Exploring the Effects of Gracilaria lemaneiformis Polysaccharides on the Fecal Microbiota and Fecal Metabolites of Fattening Pigs Based on 16S rDNA and Metabolome Sequencing
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Materials
2.3. Experimental Design and Feeding Management
2.4. Microbiological Analysis
2.5. Untargeted Metabolomics Profiling
2.6. Assessment of Antioxidant Enzymes in Serum
2.7. Measurement of Serum Immune Markers
2.8. Statistical Analysis
3. Results
3.1. Effects of GLP on Fecal Microbiota in Fattening Pigs
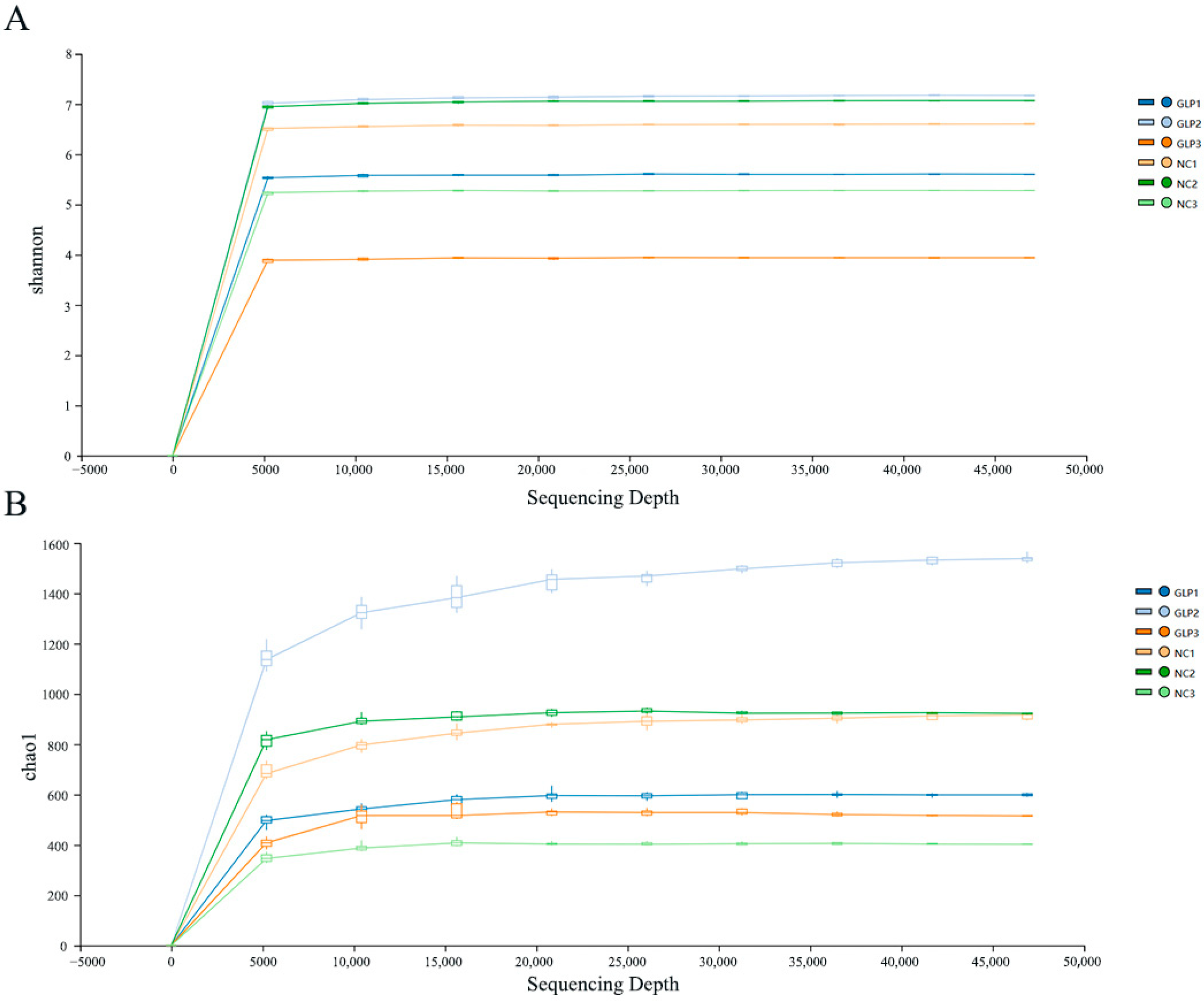
3.1.1. Sequencing Information
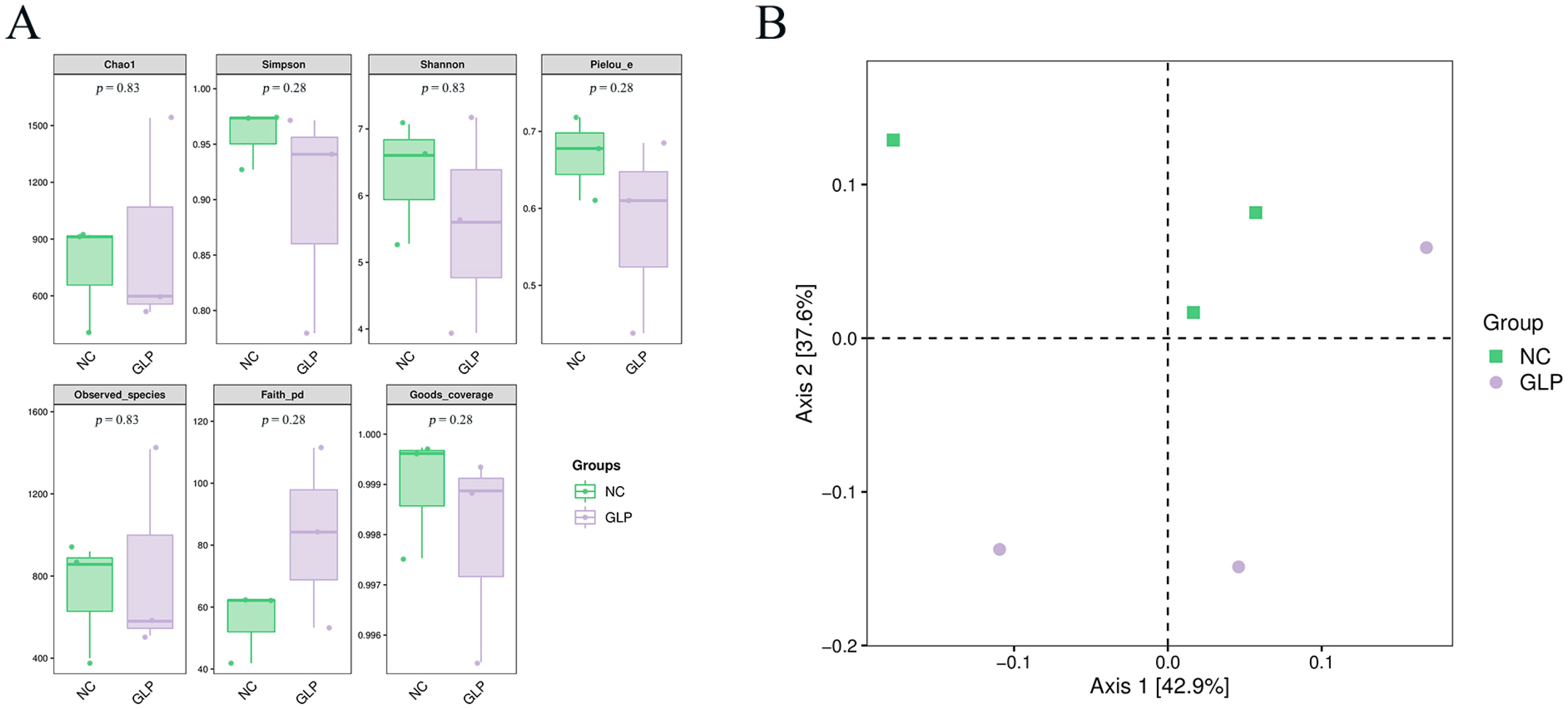
3.1.2. Alpha-Diversity Analysis
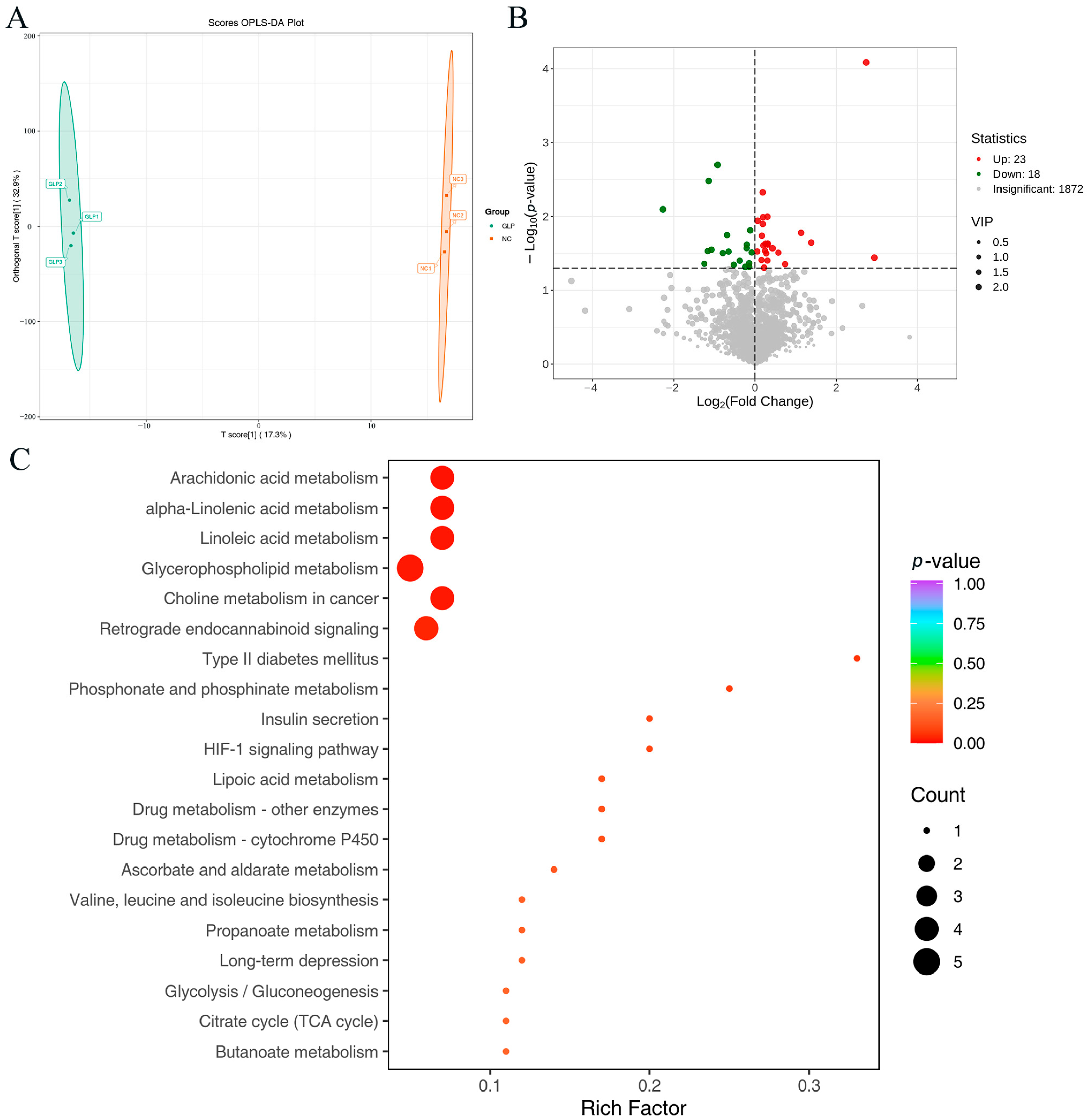
3.1.3. Beta-Diversity Analysis
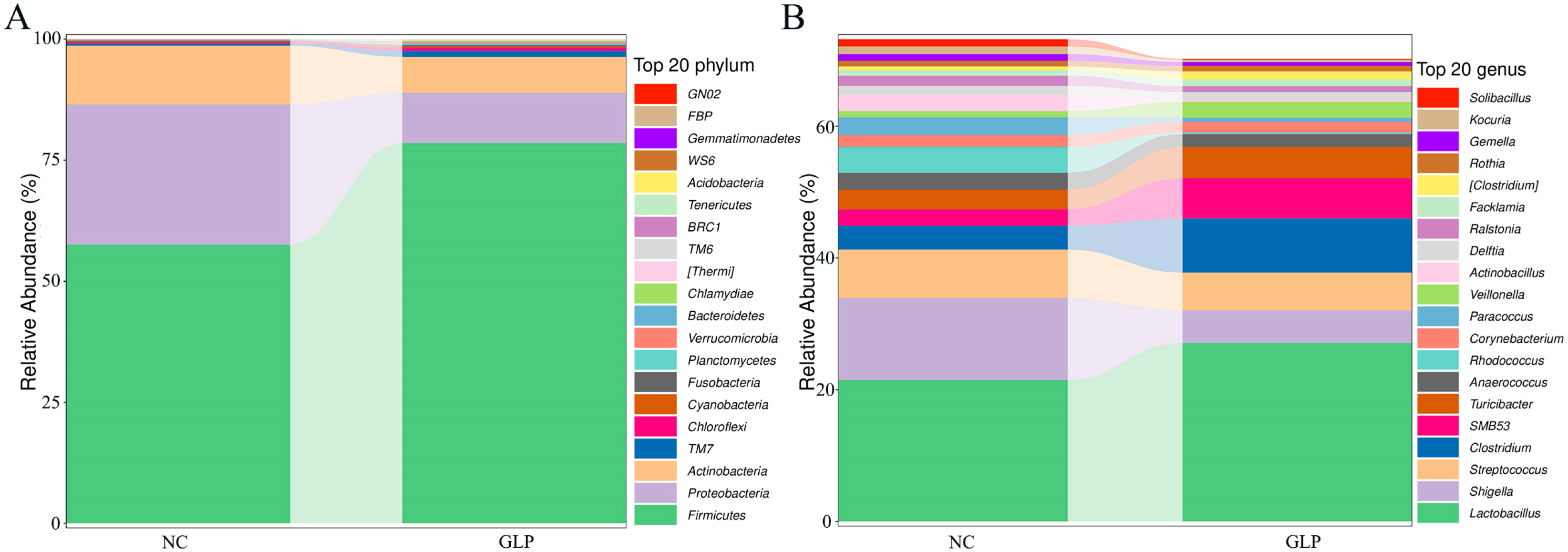
3.1.4. Differences in the Structure and Abundance of the Fecal Microbiota
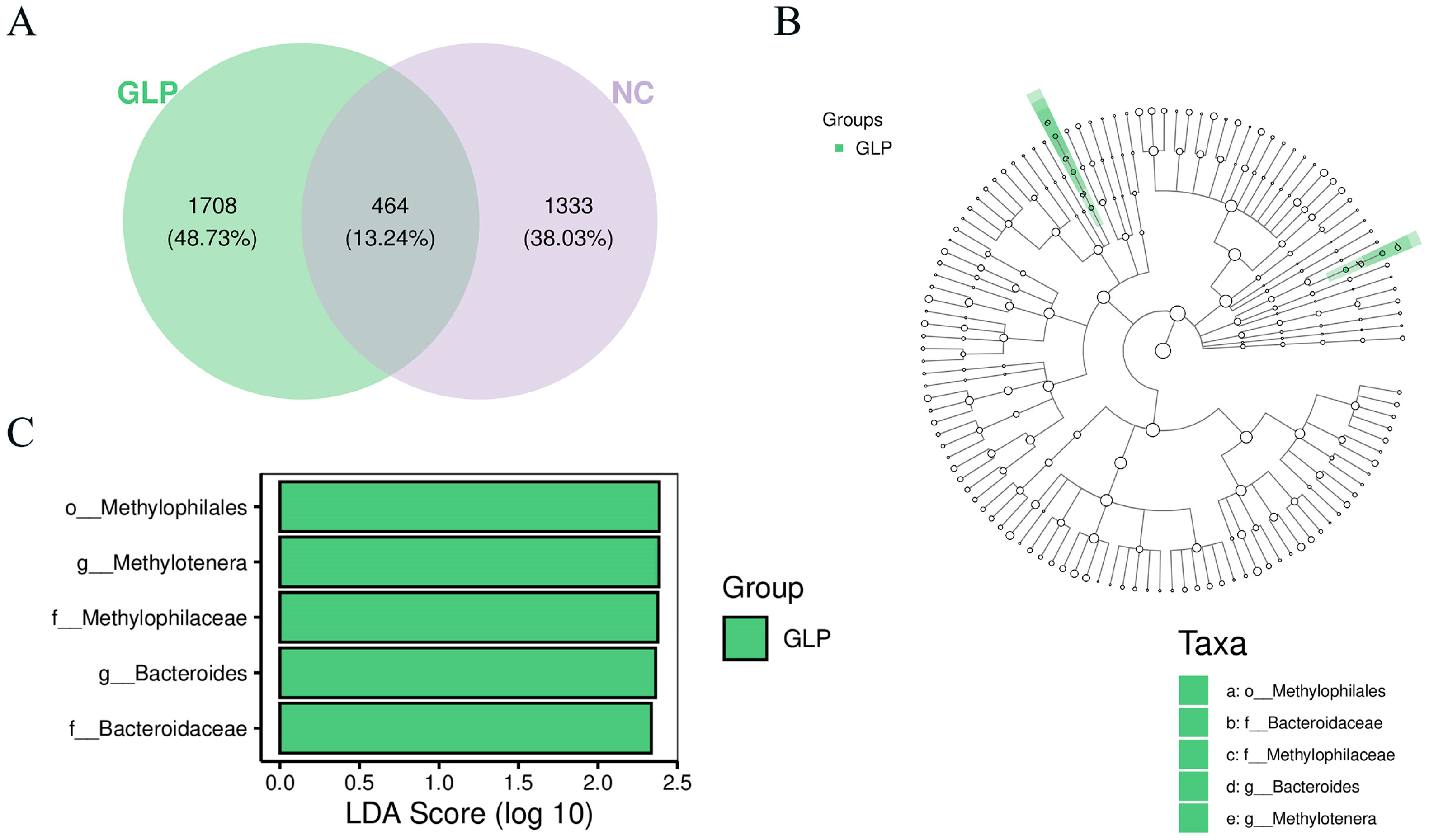
3.1.5. Analysis of Shared and Specific Fecal Microbiota
3.2. Effects of GLP on Fecal Metabolites in Fattening Pigs
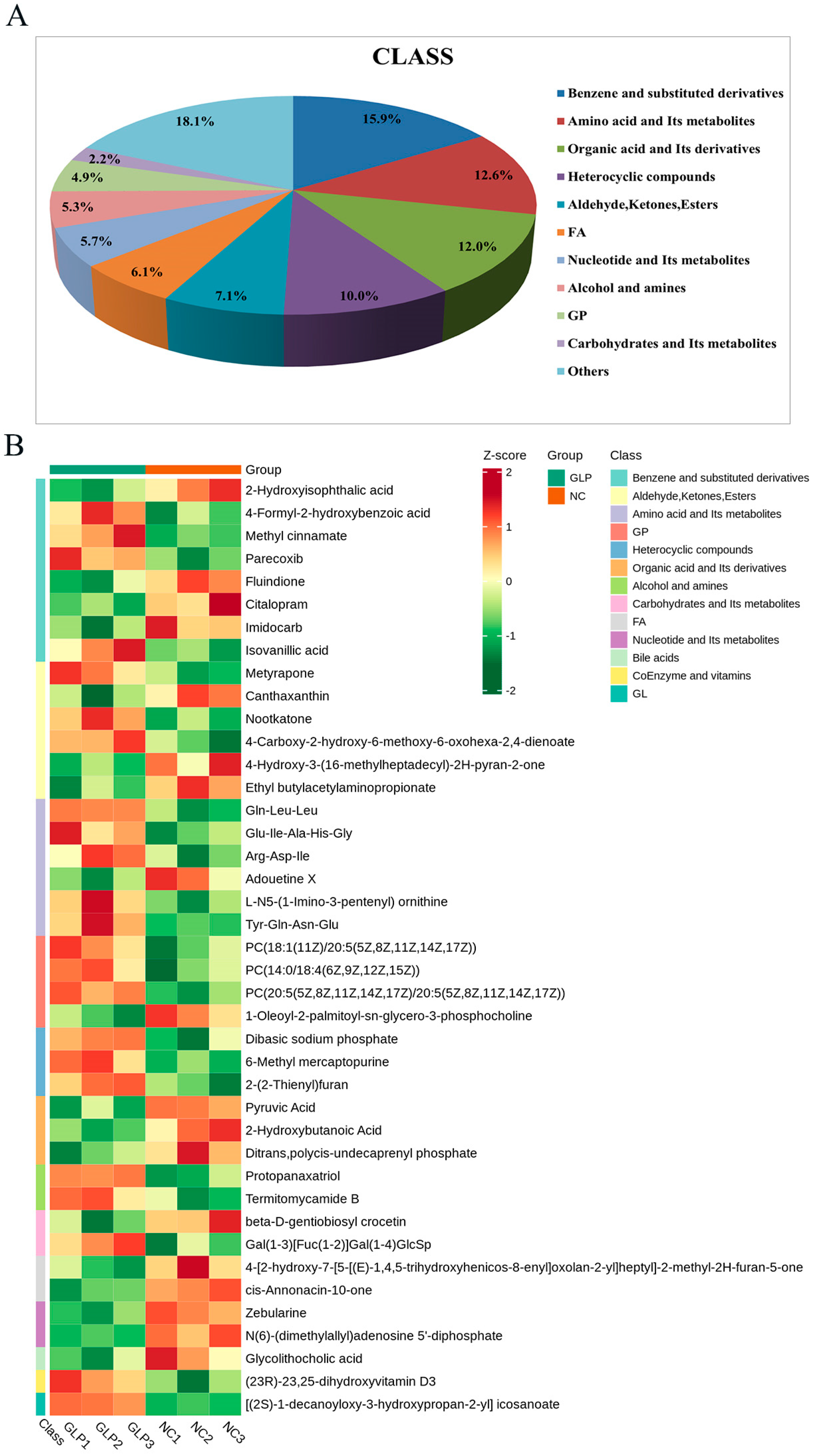
3.2.1. Major Metabolite Profiling
