A Review of Assessment of Sow Pain During Farrowing Using Grimace Scores
Simple Summary
Abstract
1. Introduction
2. Physiology of Farrowing and Parturient Pain
2.1. Farrowing Physiology
2.2. ”Normal” and “Abnormal” Pain During Farrowing
2.3. Implications of Parturient Pain
2.4. Factors Affecting Parturient Pain
2.5. Treatment of Parturient Pain
3. Measuring Pain in Domestic Animals
3.1. Pain as a Welfare Issue
3.2. Measuring and Quantifying Pain
3.3. Facial Grimace Scales
4. Measuring Pain in Farrowing Sows
4.1. Existing Methods of Pain Measurement During Farrowing
4.2. Limitations in the Understanding of Pain in Sows
4.3. The Sow Grimace Scale
4.4. Comparing Grimace Scales
4.5. Validity and Reliability of Grimace Scales
4.6. Validation of the Sow Grimace Scale
4.7. On-Farm Implementation of the Sow Grimace Scale
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FAU | Facial Action Units |
| IPI | Inter-piglet interval |
| NSAID | Non-steroidal anti-inflammatory drug |
References
- Dalgleish, M.; Whitelaw, A. State of the Industry Report 2021; Australian Pork Ltd.: Barton, ACT, Australia, 2021; Available online: https://australianpork.com.au/sites/default/files/2021-10/APLStateofIndustry-Report.pdf (accessed on 1 April 2025).
- Athorn, R.; Plush, K. Best Practice Gilt Management for Fertility and Longevity; Australian Pork Ltd.: Barton, ACT, Australia, 2019; Available online: https://australianpork.com.au/sites/default/files/2021-06/2019-09_Best_Practice_Gilt_Management_for_Fertility_and_Longevity.pdf (accessed on 10 April 2025).
- Oliviero, C.; Peltoniemi, O. Troubled process of parturition of the domestic pig. In Animal Reproduction in Veterinary Medicine; Aral, F., Payan-Carreira, R., Quaresma, M., Eds.; InTechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Alonso, M.E.; González-Montaña, J.R.; Lomillos, J.M. Consumers’ concerns and perceptions of farm animal welfare. Animals 2020, 10, 385. [Google Scholar] [CrossRef]
- Niven, C.; Gijsbers, K. A study of labour pain using the MCGILL pain questionnaire. Soc. Sci. Med. 1984, 19, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Burnes, J.; Muns, R.; Barrios-García, H.; Villanueva-García, D.; Domínguez-Oliva, A.; Mota-Rojas, D. Parturition in mammals: Animal models, pain and distress. Animals 2021, 11, 2960. [Google Scholar] [CrossRef] [PubMed]
- Mainau, E.; Manteca, X. Pain and discomfort caused by parturition in cows and sows. Appl. Anim. Behav. Sci. 2011, 135, 241–251. [Google Scholar] [CrossRef]
- Walls, A.; Hatze, B.; Lomax, S.; Bathgate, R. Defining “Normal” in pig parturition. Animals 2022, 12, 2754. [Google Scholar] [CrossRef]
- Crook, A. Introduction: Pain: An issue of animal welfare. In Pain Management in Veterinary Practice; Egger, C.M., Love, L., Doherty, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-1-1189-9919-6. [Google Scholar] [CrossRef]
- Mellor, D.J. Updating animal welfare thinking: Moving beyond the “Five Freedoms” towards “A life worth living”. Animals 2016, 6, 21. [Google Scholar] [CrossRef]
- Mogil, J.S.; Pang, D.S.J.; Dutra, G.G.S.; Chambers, C.T. The development and use of facial grimace scales for pain measurement in animals. Neurosci. Biobehav. Rev. 2020, 116, 480–493. [Google Scholar] [CrossRef]
- Navarro, E.; Mainau, E.; Manteca, X. Development of a facial expression scale using farrowing as a model of pain in sows. Animals 2020, 10, 2113. [Google Scholar] [CrossRef]
