animals 2025, 15, 233. https://doi.org/10.3390/ani15020233
Nielsen FK, Hansen RJ, Muurmann AT, Bahrndorff S, Eriksen NT. Metabolic Performance of Mealworms and Black Soldier Fly Larvae Reared on Food and Agricultural Waste and By-Products. Animals. 2025; 15(2):233. https://doi.org/10.3390/ani15020233
Chicago/Turabian StyleNielsen, Frederik Kjær, Rasmus Juhl Hansen, Asmus Toftkær Muurmann, Simon Bahrndorff, and Niels Thomas Eriksen. 2025. "Metabolic Performance of Mealworms and Black Soldier Fly Larvae Reared on Food and Agricultural Waste and By-Products" Animals 15, no. 2: 233. https://doi.org/10.3390/ani15020233
APA StyleNielsen, F. K., Hansen, R. J., Muurmann, A. T., Bahrndorff, S., & Eriksen, N. T. (2025). Metabolic Performance of Mealworms and Black Soldier Fly Larvae Reared on Food and Agricultural Waste and By-Products. Animals, 15(2), 233. https://doi.org/10.3390/ani15020233