3.2.2. Differentially Accumulated Metabolite Analysis
3.2.3. Differences in Metabolic Pathways Between NC and GLP Groups
3.3. Effect of GLP on Antioxidant Capacity in Fattening Pigs
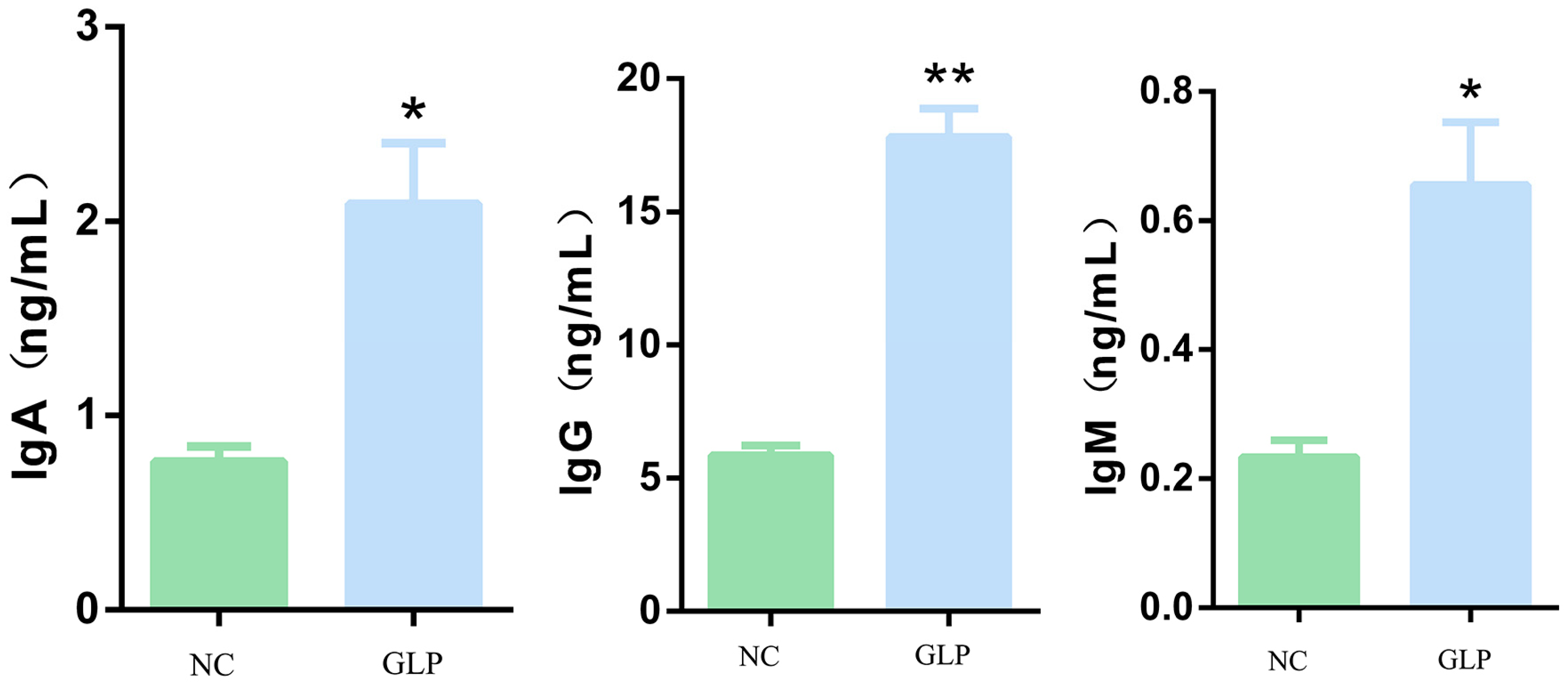
3.4. Effects of GLP on Serum Immunological Indicators in Fattening Pigs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krajmalnik-Brown, R.; Ilhan, Z.E.; Kang, D.W.; DiBaise, J.K. Effects of gut microbes on nutrient absorption and energy regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.B.; Jeong, H.W.; Cho, D.; Lee, B.J.; Lee, J.H.; Choi, J.Y.; Bae, I.H.; Lee, S.J. Fermented green tea extract alleviates obesity and related complications and alters gut microbiota composition in diet-induced obese mice. J. Med. Food 2015, 18, 549–556. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Zhao, P.; Shao, Q.; Ma, Y.; Bai, D.; Liao, C.; He, L.; Huang, S.; Wang, X. Effects of dietary Glycyrrhiza polysaccharide supplementation on growth performance, intestinal antioxidants, immunity and microbiota in weaned piglets. Anim. Biotechnol. 