- Morton, D.B.; Griffiths, P.H.M. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef]
- Ison, S.H.; Jarvis, S.; Rutherford, K.M.D. The identification of potential behavioural indicators of pain in periparturient sows. Res. Vet. Sci. 2016, 109, 114–120. [Google Scholar] [CrossRef]
- Gilbert, C.L.; Lawrence, A.B.; Forsling, M.L.; Goode, J.A.; McGrath, T.J.; McLean, K.A.; Petherick, J.C. Maternal plasma vasopressin, oxytocin and cortisol concentrations following foetal ejection in the pig. Anim. Reprod. Sci. 1996, 43, 137–150. [Google Scholar] [CrossRef]
- Mainau, E.; Ruiz-de-la-Torre, J.L.; Dalmau, A.; Salleras, J.M.; Manteca, X. Effects of meloxicam (Metacam®) on post-farrowing sow behaviour and piglet performance. Animal 2012, 6, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.; Kinch, R.; Dobkin, P.; Lebrun, M.; Taenzer, P. Severity of labour pain: Influence of physical as well as psychologic variables. Can. Med. Assoc. J. 1984, 130, 579–584. [Google Scholar] [PubMed]
- Noakes, D.E.; Parkinson, T.J.; England, G.C.W.; Arthur, G.H. (Eds.) Arthur’s Veterinary Reproduction and Obstetrics, 8th ed.; Harcourt Publishers Limited: Hitchin, UK, 2001; ISBN 978-0-7020-2556-3. [Google Scholar] [CrossRef]
- Lowe, N.K. The nature of labor pain. Am. J. Obstet. Gynecol. 2002, 186, 16–24. [Google Scholar] [CrossRef]
- Cowart, R.P. Parturition and dystocia in swine. In Current Therapy in Large Animal Theriogenology, 2nd ed.; Youngquist, R.S., Threlfall, W.R., Eds.; Elsevier Inc: Amsterdam, The Netherlands, 2007; pp. 778–784. ISBN 978-0-7216-9323-1. [Google Scholar] [CrossRef]
- Australian Government Department of Health and Aged Care. Pregnancy, Birth and Baby (PB&B). Giving Birth—Third Stage of Labour. Available online: https://www.pregnancybirthbaby.org.au/giving-birth-third-stage-of-labour (accessed on 1 April 2025).
- Ison, S.H.; Jarvis, S.; Hall, S.A.; Ashworth, C.J.; Rutherford, K.M.D. Periparturient behaviour and physiology: Further insight into the farrowing process for primiparous and multiparous sows. Front. Vet. Sci. 2018, 5, 122. [Google Scholar] [CrossRef]
- Ison, S.H.; Rutherford, K.M.D. Attitude of farmers and veterinarians towards pain and the use of pain relief in pigs. Vet. J. 2014, 202, 622–627. [Google Scholar] [CrossRef]
- Edwards, S.A.; Malkin, S.J.; Spechter, H.H. An analysis of piglet mortality with behavioural observations. In Proceedings of the British Society of Animal Production; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar] [CrossRef]
- Haussmann, M.F.; Lay, D.C.; Buchanan, H.S.; Hopper, J.G. Butorphanol tartrate acts to decrease sow activity, which could lead to reduced pig crushing. J. Anim. Sci. 1999, 77, 2054–2059. [Google Scholar] [CrossRef]
- Schmitt, O.; Baxter, E.M.; Boyle, L.A.; O’Driscoll, K. Nurse sow strategies in the domestic pig: II. Consequences for piglet growth, suckling behaviour and sow nursing behaviour. Animal 2019, 13, 590–599. [Google Scholar] [CrossRef]
- Ahlström, S.; Jarvis, S.; Lawrence, A.B. Savaging gilts are more restless and more responsive to piglets during the expulsive phase of parturition. Appl. Anim. Behav. Sci. 2002, 76, 83–91. [Google Scholar] [CrossRef]
- Fraser, D.; Phillips, P.A. Lethargy and low water intake by sows during early lactation: A cause of low piglet weight gains and survival? Appl. Anim. Behav. Sci. 1989, 24, 13–22. [Google Scholar] [CrossRef]
- Oliviero, C.; Heinonen, M.; Valros, A.; Peltoniemi, O. Environmental and sow-related factors affecting the duration of farrowing. Anim. Reprod. Sci. 2010, 119, 85–91. [Google Scholar] [CrossRef]
- Canario, L.; Cantoni, E.; Le Bihan, E.; Caritez, J.C.; Billion, Y.; Bidanel, J.P.; Foulley, J.L. Between-breed variability of stillbirth and its relationship with sow and piglet characteristics. J. Anim. Sci. 2006, 84, 3185–3196. [Google Scholar] [CrossRef]
- van Dijk, A.J.; van Rens, B.T.T.M.; van der Lende, T.; Taverne, M.A.M. Factors affecting duration of the expulsive stage of parturition and piglet birth intervals in sows with uncomplicated, spontaneous farrowings. Theriogenology 2005, 64, 1573–1590. [Google Scholar] [CrossRef] [PubMed]
- Nam, N.H.; Sukon, P. Risk factors associated with dystocia in swine. Vet. World 2021, 14, 1835–1839. [Google Scholar] [CrossRef] [PubMed]
- Mainau, E.; Dalmau, A.; Ruiz-de-la-Torre, J.L.; Manteca, X. A behavioural scale to measure ease of farrowing in sows. Theriogenology 2010, 74, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- van Rens, B.T.T.M.; van der Lende, T. Parturition in gilts: Duration of farrowing, birth intervals and placenta expulsion in relation to maternal, piglet and placental traits. Theriogenology 2004, 62, 331–352. [Google Scholar] [CrossRef]
- Zaremba, W.; Udluft, T.; Failing, K.; Bostedt, H. Analysis of the course of birth and the early postpartal period in pigs after hormonal partus induction with special consideration of complication rate. Anim. Vet. Sci. 2019, 7, 29–39. [Google Scholar] [CrossRef]
- Lawrence, A.B.; Petherick, J.C.; McLean, K.A.; Deans, L.A.; Chirnside, J.; Gaughan, A.; Clutton, E.; Terlouw, E.M.C. The effect of environment on behaviour, plasma cortisol and prolactin in parturient sows. Appl. Anim. Behav. Sci. 1994, 39, 313–330. [Google Scholar] [CrossRef]
- Oliviero, C.; Heinonen, M.; Valros, A.; Hälli, O.; Peltoniemi, O.A.T. Effect of the environment on the physiology of the sow during late pregnancy, farrowing and early lactation. Anim. Reprod. Sci. 2008, 105, 365–377. [Google Scholar] [CrossRef]
- Ison, S.H.; Jarvis, S.; Rutherford, K.M.D. A survey of sow management at farrowing in the UK. Anim. Welf. 2016, 25, 309–317. [Google Scholar] [CrossRef]
- Kuller, W.; Sietsma, S.; Hendriksen, S.; Sperling, D. Use of paracetamol in sows around farrowing: Effect on health and condition of the sow, piglet mortality, piglet weight and piglet weight gain. Porc. Health Manag. 2021, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Schoos, A.; Chantziaras, I.; Vandenabeele, J.; Biebaut, E.; Meyer, E.; Cools, A.; Devreese, M.; Maes, D. Prophylactic use of meloxicam and paracetamol in perpartal sows suffering from postpartum dysgalactia syndrome. Front. Vet. Sci. 2020, 8, 603719. [Google Scholar] [CrossRef]
- Björkman, S.; Grahofer, A. Tools and protocols for managing hyperprolific sows at parturition: Optimizing piglet survival and sows’ reproductive health. In Animal Reproduction in Veterinary Medicine; Aral, F., Payan-Carreira, R., Quaresma, M., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83881-938-5. [Google Scholar] [CrossRef]