2023, 34, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- Aito-Inoue, M.; Lackeyram, D.; Fan, M.Z.; Sato, K.; Mine, Y. Transport of a tripeptide, Gly-Pro-Hyp, across the porcine intestinal brush-border membrane. J. Pept. Sci. 2007, 13, 468–474. [Google Scholar] [CrossRef]
- Lee, J.; González-Vega, J.C.; Htoo, J.K.; Nyachoti, C.M. Effects of dietary crude protein content and resistant starch supplementation on growth performance, intestinal histomorphology and microbial metabolites in weaned pigs. Arch. Anim. Nutr. 2024, 78, 192–207. [Google Scholar] [CrossRef]
- Huang, S.; Pang, D.; Li, X.; You, L.; Zhao, Z.; Cheung, P.C.; Zhang, M.; Liu, D. A sulfated polysaccharide from Gracilaria Lemaneiformis regulates cholesterol and bile acid metabolism in high-fat diet mice. Food Funct. 2019, 10, 3224–3236. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, C.; Zhang, P.; Ai, C.; Song, S. Digestion characteristics of polysaccharides from Gracilaria lemaneiformis and its interaction with the human gut microbiota. Int. J. Biol. Macromol. 2022, 213, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Hu, X.; Xiang, H.; Chen, S.; Li, L.; Qi, B.; Li, C.; Liu, S.; Yang, X. Structural characterization and hypolipidemic activity of Gracilaria lemaneiformis polysaccharide and its degradation products. Food Chem. X. 2022, 14, 100314. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Hu, X.; Liu, S.; Pan, C.; Chen, S.; Li, L.; Qi, B.; Yang, X. Insights on preparation, structure and activities of Gracilaria lemaneiformis polysaccharide. Food Chem. X 2021, 12, 100153. [Google Scholar] [CrossRef]
- Jiang, S.; Yang, C.; Xiao, Y.; Zheng, S.; Jiang, Q.; Chen, J. Effects of Polysaccharides-Rich Extract from Gracilaria lemaneiformis on Growth Performance, Antioxidant Capacity, Immune Function, and Meat Quality in Broiler Chickens. J. Poult. Sci. 2023, 60, 2023018. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, L.; Ma, Q.; Hua, L.; Sun, J.; Zhang, J.; Li, W.; Ren, Q.; Yan, X.; Xing, B. Effects of Gracilaria lemaneiformis Polysaccharides on Growth Performance, Carcass Traits and Meat Quality of Finishing Pigs. Chin. J. Anim. Nutr. 2023, 35, 4238–4246. Available online: https://kns.cnki.net/kcms2/article/abstract?v=iAN2XHIMbKvb2Co9gSYb4rDx1aUIlAor4_w5Q3IZSEvyIE8uFuc6K_45UtYoHuateJ5MYhfuSvoG8F4qzwca62BYpKKzGiX_DpprDZKeLhi4DIGfNEJLvlruASccI7kynV3OJqHKrNyMOjFUaiBeSGGrjOJTrzLXmB3ECUn5tfktLpnogC4Z2ZLtEnz6aXHF&uniplatform=NZKPT&language=CHS (accessed on 23 November 2024). (In Chinese).
- Zhang, X.; Aweya, J.J.; Huang, Z.; Kang, Z.; Bai, Z.; Li, K.; He, X.; Liu, Y.; Chen, X.; Cheong, K.L. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohydr. Polym. 2020, 234, 115894. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Li, D.; Wang, K.; Qiao, S.; Jia, G.; Jiang, Z.; Chen, Z.; Lin, Y.; Wu, D.; Zhu, X.; Xiong, B.; et al. Pig Feeding Standards. In Proceedings of the First Academic Seminar of the Swine Husbandry Professional Committee of Shandong Animal Husbandry and Veterinary Medicine Society, Jinan, China, 18 September 2007; pp. 288–321. Available online: https://kns.cnki.net/kcms2/article/abstract?v=UjEBX92ALNFKVeww0yvF_wVlU8nfsDjKBdLRCHY3oNlJ5etDXTi0ibupmUTA-LjEjS7JsqkS9EwdYiP0-VlIukUE8lQztLAMCMuu1x5pvyI5-7G9N7wmVzl4-mItp88o3ucxHZzUaLO7mdhwIzA1TZvLxO7KjLj4aPQ6-xPNS1HYfYAj24po_VT0g0nvbaXmWIR5r-8zhYA=&uniplatform=NZKPT&language=CHS (accessed on 23 November 2024).