- Hill, S.V.; del Rocio Amezcua, M.; Ribeiro, E.S.; O’Sullivan, T.L.; Friendship, R.M. Defining the effect of oxytocin use in farrowing sows on stillbirth rate: A systemic review with a meta-analysis. Animals 2022, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Merskey, H. Pain terms: A list with definitions and a note on usage. Recommended by the International Association for the Study of Pain (IASP) Subcommittee on Taxonomy. Pain 1979, 6, 249–252. [Google Scholar]
- Short, C.E. Fundamentals of pain perception in animals. Appl. Anim. Behav. Sci. 1998, 59, 125–133. [Google Scholar] [CrossRef]
- Sneddon, L.U.; Elwood, R.W.; Adamo, S.A.; Leach, M.C. Defining and assessing animal pain. Anim. Behav. 2014, 97, 201–212. [Google Scholar] [CrossRef]
- McLennan, K.M. Why pain is still a welfare issue for farm animals, and how facial expression could be the answer. Agriculture 2018, 8, 127. [Google Scholar] [CrossRef]
- Mellor, D.J.; Reid, C.S.W. Concepts of animal well-being and predicting the impact of procedures on experimental animals. In Improving the Well-Being of Animals in the Research Environment; Baker, R.M., Jenkin, G., Mellor, D.J., Eds.; Australian and New Zealand Council for the Care of Animals in Research and Teaching (ANZCCART): Glen Osmond, SA, Australia, 1994; pp. 3–18. ISBN 978-0-6461-8116-5. [Google Scholar]
- Whittaker, A.L.; Marsh, L.E. The role of behavioural assessment in determining ‘positive’ affective states in animals. CABI Rev. 2019, 14, 1–13. [Google Scholar] [CrossRef]
- Murrell, J.C.; Johnson, C.B. Neurophysiological techniques to assess pain in animals. J. Vet. Pharmacol. Ther. 2006, 29, 325–335. [Google Scholar] [CrossRef]
- Prunier, A.; Mounier, L.; Le Neindre, P.; Leterrier, C.; Mormède, P.; Paulmier, V.; Prunet, P.; Terlouw, C.; Guatteo, R. Identifying and monitoring pain in farm animals: A review. Animal 2012, 7, 998–1010. [Google Scholar] [CrossRef]
- Anil, S.S.; Anil, L.; Deen, J. Challenges of pain assessment in domestic animals. J. Am. Vet. Med. Assoc. 2002, 220, 313–319. [Google Scholar] [CrossRef]
- Darwin, C. The Expression of the Emotions in Man and Animals; Cambridge University Press: Cambridge, UK, 1872; ISBN 978-1-1398-3381-3. [Google Scholar] [CrossRef]
- Ekman, P.; Friesen, W.V. Facial Action Coding System: Investigator’s Guide; APA PsycTests: Washington, DC, USA, 1978. [Google Scholar] [CrossRef]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; Clarke, S.E.; Drummond, T.E.; Echols, S.; Glick, S.; Ingrao, J.; Klassen-Ross, T.; LaCroix-Fralish, M.L.; et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 2010, 7, 447–449. [Google Scholar] [CrossRef]
- Sotocina, S.G.; Sorge, R.E.; Zaloum, A.; Tuttle, A.H.; Martin, L.J.; Wieskopf, J.S.; Mapplebech, J.C.S.; Wei, P.; Zhan, S.; Zhang, S.; et al. The rat grimace scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 2011, 7, 1744–8069. [Google Scholar] [CrossRef]
- Keating, S.C.J.; Thomas, A.A.; Flecknell, P.A.; Leach, M.C. Evaluation of EMLA cream for preventing pain during tattooing of rabbits: Changes in physiological, behavioural and facial expression responses. PLoS ONE 2012, 7, e44437. [Google Scholar] [CrossRef] [PubMed]
- Gleerup, K.B.; Forkman, B.; Lindegaard, C.; Andersen, P.H. An equine pain face. Vet. Anaesth. Analg. 2015, 42, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Gleerup, K.B.; Andersen, P.H.; Munksgaard, L.; Forkman, B. Pain evaluation in dairy cattle. Appl. Anim. Behav. Sci. 2015, 171, 25–32. [Google Scholar] [CrossRef]
- Guesgen, M.J.; Beausoleil, N.J.; Leach, M.; Minot, E.O.; Stewart, M.; Stafford, K.J. Coding and quantification of a facial expression for pain in lambs. Behav. Process. 2016, 132, 49–56. [Google Scholar] [CrossRef]
- Di Giminiani, P.; Brierley, V.L.M.H.; Scollo, A.; Gottardo, F.; Malcolm, E.M.; Edwards, S.A.; Leach, M.C. The assessment of facial expressions in piglets undergoing tail docking and castration: Toward the development of the piglet grimace scale. Front. Vet. Sci. 2016, 3, 100. [Google Scholar] [CrossRef]
- Viscardi, A.V.; Hunniford, M.; Lawlis, P.; Leach, M.; Turner, P.V. Development of a piglet grimace scale to evaluate piglet pain using facial expressions following castration and tail docking: A pilot study. Front. Vet. Sci. 2017, 4, 51. [Google Scholar] [CrossRef]
- Reijgwart, M.L.; Schoemaker, N.J.; Pascuzzo, R.; Leach, M.C.; Stodel, M.; de Nies, L.; Hendriksen, C.F.M.; van der Meer, M.; Vinke, C.M.; van Zeeland, Y.R. The composition and initial evaluation of a grimace scale in ferrets after surgical implantation of a telemetry probe. PLoS ONE 2017, 12, e0187986. [Google Scholar] [CrossRef]
- Evangelista, M.C.; Watanabe, R.; Leung, V.S.Y.; Monteiro, B.P.; O’Toole, E.; Pang, D.S.J.; Steagall, P.V. Facial expressions of pain in cats: The development and validation of a feline grimace scale. Sci. Rep. 2019, 9, 19128. [Google Scholar] [CrossRef]
- Domínguez-Oliva, A.; Olmos-Hernández, A.; Hernández-Ávalos, I.; Lecona-Butrón, H.; Mora-Medina, P.; Mota-Rojas, D. Rat grimace scale as a method to evaluate animal welfare, nociception, and quality of the euthansia method of Wistar rats. Animals 2023, 13, 3161. [Google Scholar] [CrossRef] [PubMed]
- Matsumiya, L.C.; Sorge, R.E.; Sotocinal, S.G.; Tabaka, J.M.; Wieskopf, J.S.; Zaloum, A.; King, O.D.; Mogil, J.S. Using the mouse grimace scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 42–49. [Google Scholar] [PubMed]
- McLennan, K.M.; Miller, A.L.; Dalla Costa, E.; Stucke, D.; Corke, M.J.; Broom, D.M.; Leach, M.C. Conceptual and methodological issues relating to pain assessment in mammals: The development and utilisation of pain facial expression scales. Appl. Anim. Behav. Sci. 2019, 217, 1–15. [Google Scholar] [CrossRef]
- Herskin, M.S.; Di Giminiani, P. Pain in pigs: Characterisation, mechanisms and indicators. In Advances in Pig Welfare; Špinka, M., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 325–355. ISBN 978-0-08-101012-9. [Google Scholar] [CrossRef]
- Walls, A. An Investigation of Sow Parturition: Developing Tools, Technologies and Protocols to Improve Sow and Piglet Welfare. Unpublished. Doctoral Dissertation, University of Sydney, Sydney, Australia, 2024. [Google Scholar]
- Randall, G.C.B.; Kendall, J.Z.; Tsang, B.K.; Taverne, M.A.M. Endocrine changes following infusion of fetal pigs with corticotropin in litters of reduced numbers. Anim. Reprod. Sci. 1990, 23, 109–122. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Martínez-Burnes, J.; Napolitano, F.; Domínguez-Muñoz, M.; Guerrero-Legarreta, I.; Mora-Medina, P.; Ramírez-Necoechea, R.; Lezama-García, K.; Gonález-Lozano, M. Dystocia: Factors affecting parturition in domestic animals. CABI Rev. 2020, 15, 1–16. [Google Scholar] [CrossRef]
- Viscardi, A.V.; Turner, P.V. Use of meloxicam or ketoprofen for piglet pain control following surgical castration. Front. Vet. Sci. 2018, 5, 299. [Google Scholar] [CrossRef]