- GB/T 6432-2018; National Feed Industry Standardization Technical Committee. Determination of Crude Protein in Feeds-Kjeldahl Method. Standards Press of China: Beijing, China, 2018. Available online: https://kns.cnki.net/kcms2/article/abstract?v=iAN2XHIMbKvxIr8AxTd1xJ_jNPy09w9hq62kQQD3Nqe8U3d3WmpMW81dErhxsZ51TODwseAT7c5OX21k3UmHW9sOVLx2ongDvJdtV4haXoV6IhoqhjkEv4GF2zHH0oLA72jKhUmJrmJK2EqTMEK9X1EC2xQnyXiDazoh-c5GNHGgjb2cHOMTZw==&uniplatform=NZKPT&language=CHS (accessed on 23 November 2024).
- GB/T 6434-2022; National Feed Industry Standardization Technical Committee. Determination of Crude Fiber Content in Feeds. Standards Press of China: Beijing, China, 2022. Available online: https://kns.cnki.net/kcms2/article/abstract?v=iAN2XHIMbKt2g158IqM5KBfQhD9zb2CHJvPtme9XhXF5jGUAgMozLbgu9oRIVcukdB8SVpZd2f6gxAi4DiTUKcn8zjE836pneGCz9DgFeOCJxwH64H2pmRJVCfv7KZ_mHooTzuy-xU42Il8F2s3bAIipAn6htkKIJVu2a9wNOgv1FMiYOLaEGw==&uniplatform=NZKPT&language=CHS (accessed on 23 November 2024).
- GB/T 6438-2007; National Feed Industry Standardization Technical Committee. Animal Feeding Stuffs—Determination of Crude Ash. Standards Press of China: Beijing, China, 2007. Available online: https://kns.cnki.net/kcms2/article/abstract?v=iAN2XHIMbKsc99lTqbQy5Upz-jin6fAiVHq2x9-vTwEnmZp1AwOagp2IL8FTRxA1cJw2o2JbicTqqOuqlfrV1fxqoU2eQF4psWpn_dZXjVGMQSe25HGIgS7iTGkFFGzAeWftrlM7NVSmcug8zdGOAnvUrRgBI0oq0LDw-fLkBf6GpJEzKRG-ZA==&uniplatform=NZKPT&language=CHS (accessed on 23 November 2024).
- GB/T 6436-2018; National Feed Industry Standardization Technical Committee. Determination of Calcium in Feeds. Standards Press of China: Beijing, China, 2018. Available online: https://kns.cnki.net/kcms2/article/abstract?v=iAN2XHIMbKu4KAjm27un46pqk6wHgnwruco2tLDvvgbYToMkhuEg_K1wxdbXEGG9BbYeAnETZAkZVvTNTsBOjq_z9PoFxbx9jZJu-R4KYxu7SXqF2bcWXvhTCcCfZCcXfXBru27Zk0daBO_L4r9vszSqmwomGV6lNdg0ITcxtQJkqLPLcG-8cg==&uniplatform=NZKPT&language=CHS (accessed on 23 November 2024).
- GB/T 6437-2018; National Feed Industry Standardization Technical Committee. Determination of Phosphorus in Feeds—Spectrophotometry. Standards Press of China: Beijing, China, 2018. Available online: https://kns.cnki.net/kcms2/article/abstract?v=iAN2XHIMbKsy59ttHCBU8jD6RSDCl4PT54fn1dN2PITCATCE3vIA5-kBvvlJvlZ8fNj60tYwmhsOceqfezdLAgSTyUHwgwWzZiWaGkimw4uWGpwvsMTlAopbbeQaiyi4akTR2gVYnqwEFtTS4Sced_yz6BqJT1N0Emn8tV7gaUPXlYFZCdSabA==&uniplatform=NZKPT&language=CHS (accessed on 23 November 2024).
- GB/T 18246-2019; National Feed Industry Standardization Technical Committee. Determination of Amino Acids in Feeds. Standards Press of China: Beijing, China, 2019. Available online: https://kns.cnki.net/kcms2/article/abstract?v=iAN2XHIMbKvxUhFH4J3ul8xB1lXX6AyoNISk7z2CpGBzD1mVKYJcibWuAjBAOyHZLPsjVT8akxpNuWC5Aqsq9Kc2PNb6CHeRsSBszRgeohByZbIzSPD9UWTTU2fIPoKt9Lg2NXKl5spnQAk1nNj9dwb1GKvibocmiSGt7K0jK9l99Iq_7zJxzg==&uniplatform=NZKPT&language=CHS (accessed on 23 November 2024).
- Noblet, J.; Perez, J.M. Prediction of digestibility of nutrients and energy values of pig diets from chemical analysis. J. Anim. Sci. 1993, 71, 3389–3398. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Thomann, J.; Ley, L.; Klaiber, A.; Liechti, M.E.; Duthaler, U. Development and validation of an LC-MS/MS method for the quantification of mescaline and major metabolites in human plasma. J. Pharm. Biomed. Anal. 2022, 220, 114980. [Google Scholar] [CrossRef]
- Goethals, S.; Rombouts, C.; Hemeryck, L.Y.; Van Meulebroek, L.; Van Hecke, T.; Vossen, E.; Van Camp, J.; De Smet, S.; Vanhaecke, L. Untargeted Metabolomics to Reveal Red versus White Meat-Associated Gut Metabolites in a Prudent and Western Dietary Context. Mol. Nutr. Food Res. 2020, 64, e2000070. [Google Scholar] [CrossRef]
- Khanal, S.; Bai, Y.; Ngo, W.; Nichols, K.K.; Wilson, L.; Barnes, S.; Nichols, J.J. Human meibum and tear film derived cholesteryl and wax esters in meibomian gland dysfunction and tear film structure. Ocul. Surf. 2022, 23, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, P.; Yang, X.; Chen, M.; Dong, Y.; Li, J. A cross-sectional study identifying disparities in serum metabolic profiles among hypertensive patients with ISH, IDH and SDH subtypes. Front. Cardiovasc. Med. 2023, 10, 1102754. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, X.; Tan, Q.; Xiao, Q.; Mei, S. A Comparative Metabolomics Study of Flavonoids in Radish with Different Skin and Flesh Colors (Raphanus sativus L.). J. Agric. Food Chem. 2020, 68, 14463–14470. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xie, Y.; Zajac, A.M.; Hu, Y.; Aroian, R.V.; Urban, J.F., Jr.; Li, R.W. Gut microbial signatures associated with moxidectin treatment efficacy of Haemonchus contortus in infected goats. Vet. Microbiol. 2020, 242, 108607. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, P.; Figat, S.; O’Doherty, J.V. The effect of dietary laminarin and fucoidan in the diet of the weanling piglet on performance, selected faecal microbial populations and volatile fatty acid concentrations. Animal 2010, 4, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Le Bon, M.; Connerton, I.F.; Mellits, K.H. Common colonic community indicators of the suckling pig microbiota where diversity and abundance correlate with performance. FEMS Microbiol. Ecol. 2022, 98, fiac048. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Achi, S.C.; Ibeawuchi, S.R.; Anandachar, M.S.; Gementera, H.; Chaudhury, U.; Usmani, F.; Vega, K.; Sayed, I.M.; Das, S. The crosstalk between microbial sensors ELMO1 and NOD2 shape intestinal immune responses. Virulence 2023, 14, 2171690. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.J.; Brown, J.M.; Murray, R.D.; Getty, B.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Characterization of novel bovine gastrointestinal tract Treponema isolates and comparison with bovine digital dermatitis treponemes. Appl. Environ. Microbiol. 2011, 77, 138–147. [Google Scholar] [CrossRef]
- Yu, J.; Li, C.; Li, X.; Liu, K.; Liu, Z.; Ni, W.; Zhou, P.; Wang, L.; Hu, S. Isolation and functional analysis of acid-producing bacteria from bovine rumen. PeerJ 2023, 11, e16294. [Google Scholar] [CrossRef] [PubMed]
- Kailasapathy, K.; Chin, J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000, 78, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; She, Y.; Deng, M.; Guo, Y.; Li, Y.; Liu, G.; Zhang, H.; Sun, B.; Liu, D. The Effect of Capsaicin on Growth Performance, Antioxidant Capacity, Immunity and Gut Micro-Organisms of Calves. Animals 2023, 13, 2309. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Lu, S.Y.; Liu, Y.; Tang, S.; Zhang, W.; Yu, Q.; Shi, C.; Cheong, K.L. Gracilaria lemaneiformis polysaccharides alleviate colitis by modulating the gut microbiota and intestinal barrier in mice. Food Chem. X 2021, 13, 100197. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Kawata, A.; Suzuki, S.; Fujisawa, S. Cytotoxicity and Pro-/Anti-inflammatory Properties of Cinnamates, Acrylates and Methacrylates Against RAW264.7 Cells. In Vivo 2018, 32, 1309–1322. [Google Scholar] [CrossRef]
- Lilin, E.; Li, W.; Hu, Y.; Deng, L.; Yao, J.; Zhou, X. Methyl cinnamate protects against dextran sulfate sodium-induced colitis in mice by inhibiting the MAPK signaling pathway. Acta Biochim. Biophys. Sin. 2023, 55, 1806–1818. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, J.; Deng, J.; Duan, Z.; Zhu, C.; Fan, D. The protective effect of protopanaxatriol-type saponin on intestinal health in antibiotic-treated mice. Food Funct. 2019, 10, 4124–4133. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, T.; Xia, X.; Fu, C.; Wang, X.; Zhao, Y. Dietary Ginsenoside T19 Supplementation Regulates Glucose and Lipid Metabolism via AMPK and PI3K Pathways and Its Effect on Intestinal Microbiota. J. Agric. Food Chem. 2020, 68, 14452–14462. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, C.; Shu, Z.; Wang, X.; Zhao, Y.; Song, W.; Chen, X.; Jin, M.; Xiu, Y.; Guo, X.; et al. Isovanillic acid protects mice against Staphylococcus aureus by targeting vWbp and Coa. Future Microbiol. 2023, 18, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Ghani, N.; Zhao, X.; Good, J.; Huang, A.; Wrona, H.L.; Liu, J.; Liu, C.J. Dietary pyruvate targets cytosolic phospholipase A2 to mitigate inflammation and obesity in mice. Protein Cell 2024, 15, 661–685. [Google Scholar] [CrossRef]