- Vullo, C.; Barbieri, S.; Catone, G.; Graïc, J.-M.; Magaletti, M.; Di Rosa, A.; Motta, A.; Tremolada, C.; Canali, E.; Dalla Costa, E. Is the piglet grimace scale (PGS) a useful welfare indicator to assess pain after cryptorchidectomy in growing pigs? Animals 2020, 10, 412. [Google Scholar] [CrossRef]
- Lou, M.E.; Porter, S.T.; Massey, J.S.; Ventura, B.; Deen, J.; Li, Y. The application of 3D landmark-based geometric morphometrics towards refinement of the piglet grimace scale. Animals 2022, 12, 1944. [Google Scholar] [CrossRef]
- Evangelista, M.C.; Monteiro, B.P.; Steagall, P.V. Measurement properties of grimace scales for pain assessment in nonhuman mammals: A systemic review. Pain 2022, 163, 697–714. [Google Scholar] [CrossRef]
- Yamamoto, K.; Tatsutani, S.; Ishida, T. Detection of nausea-like response in rats by monitoring facial expression. Front. Pharmacol. 2017, 7, 534. [Google Scholar] [CrossRef] [PubMed]
- Tuyttens, F.A.M.; Stadig, L.; Heerkens, J.L.T.; Van laer, E.; Buijs, S.; Ampe, B. Opinion of applied ethologists on expectation bias, blinding observers and other debiasing techniques. Appl. Anim. Behav. Sci. 2016, 181, 27–33. [Google Scholar] [CrossRef]
- Rutherford, K.M.D. Assessing pain in animals. Anim. Welf. 2002, 11, 31–53. [Google Scholar] [CrossRef]
- Norring, M.; Wikman, I.; Hokkanen, A.-H.; Kujala, M.V.; Hänninen, L. Empathic veterinarians score cattle pain higher. Vet. J. 2014, 200, 186–190. [Google Scholar] [CrossRef]
- Ellingsen, K.; Zanella, A.J.; Bjerkås, E.; Indrebø, A. The relationship between empathy, perception of pain and attitudes toward pets among Norwegian dog owners. Anthrozoös 2010, 23, 231–243. [Google Scholar] [CrossRef]
- Hellyer, P.W.; Frederick, C.; Lacy, M.; Salman, M.D.; Wagner, A.E. Attitudes of veterinary medical students, house officers, clinical faculty and staff toward pain management in animals. J. Am. Vet. Med. Assoc. 1999, 214, 238–244. [Google Scholar] [CrossRef]
- Huxley, J.N.; Whay, H.R. Current attitudes of cattle practitioners to pain and the use of analgesics in cattle. Vet. Rec. 2006, 159, 662–668. [Google Scholar] [CrossRef]
- Monteiro, B.P.; Lee, N.H.Y.; Steagall, P.V. Can cat caregivers reliably assess acute pain in cats using the feline grimace scale? A large bilingual global survey. J. Feline Med. Surg. 2023, 25, 1098612X221145499. [Google Scholar] [CrossRef]
- Trindade, P.H.E.; Lopez-Soriano, M.; Merenda, V.R.; Tomacheuski, R.M.; Pairis-Garcia, M.D. Effect of the observer’s gender bias monitoring acute pain using a validated behaviour scale in castrated piglets. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Broomé, S.; Feighelstein, M.; Zamansky, A.; Carreira Lencioni, G.; Haubro Andersen, P.; Pessanha, F.; Mahmoud, M.; Kjellström, H.; Ali Salah, A. Going deeper than tracking: A survey of computer-vision based recognition of animal pain and emotions. Int. J. Comput. Vis. 2023, 131, 572–590. [Google Scholar] [CrossRef]
- Nie, L.; Li, B.; Jiao, F.; Shao, J.; Yand, T.; Liu, Z. ASPP-YOLOv5: A study on constructing pig facial expression recognition for heat stress. Comput. Electron. Agric. 2023, 214, 108346. [Google Scholar] [CrossRef]
- Australian Pork Limited. Submission into the National Agricultural Workforce Strategy. August 2020. Available online: https://australianpork.com.au/sites/default/files/2022-02/APL%20NAWS%20Submission%20%281%29.pdf (accessed on 1 April 2025).
- Eckersall, P.D.; Saini, P.K.; McComb, C. The acute phase response of acid soluble glycoprotein, α1-acid glycoprotein. Ceruloplasmin, haptoglobin and C-reactive protein, in the pig. Vet. Immunol. Immunopathol. 1996, 51, 377–385. [Google Scholar] [CrossRef]
- Haga, H.A.; Ranheim, B. Castration of piglet: The analgesic effects of intratesticular and infrafunicular lidocaine injection. Vet. Anaesth. Analg. 2005, 32, 1–9. [Google Scholar] [CrossRef]
- Onuma, K.; Watanabe, M.; Sasaki, N. The grimace scale: A useful tool for assessing pain in laboratory animals. Exp. Anim. 2024, 73, 234–245. [Google Scholar] [CrossRef]
- Weary, D.M.; Niel, L.; Flower, F.C.; Fraser, D. Identifying and preventing pain in animals. Appl. Anim. Behav. Sci. 2006, 100, 64–76. [Google Scholar] [CrossRef]
| Animal | Cause of Pain | Facial Action Units (FAUs) |
|---|---|---|
| Mouse [54] | Injection of nociceptive compounds | Orbital tightening, nose bulge, cheek bulge, ear position, whisker change. |
| Rat [55] | Injection of nociceptive compounds | Orbital tightening, nose/cheek flattening, ear changes, whisker change. |
| Rabbit [56] | Ear tattooing | Orbital tightening, cheek flattening, pointed nose, whisker change. |
| Horse [57] | Tourniquet or topical capsaicin | Angled eye, withdrawn and tense stare, asymmetrical/low ears, square-like nostrils, muzzle tension, tension of the mimic muscles. |
| Cow [58] | Range of painful conditions | Orbital tightening, tense stare, tense and backwards ears, tension of facial muscles, strained or dilated nostrils, tension of the lips. |
| Sheep [59] | Tail docking | Orbital tightening, nose features, mouth features, cheek flattening, ear posture. |
| Piglet [60,61] | Castration and tail docking | Orbital tightening, cheek tightening/nose bulge, ear position. |