- Ren, M.; Wang, Y.; Lin, L.; Li, S.; Ma, Q. α-Linolenic Acid Screened by Molecular Docking Attenuates Inflammation by Regulating Th1/Th2 Imbalance in Ovalbumin-Induced Mice of Allergic Rhinitis. Molecules 2022, 27, 5893. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, Y.; Mai, J.; Guo, G.; Meng, J.; Fang, X.; Chen, X.; Liu, C.; Zhong, S. Comprehensive Metabolic Profiling of Inflammation Indicated Key Roles of Glycerophospholipid and Arginine Metabolism in Coronary Artery Disease. Front. Immunol. 2022, 13, 829425. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, S.; Hou, L.; Li, K.; Wang, L.; Gao, K.; Yang, X.; Jiang, Z. Supplemental Choline Modulates Growth Performance and Gut Inflammation by Altering the Gut Microbiota and Lipid Metabolism in Weaned Piglets. J. Nutr. 2021, 151, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Bakky, M.A.H.; Tran, N.T.; Zhang, Y.; Hu, H.; Lin, H.; Zhang, M.; Liang, H.; Zhang, Y.; Li, S. Effects of dietary supplementation of Gracilaria lemaneiformis-derived sulfated polysaccharides on the growth, antioxidant capacity, and innate immunity of rabbitfish (Siganus canaliculatus). Fish. Shellfish. Immunol. 2023, 139, 108933. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Y.; Ma, L.; Gu, F.; Liao, K.; Liu, Y.; Zhang, Y.; Liu, H.; Hong, Y.; Cao, M.; et al. Sulfate oligosaccharide of Gracilaria lemaneiformis modulates type 1 immunity by restraining T cell activation. Carbohydr. Polym. 2022, 288, 119377. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Long, W.; Zhang, J.; Jian, C.; Chen, J.; Huang, J.; Li, S.; Zhang, J.; Wang, L.; Chen, Y.; et al. Integrated multi-omics revealed that dysregulated lipid metabolism played an important role in RA patients with metabolic diseases. Arthritis Res. Ther. 2024, 26, 188. [Google Scholar] [CrossRef]
- Berg, E.P.; Maddock, K.R.; Linville, M.L. Creatine monohydrate supplemented in swine finishing diets and fresh pork quality: III. Evaluating the cumulative effect of creatine monohydrate and alpha-lipoic acid. J. Anim. Sci. 2003, 81, 2469–2474. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.B.; Soto, J.A.; Stein, H.H. Effects on nitrogen balance and metabolism of branched-chain amino acids by growing pigs of supplementing isoleucine and valine to diets with adequate or excess concentrations of dietary leucine. J. Anim. Sci. 2020, 98, skaa346. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Hao, R.; Li, Q.; Zhao, J.; Li, H.; Wang, W.; Gao, J. Effects of grape seed procyanidins on growth performance, immune function and antioxidant capacity in weaned piglets. Livest. Sci. 2015, 178, 237–242. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, Y.; Li, J.; Qu, H.; Zhao, Y.; Wen, C.; Zhou, Y. Dietary β-sitosterol regulates serum lipid level and improves immune function, antioxidant status, and intestinal morphology in broilers. Poult. Sci. 2020, 99, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Tran, G.B.; Nguyen, C.T. Anti-oxidative effects of superoxide dismutase 3 on inflammatory diseases. J. Mol. Med. 2020, 98, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M. Nutritional strategies to optimize dairy cattle immunity. J. Dairy. Sci. 2016, 99, 4967–4982. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, Y.; Li, J.; Qu, H.; Zhao, Y.; Wen, C.; Zhou, Y. Dietary β-Sitosterol Improves Growth Performance, Meat Quality, Antioxidant Status, and Mitochondrial Biogenesis of Breast Muscle in Broilers. Animals 2019, 9, 71. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | Content |
|---|---|
| Corn | 58.00 |
| Soybean meal | 14.00 |
| Wheat bran | 9.00 |
| Grass meal | 15.00 |
| Premix a | 4.00 |
| Total | 100.00 |
| Nutrient levels b | |
| Digestive energy/(MJ/kg) | 11.33 |
| Crude protein | 13.13 |
| Crude fiber | 9.10 |
| Crude ash | 8.30 |
| Calcium | 0.60 |
| Phosphorus | 0.50 |
| Lysine | 0.90 |
| Type | Df | Sums of Squares | R2 | F | p-Value |
|---|---|---|---|---|---|
| Group | 1 | 0.046453 | 0.256689 | 1.381328 | 0.400000 |
| Residual | 4 | 0.134517 | 0.743311 | — | — |
| Total | 5 | 0.180970 | 1.000000 | — | — |
| Items | NC | GLP | p-Value |
|---|---|---|---|
| Firmicutes | 57.56 ± 5.13 | 78.57 ± 2.72 | 0.00 |
| Proteobacteria | 28.98 ± 2.32 | 10.34 ± 0.35 | 0.00 |
| Actinobacteriota | 12.09 ± 1.08 | 7.43 ± 1.81 | 0.09 |
| Lactobacillus | 21.47 ± 0.20 | 27.09 ± 0.33 | 0.02 |
| Shigella | 12.46 ± 0.65 | 4.67 ± 0.75 | 0.00 |
| Streptococcus | 7.36 ± 1.39 | 5.36 ± 1.01 | 0.31 |
| Clostridium | 3.61 ± 0.47 | 8.24 ± 0.49 | 0.00 |
| SMB53 | 2.47 ± 0.54 | 6.10 ± 2.09 | 0.05 |
| Turicibacter | 2.97 ± 0.62 | 4.72 ± 1.91 | 0.43 |
| Items | NC | GLP | p-Value |
|---|---|---|---|
| SOD, U/mL | 64.91 ± 1.10 | 69.48 ± 3.30 | 0.24 |
| GSH-Px, U/mL | 599.80 ± 9.18 | 680.81 ± 17.37 | 0.00 |
| CAT, U/mL | 118.63 ± 8.83 | 131.80 ± 5.92 | 0.24 |
| MDA, nmol/mL | 0.812 ± 0.15 | 0.74 ± 0.09 | 0.70 |
| T-AOC, U/mL | 6.92 ± 0.30 | 10.08 ± 0.68 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Ma, Q.; Wang, H.; Yan, X.; Wang, L.; Xing, B.; Lu, Q.; Wang, J. Exploring the Effects of Gracilaria lemaneiformis Polysaccharides on the Fecal Microbiota and Fecal Metabolites of Fattening Pigs Based on 16S rDNA and Metabolome Sequencing. Animals 2025, 15, 153. https://doi.org/10.3390/ani15020153
Jia M, Ma Q, Wang H, Yan X, Wang L, Xing B, Lu Q, Wang J. Exploring the Effects of Gracilaria lemaneiformis Polysaccharides on the Fecal Microbiota and Fecal Metabolites of Fattening Pigs Based on 16S rDNA and Metabolome Sequencing. Animals. 2025; 15(2):153. https://doi.org/10.3390/ani15020153
Chicago/Turabian StyleJia, Mingyang, Qiang Ma, Hongjun Wang, Xiangzhou Yan, Lei Wang, Baosong Xing, Qingxia Lu, and Jing Wang. 2025. "Exploring the Effects of Gracilaria lemaneiformis Polysaccharides on the Fecal Microbiota and Fecal Metabolites of Fattening Pigs Based on 16S rDNA and Metabolome Sequencing" Animals 15, no. 2: 153. https://doi.org/10.3390/ani15020153
APA StyleJia, M., Ma, Q., Wang, H., Yan, X., Wang, L., Xing, B., Lu, Q., & Wang, J. (2025). Exploring the Effects of Gracilaria lemaneiformis Polysaccharides on the Fecal Microbiota and Fecal Metabolites of Fattening Pigs Based on 16S rDNA and Metabolome Sequencing. Animals, 15(2), 153. https://doi.org/10.3390/ani15020153