| Ferrett [62] | Intraperitoneal telemetry probe implantation | Orbital tightening, nose bulging, cheek bulging, ear changes. |
| Cat [63] | Admitted to veterinary hospital with abdominal pain | Whisker retraction, orbital tightening, muzzle tension, ear position, whisker position, head position. |
| Sow [12] | Farrowing | Tension above eyes, snout angle, neck tension, temporal tension and ear position, cheek tension. |
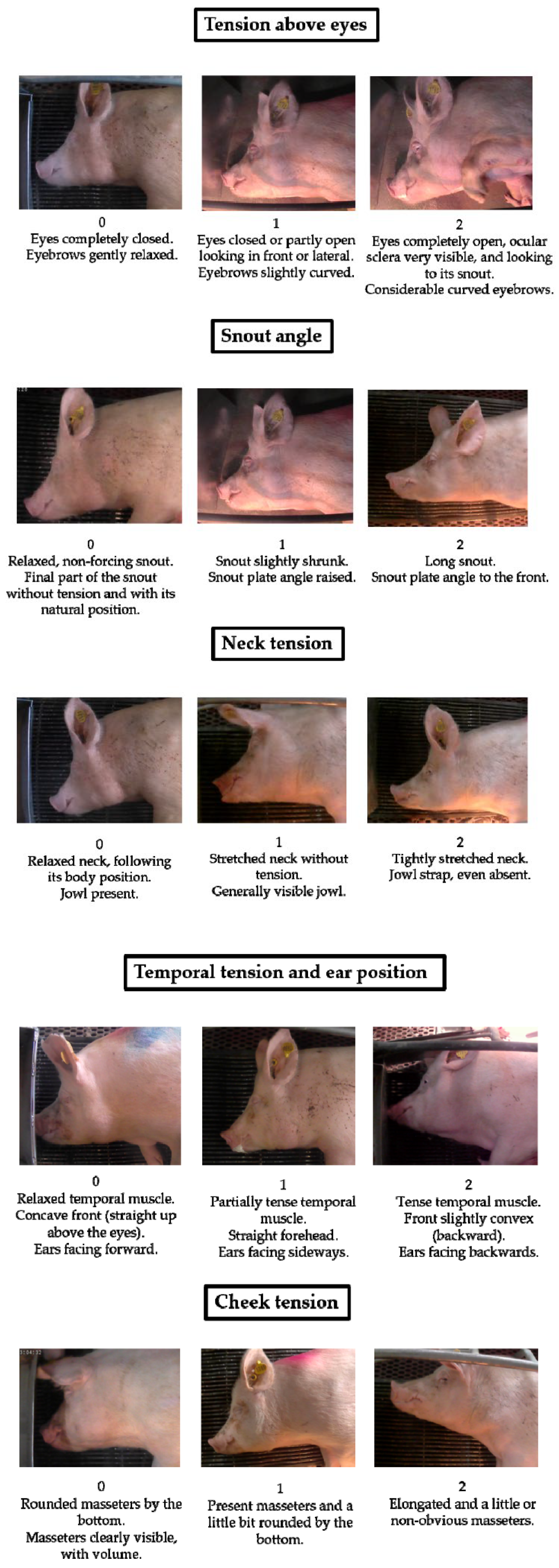
| Animal | Cheek FAU | Pain not Present | Moderate Pain | Severe Pain |
|---|---|---|---|---|
| Mouse [54] | “Cheek bulge” |  |  |  |
| Ferret [62] | “Cheek bulging” |  |  |  |
| Rat [55] | “Nose/cheek flattening” |  |  |  |
| Sheep [59] | “Cheek flattening” |  |  |  |
| Piglet [60] | “Cheek tension” |  |  |  |
| Sow [12] | “Cheek tension” | 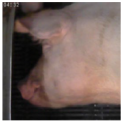 | 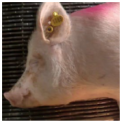 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmer, L.; Lomax, S.; Bathgate, R. A Review of Assessment of Sow Pain During Farrowing Using Grimace Scores. Animals 2025, 15, 2915. https://doi.org/10.3390/ani15192915
Palmer L, Lomax S, Bathgate R. A Review of Assessment of Sow Pain During Farrowing Using Grimace Scores. Animals. 2025; 15(19):2915. https://doi.org/10.3390/ani15192915
Chicago/Turabian StylePalmer, Lucy, Sabrina Lomax, and Roslyn Bathgate. 2025. "A Review of Assessment of Sow Pain During Farrowing Using Grimace Scores" Animals 15, no. 19: 2915. https://doi.org/10.3390/ani15192915
APA StylePalmer, L., Lomax, S., & Bathgate, R. (2025). A Review of Assessment of Sow Pain During Farrowing Using Grimace Scores. Animals, 15(19), 2915. https://doi.org/10.3390/ani15192915






